呈现强链内铁磁耦合的EO-叠氮与羧基双重桥连一维铜链化合物:合成、晶体结构、磁性及DFT计算
马晓慧李菲菲岑培培刘翔宇*,周惠良罗树常吴玥暐张诚诚宋伟明*,
呈现强链内铁磁耦合的EO-叠氮与羧基双重桥连一维铜链化合物:合成、晶体结构、磁性及DFT计算
马晓慧1李菲菲1岑培培1刘翔宇*,1周惠良1罗树常*,2吴玥暐1张诚诚1宋伟明*,1
(1宁夏大学化学化工学院,煤炭高效利用与绿色化工国家重点实验室,银川750021)
(2贵州工程应用技术学院化工系,毕节551700)
合成了一例以取代苯甲酸衍生物为辅助配体的叠氮铜化合物[Cu(4-Fb)(N3)(H2O)]n(1)(4-Fb=4-formylbenzoate),并对其结构和磁性进行了表征。单晶结构研究表明,化合物1中的最小不对称单元包含一个晶体学独立的Cu离子,中心离子呈现了扭曲的四棱锥几何构型。相邻的Cu离子之间通过交替的μ-1,1-(EO)-叠氮和syn,syn-羧酸双重桥连接成一维线性金属链。磁性研究揭示,双重桥的超交换反补偿效应导致目标化合物中链内相邻的Cu离子之间表现出强的铁磁耦合作用(J=72.1 cm-1)。但是并没有观察到铁磁有序和慢磁弛豫现象。作为影响磁性能的重要结构参数,化合物中Cu-N-Cu的角度(113.34°)与已报道的含双重桥的叠氮铜体系相符。对化合物的磁构关系进行了讨论和探究。此外,密度泛函理论(DFT)计算结果为化合物中相邻Cu离子间的铁磁耦合作用提供了定性的理论解释。
叠氮铜;取代苯甲酸;晶体结构;铁磁性;DFT计算
0 Introduction
Themolecularmagnetismhasbeenahot research topic,in which great progress has been achieved[1-2].Research on the structural design and synthesis of molecule-based magnetic materials,including single-molecule magnets(SMMs)[3]and singlechain magnets(SCMs)[4],has made tremendous strides as a significant intersection between chemical synthesis and materials science and continues to be a productive area due to their intriguing structures, unique physical characteristics,and promising novel applications,such as magnetic sensors,magnetic switches and multifunctional magnetic devices[5-6].Since the first example of SCMs in 2001[4a],one-dimensional (1D)molecular assemblies have received considerable attention for the construction of new molecule-based magnets.Such materials are usually assembled by combining paramagnetic centers with suitable organic ligands that regulate the architectures and transmit magnetic exchange coupling[7].
A popular approach for constructing these types of materials is to employ short ligands capable of efficiently transmitting the magnetic coupling[8].In this sense,the azido ligand with three donor atoms is able to link metal ions in different coordination modes (Scheme 1),which induces rich structural diversity as well as a range of different magnetic properties in the azido-metal complexes[9-14].Especially,the azido-based Cucoordination polymers are among the most important kinds of azido-metal complexes owing to the superiority for understanding the fundamental nature ofmagneticinteractionsandmagneto-structural relationships on the molecular level[15].It is wellestablished that μ-1,3(end-to-end,EE)modes usually propagate antiferromagnetic while μ-1,1(end-on,EO) modes are usually ferromagnetic in the azido-Cucases,although the coupling between metal ions bridged by EO-azido ligands can be antiferromagnetic in the presence of other bridging ligands or for very large metal-N-metal angle[16].It has been suggested that the strongest ferromagnetic coupling in the EO-azido linker occurs at a Cu-N-Cu bond angle close to 108°,and so an antiferromagnetic interaction would probably be found for larger Cu-N-Cu bond angles[17]. Consequently,an effective strategy for tuning the structures of azido-Cucompounds with notable magnetic properties is to introduce coligands into the systems.The most common coligands are carboxylatecontaining organics in which the carboxyl links the Cuions to generate various systems and adopts different bridging modes to transmit diverse superexchange interactions[18].A series of azido/carboxylate/ Cucompounds involving the combinations of μ-1,1-azido ligands and syn,syn-carboxylates have been previously prepared and performed intriguing structures and magnetisms[19].Most importantly,molecular orbital calculations dramatically support the counter complementary effect enforced by the carboxylateligand,which weakens the effect of the antiferromagnetic azido ligand to the point where dominant ferromagnetic behavior is obtained[20].It still remains significant challenge to design and configure the desired system for clarifying the complicated and crucial issues of azido-Cucompounds,such as the key factors influencing the magnetic interactions,the regulation of magneto-structural correlation,and the mechanism of magnetization.
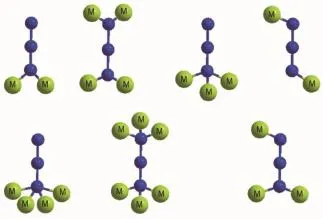
Scheme 1Coordination modes of bridging azido
Based on the considerations above,we report the synthesis,structural characterization,and magnetic properties of a 1D azido-Cucoordination polymer, [Cu(4-Fb)(N3)(H2O)]n(1)(4-Fb=4-formylbenzoate),in which the intrachain Cuions are connected by a mixed-bridge of syn,syn-carboxylate and μ-1,1-azido ligand with Cu-N-Cu angle of 113.34°.Magnetic investigations suggest that compound 1 shows strong ferromagnetic coupling between neighboring Cuions, which is further explored by density functional theory (DFT)calculations as well.
1 Experimental
1.1 Physical measurements
Elemental analysis(C,H,N)was performed on a Perkin-Elmer 2400 CHN elemental analyzer.The FTIR spectra were recorded in the range of 400~4 000 cm-1using KBr pellets on an EQUINOX55 FT/IR spectrophotometer.The phase purity of the bulk or polycrystalline samples were verified by powder X-ray diffraction(PXRD)measurements performed on a Rigaku RU200 diffractometer at 60 kV,300 mA and Cu Kα radiation(λ=0.071 073 nm),with a scan speed of 5°·min-1,a step size of 0.02°and a scan range of 5°~50°(2θ).Temperature-dependent magnetic measurement was obtained on poly-crystalline sample using a Quantum Design MPMS-XL7 SQUID magnetometer at temperatures range 1.9~300 K with an applied field of 1 000 Oe(restrained in eicosane to prevent torqueing at high fields).Magnetization measurements were taken at 2.0 K from 0 to 50 kOe.All data were corrected for diamagnetism estimated from Pascal′s constants,and an experimental correction for the sample holder was applied.
1.2 Materials and methods
All of the solvents and reagents for synthesis are of analytical grade and are commercially available. Cu(NO3)2·3H2O,4-formylbenzoic acid(4-Fba)and NaN3were purchased from commercial sources and used without further purification.
Caution!Although we have not experienced any problems in our experiments,azido and its compounds are potentially explosive;only a small amount of material should be prepared and handled with care.
1.3 Preparation of[Cu(4-Fb)(N3)(H2O)]n(1)
Compound1washydrothermallysynthesized under autogenous pressure.A mixture of Cu(NO3)2· 3H2O(0.051 g,0.3 mmol),4-Fba(0.045 g,0.3 mmol), NaN3(0.033 g,0.5 mmol)and H2O(8 mL)was sealed in a 15 mL Teflon-lined autoclave and heated to 120℃.After being maintained for 3 days,the reaction vessel was cooled to 20℃in 12 h.Green crystals were collected(Yield:80%,based on Cu).Anal.Calcd.for CuC8H7N3O4(%):C,35.23;H,2.58;N,15.41.Found(%): C,35.21;H,2.57;N,15.39.IR(KBr,cm-1):3 414 (m),2 094(m),1 662(m),1 608(s),1 375(s),1 317 (w),1 225(s),1 179(s),1 063(s),1 037(s),867(w),774 (w),688(m),584(w).
1.4 Crystallographic data collection and refinement
Suitable single crystal of the compound was mounted on glass fibers for X-ray measurements. Reflection data were collected at room temperature on a Bruker SMART APEX-CCD-based diffractometer using graphite mono-chromated Mo Kα radiation(λ= 0.071 073 nm).An empirical absorption correction was applied using the SADABS program[21].Data processing was accomplished with the SAINT processing program.The structures were solved by the direct methods and refined with full-matrix least-squares on F2using SHELXTL 97 program[22].All non-hydrogen atoms were refined with anisotropic displacement parameters.Hydrogen atoms were placed in geometrically calculated positions.Selected crystallographic data and structural refinement details for1 are summarized in Table 1.Selected bond lengths and bond angles,and the hydrogen bonds of compound 1are listed in Table S1 and S2,respectively.
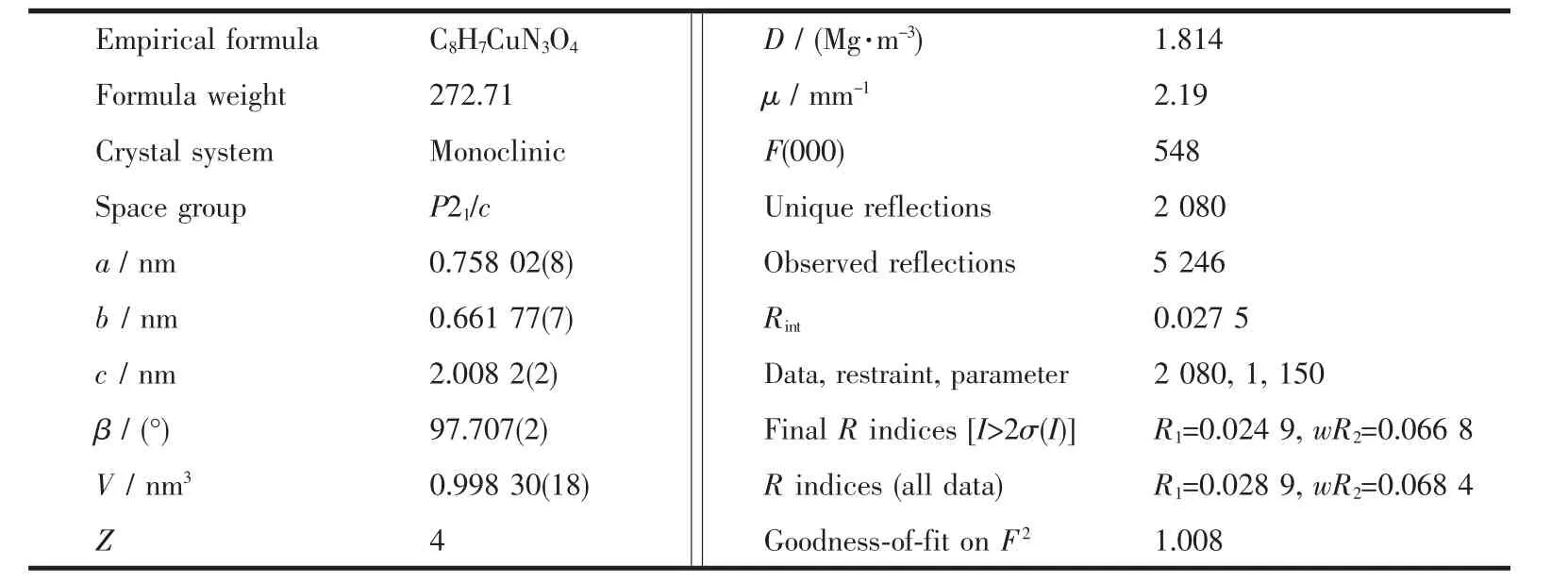
Table1 Selected crystallographic data and structure refinement for compound 1
CCDC:1496426.
1.5 Computational methodology
The following computational methodology was used to calculate the coupling constant in the title compound[23].The spin Hamiltonian suggested originally by Heisenberg can be written as Hˆ=-∑(i>j)JSiSj(where Siand Sjare the spin operators of the paramagnetic centers,Si=Sj=1/2 for Cuion;and the J constant is the coupling constant between the paramagnetic spin carriers),which can be employed to express the exchange coupling between two transition metal ions, the full Hamiltonian matrix for the entire system can be established.The J value was calculated from the energy difference of the two spin states:the broken symmetry(BS)state and the triplet state(HS),the broken symmetry approach along with electron correlations has been widely used to investigate magnetic properties in a large number of magnetic systems[24].The J value was calculated using the following equation:

where EBSis the energy of the broken symmetry singlet state and EHSis the energy of the triplet state.
The DFT calculations are implemented with the ORCA 3.0.2 package[25].The BP86 functional proposed by Becke[26]and Perdew[27a]and hybrid B3LYP functional built by Becke[27b]were applied in the calculations,respectively.The double-ξ quality plus polarization def2-SVP basis set and polarized triple-quality basis sets of def2-TZVP,TZVP,and TZV proposed by Ahlrichs and co-workers were respectively performed for all atoms[28].The calculation model for the compound was built from the experimental results.
2 Results and discussion
2.1 Crystal structure of 1
Single-crystal X-ray diffraction analysis reveals that compound 1 crystallizes in the monoclinic space group P21/c.The asymmetrical unit of compound 1 is composed by one Cucation,one azido ligand,one 4-Fb ligand and one coordinated water molecule.The penta-coordinated Cucation in the center presents a distorted tetragonal pyramid geometry(Fig.1b).The bottom square is formed by two nitrogen atoms(Cu1-N1 0.199 38 nm,Cu1-N1i0.199 46 nm)from two azido ligands and two oxygen atoms(Cu1-O1 0.194 08 nm,Cu1-O2i0.195 66 nm)from two carboxylate groupsof4-Fbligands.Theapicalpositionis occupied by one oxygen atom(Cu1-O3 0.233 92 nm) from coordinated water molecule(Fig.1a).Adjacent Cucations are mediated by EO-azido,μ2-bridging bidentate carboxylate groups,with a Cu-N-Cu angle of 113.34°and a Cu-Cu distance of 0.333 2 nm to yield a well-isolated 1D copper chain(Fig.1c).And then, the linear metal chains are integrated by interchain hydrogen-bonding between the O atom in the coordinated water and the terminal N atom in the azido anion(O4…N3 0.268 nm)(Fig.1d),constructing the supramolecular network of 1.In addition,the azido moieties are quasi linear with N1-N2-N3 angles of 178.8°,and the bond length of N1-N2(0.121 8 nm)is slightly longer than N2-N3(0.114 2 nm).The nearest distance of interchain Cuions is 0.758 0 nm.
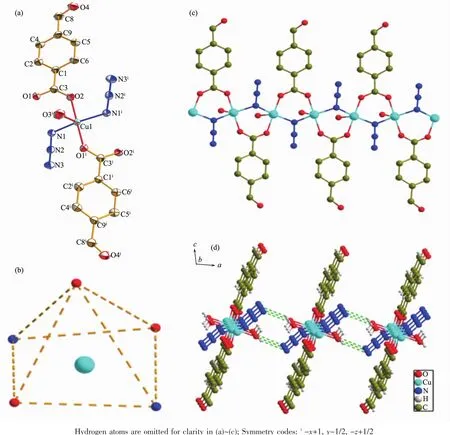
Fig.1 (a)Structure of 1 with 50%thermal ellipsoids;(b)Simplified tetragonal pyramidal geometry of the center Cucation for 1;(c)1D chain with carboxylate and azido bridges for 1;(d)Hydrogen bonding formed by azido and water molecules between adjacent chains in 1
2.2 Magnetic studies
The crystalline sample of 1 was all phase-pure, as confirmed by PXRD(Fig.S1).According to the obtained data,a dominant ferromagnetic coupling between the Cucations in compound 1 can be suggested.
The magnetic properties of 1 are shown in Fig.2 in the form of a χMT versus T plot(χMis the molar magnetic susceptibility per Cucation).χMT values are observed as 0.54 cm3·K·mol-1for 1 at 300 K, larger than the spin-only value(0.375 cm3·K·mol-1) for an isolated Cucation(S=1/2).Upon cooling,the χMT values increase gradually,and firstly the value increases to 3.99 cm3·K·mol-1at 4 K,suggesting ferromagnetic exchange between Cucations and finally drops to 2.01 cm3·K·mol-1at 1.9 K.The χMT vs T curve illustrates that strongly coupled ferromagneticsystemaccompanieswithantiferromagnetic interaction between the azido-Cuchains in the compound 1,especially at low temperature.The parameters fitted by the Curie-Weiss law above are obtained to be C=0.472 cm3·K·mol-1and θ=43.04 K. The positive θ value supports strong ferromagnetic coupling between the intrachain Cuions.Considering themean-fieldapproximation forinterchain coupling zJ′(Eq.2),the temperature-dependent magn-etic susceptibility data of 1 can be simulated with the formula proposed by Baker et al.(Eq.3)[29]for a ferromagnetic Cuchain(S=1/2)which is achieved from the high temperature series expansion.

Fig.2 χMT vs T and 1/χMvs T plots for compound 1
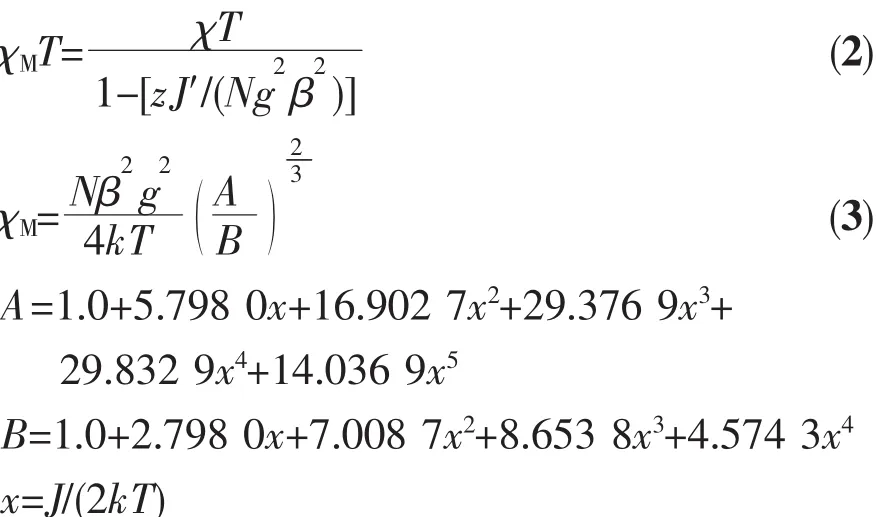
The best fit of the magnetic susceptibility data resulted in:g=2.27,J=72.1 cm-1,zJ′=-0.71 cm-1and R=5.75×10-5(g is the Zeeman factor of the metal ion, J describes the intrachain magnetic interaction,zJ′describes the interchain antiferromagnetic interaction and R accounts for the agreement factor defined as R=∑[(χMT)obsd-(χMT)calcd]2/∑[(χMT)obsd]2).The large J value supports strong ferromagnetic coupling between the Cucenters.The small negative zJ′value indicates the presence of interchain antiferromagnetic interactions,according with the drop in the χMT product at low temperature.In principle,it seems that intrachain antiferromagnetic coupling would be predicted for 1 due to the cooperation of EO-azido with the Cu-N-Cu angle of 113.34°(larger than the critical value of 108°) andthecarboxylategroupwithsyn-synmode. However,according to the proposition from Thompson et al.and Escuer et al.,the counter-complementarity function derived from two kinds of ligands may expound the strong ferromagnetic interaction,not just the total of the two isolated components[20].
AsshowninFig.3,theisothermalfielddependent magnetization M(H)values at 2 K and fields up to 50 kOe are measured for 1.The magnetization curveincreaseslinearlyunderverylowfield, subsequently climbs up quickly until 10 kOe and rises up gradually to 50 kOe with an effective moment of 1.06Nβ at the high fields,slightly higher than the saturation value(1.0Nβ)of one Cucation.Notably, the S-shaped curve emerges with a critical field of 1 200 Oe at low field(Fig.3,inset),signifying weakly interchain antiferromagnetic exchange and 1 might sustain decoupling effect of external field.
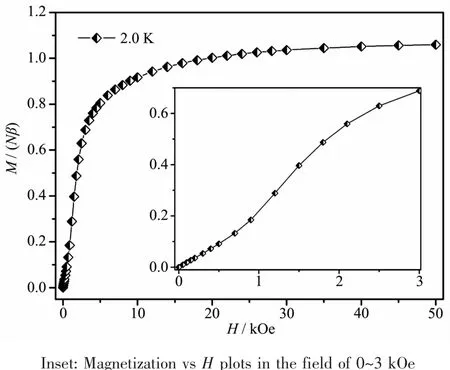
Fig.3 Magnetization vs H plots for 1
The dc magnetization is determined at 2,2.5, and 4 K within-7~7 kOe,which is shown in Fig.S2. Whenthetemperaturegoesdownto2K,no hysteresis loop emerges.The field-cooled(FC)and zero-field-cooled(ZFC)magnetization measurements were performed at a low applied field of 10 Oe below the temperature of 20 K(Fig.S3),the FC/ZFC plots with coincident pattern increase rapidly until the temperature drops to 2 K,indicating the absence of the ferromagnetic ordering.In addition,under the oscillatingfieldof3.5Oe,thezero-fieldAC susceptibility experiments for 1 were determined in the range of 1.9~25 K at various frequencies of 1,10, 33,100,333 and 1 000 Hz(Fig.S4).In-phase signal and no out-of-phase signal were observed until thetemperature drops to 2 K,which further verifies intrachain antiferromagnetic coupling and implies that the slow dynamics of magnetization and long-rang ferromagnetic ordering are nonexistent in compound 1. The in-phase(χ′)component of the AC magnetic susceptibility with a peak value at 4 K might explicate that compound 1 behaves as an antiferromagnet with extremely low Néel temperature.
2.3 Theoretical studies
In order to further clarify the ferromagnetic nature of the exchange interaction in compound 1,we performed a theoretical study of the isotropic coupling constants J between Cuions based on DFT calculation at BP86 and B3LYP level with the aid of ORCA program.According to the structure of 1, supposing that the dominant magnetic exchange is mediated between adjacent two Cuions through azidoandcarboxylate.Thecalculationsforthe compound were carried out with the model(for comparing)applied to the magnetic fitting by filling-in all the coordination sites of the Cuions(Fig.4).
The results of the theoretical calculation and the experimental fitting in terms of the coupling constants are listed in Table 2.Based on BP86 and B3LYP functions with def2-SVP,def2-TZVP,TZVP,and TZV basis sets,the calculated J values are unexceptionally found to be moderate positive values and close to the experimentally fitted values,which completely verifies that the strong ferromagnetic coupling is prevailing in compound 1.Obviously,the choices of the methods and basis sets for these calculations are simultaneously suitable for the title compound.Although the calculated values deviate slightly from the fitting values,in all the cases the sign and the relative magnitudes of the exchange parameters agree very well with the experimental results.It is difficult to say in general which basis set works better,but it is clear thattheresultsoftheoreticalcalculationprove qualitativelyandquantificationallythemeasuring data.Therefore,the tiny difference may result from the fact that the real compound is not scatteredentities as has been modeled but is very complicated in the whole structures.
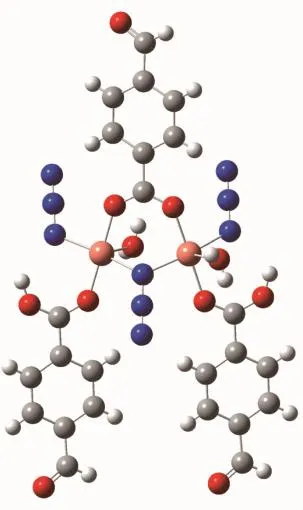
Fig.4 Magnetic cores of 1 used for computational study
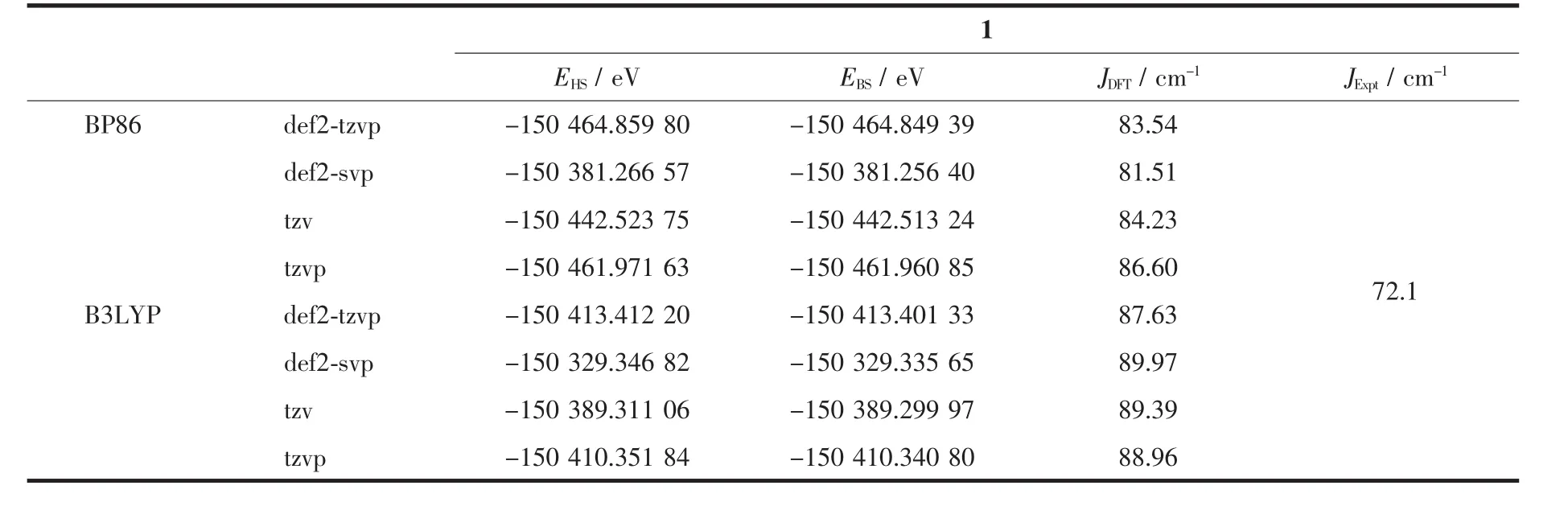
Table2 Comparison of the experimental(from fitting)and DFT studies
2.4 Discussion
In compound 1,two consecutive Cuions are bonded by syn-syn carboxylate and symmetric EO-azido bridges,constituting the 1D chain-like pattern. Intrachain Cu-Cu distances and Cu-N-Cu angles are 0.333 2 nm and 113.34°.The alternating 1D chains areinteractedthroughinterchainhydrogen-bonds derived from the water oxygen atom and the nitrogen atom of the azido group between nearby chains.The nearest interchain Cu…Cu separation is 0.758 0 nm. EO-azido is certainly one of the most interesting magnetic couplers in molecular magnetism,and the magnitude of the J parameter depends on several factors,but mainly the Cu-N-Cu angle(β).According to a number of studies on Cusystems with such bridges[30,17a],the single EO-azido motif could also mediate ferromagnetic coupling,EO-azido bridging Cuions with low β gives rise to ferromagnetic coupling,whereas the coupling is antiferromagnetic if the angle is above a critical β value which has been evaluatedtobeabout108°[19a].Inthissense, antiferromagnetic coupling would be expected for 1, owing to the presence of EO-azido bridges with a large Cu-N-Cu angle of 113.34°,together with the syn-syn carboxylate bridges featured in the chain. However,the fitting magnetic coupling parameter(J) confirmsthattheferromagneticinteractionsare enabled by the single EO-azido bridges in compound 1,probably due to the counter-complementarity effect from the syn-syn carboxylate bridge which usually transmitantiferromagneticinteractionsbetween neighbouring metal ions.
To deduce a general magneto-structural relationship,we have made a comprehensive comparison of the Cu-azido-benzoate compounds reported in recent years,as shown in Table S3[31-32,19a,33-34].EO-azido compounds with Cu-N-Cu angles of 126.8°[31a],108.2°[31a], 116.8°[31a],109.4°[31c],101.1°[31c],111.9°[31b],and 105.5°[33d]also exhibit strong ferromagnetic coupling.Similarly, in our work,the Cu-N-Cu angle is 113.34°,and the corresponding compound shows ferromagnetic interaction.The coupling constant value(J=72.1 cm-1)for compound 1 is comparable to previous results in the literature[31b,32a].
3 Conclusions
In present work,a new azido-copper compound with4-formylbenzoicacidascoligandhasbeen successfullyisolated.Structuralanalysesindicate compound 1 features a 1D two-fold bridged copper chain in which the coordination geometry of center Cuion is distorted tetragonal pyramid and the adjacent two Cuions is bridged by mixed μ-1,1(EO) -azidoandsyn-syn-carboxylateligands.Magnetic investigationsdemonstratethatthecompoundis composed of ferromagnetically coupled ferromagnetic chains.The intrachain behavior reflects how the countercomplementaryeffectimposedbythe carboxylate bridge overcomes the antiferromagnetic effect of the azido bridge resulting in an overall ferromagnetic interaction.DFT calculations qualitatively and quantificationally support the strong ferromagnetic coupling between the Cuions.
Supporting information is available at http://www.wjhxxb.cn
[1](a)Pei Y,Verdaguer M,Kahn O.J.Am.Chem.Soc.,1986, 108:7428-7430 (b)Miller J S,Calabrese J C,Rommelmann H,et al.J.Am. Chem.Soc.,1987,109:769-781
[2](a)Wang X Y,Wang Z M,Gao S.Inorg.Chem.,2008,47:5720 -5726 (b)Wang X Y,Wang L,Wang Z M,et al.J.Am.Chem.Soc., 2006,128:674-675 (c)Martín S,Barandika M G,Lezama L,et al.Inorg.Chem., 2001,40:4109-4115
[3](a)Ungur L,Lin S Y,Tang J K,et al.Chem.Soc.Rev.,2014, 43:6894-6905 (b)Gatteschi D,Sessoli R.Angew.Chem.,Int.Ed.,2003,42: 268-297 (c)Wernsdorfer W,Aliaga-Alcalde N,Hendrickson D N,et al. Nature,2002,416:406-409 (d)Woodruff D N,Winpenny R E,Layfield R A.Chem.Rev., 2013,113:5110-5148(e)Leng J D,Liu J L,Zheng Y Z,et al.Chem.Commun., 2013,49:158-160 (f)Zhang P,Zhang L,Tang J K.Dalton Trans.,2015,44:3923 -3929
[4](a)Caneschi A,Gatteschi D,Lalioti N,et al.Angew.Chem. Int.Ed.,2001,40:1760-1763 (b)Werner J,Rams M,Tomkowicz Z,et al.Inorg.Chem., 2015,54:2893-2901 (c)Vaz M G,Cassaro R A A,Akpinar H,et al.Chem.-Eur. J.,2014,20:5460-5467 (d)Wang Y Q,Cheng A L,Liu P P,et al.Chem.Commun., 2013,49:6995-6997 (e)Pardo E,Ruiz-García R,Lloret F,et al.Chem.-Eur.J., 2007,13:2054-2066 (f)Dhers S,Feltham H L,Brooker S.Coord.Chem.Rev., 2015,296:24-44
[5](a)Neville S M,Halder G J,Chapman K W,et al.J.Am. Chem.Soc.,2008,130:2869-2076 (b)Pardo E,Train C,Boubekeur K,et al.Inorg.Chem.,2012, 51:11582-11593 (c)Liu X Y,Qu X N,Zhang S,et al.Inorg.Chem.,2015,54: 11520-11525 (d)LI Hai-Qing(李海清),HUA Jing-Kun(华敬坤),ZHA Li-Qin(查丽琴),et al.Chinese J.Inorg.Chem.(无机化学学报),2015,31(7):1417-1424
[6](a)Weng D F,Wang Z M,Gao S.Chem.Soc.Rev.,2011,40: 3157-3181 (b)Ferbinteanu M,Miyasaka H,Wernsdorfer W,et al.J.Am. Chem.Soc.,2005,127:3090-3099 (c)Bogani L,Sangregorio C,Sessoli R,et al.Angew.Chem. Int.Ed.,2005,36:5967-5971 (d)Sessoli R,Powell A K.Coord.Chem.Rev.,2009,253:2328 -2341 (e)Jeremies A,Gruschinski S,Meyer M,et al.Inorg.Chem., 2016,55:1843-1853 (f)Liu X Y,Cen P P,Li F F,et al.RSC Adv.,2016,6:96103-96108
[7](a)Ferlay S,Mallah T,Ouahes R,et al.Nature,1995,378:701 -703 (b)Entley W R,Girolami G S.Science,1995,268:397-400 (c)Liu X Y,Sun L,Zhou H L,et al.Inorg.Chem.,2015,54: 8884-8886 (d)Chen M,Zhao H,Saudo E C,et al.Inorg.Chem.,2016, 55:3715-3717 (e)Liu X Y,Liu H X,Cen P P,et al.Inorg.Chim.Acta, 2016,447:12-17
[8](a)Lescouzec R,Toma L M,Vaissermann J,et al.Coord.Chem. Rev.,2005,249:2691-2729 (b)Miyasaka H,Julve M,Yamashita M,et al.Inorg.Chem., 2009,48:3420-3437 (c)Bernot K,Luzon J,Sessoli R,et al.J.Am.Chem.Soc., 2008,130:1619-1627 (d)Ding M,Wang B,Wang Z,et al.Chem.-Eur.J.,2012,18: 915-924 (e)Reger D L,Pascui A E,Smith M D,et al.Inorg.Chem., 2015,54:1487-1500 (f)SUN Lin(孙琳),LIU Huai-Xian(刘怀贤),ZHOU Hui-Liang (周惠良),et al.Chinese J.Inorg.Chem.(无机化学学报), 2015,31(6):1207-1214
[9]Kahn O.Molecular Magnetism.New York:VCH,1993.
[10](a)Ribas J,Escuer A,Monfort M,et al.Coord.Chem.Rev., 1999,1027:193-195 (b)Zeng Y F,Hu X,Liu F C,et al.Chem.Soc.Rev.,2009, 38:469-480 (c)Adhikary C,Koner S.Coord.Chem.Rev.,2010,254:2933 -2958 (d)BAI Shi-Qiang(白士强),FANG Chen-Jie(房晨婕),YAN Chun-Hua(严纯华).Chinese J.Inorg.Chem.(无机化学学报),2006,22(12):2123-2134
[11](a)Hong C S,Do Y.Angew.Chem.,Int.Ed.,1999,38:193-195 (b)Liu T F,Fu D,Gao S,et al.J.Am.Chem.Soc.,2003, 125:13976-13977
[12]Gao E Q,Bai S Q,Wang Z M,et al.J.Am.Chem.Soc., 2003,125:4984-4985
[13](a)Yoo H S,Kim I J,Yang N,et al.Inorg.Chem.,2007,46: 9054-9056 (b)Escuer A,Aroms G.Eur.J.Inorg.Chem.,2006,23:4721-4736
[14](a)Cheng M,Ding Y S,Gao E Q,et al.Dalton Trans.,2016, 45:8028-8035 (b)Schweinfurth D,Sommer M G,Atanasov M,et al.J.Am. Chem.Soc.,2015,137:1993-2005
[15](a)Liu J,Qin Y L,Qu M,et al.Dalton Trans.,2013,42:11571-11575 (b)Hu K L,Kurmoo M,Wang Z,et al.Chem.-Eur.J.,2009, 15:12050-12064 (c)Li J R,Yu Q,Sanudo C,et al.Chem.Commun.,2007,25: 2602-2604 (d)Liu X Y,Cen P P,Li H,et al.Inorg.Chem.,2014,53: 8088-8097 (e)FAN Yan(范艳),WANG Chen-Min(汪晨敏),QU Zhi-Rong(瞿志荣).Chinese J.Inorg.Chem.(无机化学学报), 2016,32(5):864-870
[16](a)Kahn O,Sikorav S,Gouteron J,et al.Inorg.Chem.,1983, 22:2877-2883 (b)Cortes R,Urtiaga M K,Lezama L,et al.Dalton Trans., 1993,24:3685-3694 (c)Thompson L K,Tandon S S.Comments Inorg.Chem., 1996,18:125-144 (d)Zhang L,Zuo J L,Gao S,et al.Angew.Chem.Int.Ed., 2000,39:3633-3635
[17](a)Ruiz E,Cano J,Alvarez S,et al.J.Am.Chem.Soc.,1998,120:11122-11129 (b)Cabrero J,Graaf C,Bordas E,et al.Chem.-Eur.J.,2003, 9:2307-2315 (c)Triki S,García C J G,Ruiz E et al.Inorg.Chem.,2005, 44:5501-5508 (d)Mialane P,Dolbecq A,Marrot J,et al.Chem.-Eur.J., 2005,11:1771-1778 (e)Nanda P K,Aromí G,Ray D.Chem.Commun.,2006,30: 3181-3183
[18](a)Shi W B,Cui A L,Kou H Z.ChemPlusChem,2014,79: 310-317 (b)Zhang S M,Chen Y H,Wang L H,et al.J.Solid State Chem.,2015,226:201-205 (c)Wang Y Q,Tan Q H,Guo X Y,et al.RSC Adv.,2016,6: 72326-72332
[19](a)Yang L,Zhang S,Liu X Y,et al.CrystEngComm,2014, 16:4194-4201 (b)Stamatatos T C,Vlahopoulou G,Raptopoulou C P,et al. Eur.J.Inorg.Chem.,2012,19:3121-3131 (c)Tangoulis V,Panagoulis D,Raptopoulou C P,et al.Dalton Trans.,2008,5:1752-1760
[20](a)Thompson L K,Tandon S S,Lloret F,et al.Inorg.Chem., 1997,36:3301-3306 (b)Escuer A,Vicente R,Mautner F A,et al.Inorg.Chem., 1997,36:1233-1236
[21]Sheldrick G M.SADABS,Program for Empirical Absorption Correction for Area Detector Data,University of Göttingen, Germany,1996.
[22]Sheldrick G M.SHELXS-97,Program for the Refinement of Crystal Structures,University of Göttingen,Germany,1997. [23](a)Ruiz E,Alemany P,Alvarez S,et al.J.Am.Chem.Soc., 1997,119:1297-1303 (b)Ruiz E,Rodríguez-Fortea A,Cano J,et al.J.Comput. Chem.,2003,24:982-989 (c)Ruiz E,Cano J,Alvarez S,et al.J.Comput.Chem.,1999, 20:1391-1400 (d)Ruiz E.Struct.Bonding,2004,113:71-102
[24](a)Sarkar S,Datta A,Mondal A,et al.J.Phys.Chem.B,2006, 110:12-15 (b)Cremades E,Ruiz E.Inorg.Chem.,2010,49:9641-9648 (c)Gole B,Chakrabarty R,Mukherjee S,et al.Dalton Trans., 2010,39:9766-9778
[25](a)Neese F.ORCA-an ab initio,Density Functional and Semiempirical Program Package,Ver.3.0.1,University of Bonn,Bonn,Germany,2013. (b)Neese F.WIREs Comput.Mol.Sci.,2012,2:73-78
[26]Becke A D.Phys.Rev.A,1988,38:3098-3100
[27](a)Perdew J P.Phys.Rev.B,1986,33:8822-8824 (b)Becke A D.J.Chem.Phys.,1993,98:5648-5652
[28](a)Schäfer A,Horn H,Ahlrichs R.J.Chem.Phys.,1992,97: 2571-2577 (b)Schfer A,Huber C,Ahlrichs R.J.Chem.Phys.,1994, 100:5829-5835
[29]Baker G A,Rushbrooke G S.Phys.Rev.,1964,135:A1272
[30](a)Tandon S S,Thompson L K,Manuel M E,et al.Inorg. Chem.,1994,33:5555-5570 (b)Thompson L K,Tandon S S,Manuel M E.Inorg.Chem., 1995,34:2356-2366 (c)Sikorav S,Bkouche-Waksman I,Kahn O.Inorg.Chem., 1984,23:490-495
[31](a)Zhao J P,Hu B W,Saudo E C,et al.Inorg.Chem.,2009, 48:2482-2489 (b)Escuer A,Vicente R,Mautner F A,et al.Inorg.Chem., 1997,36:1233-1236 (c)Zhang X M,Wang Y Q,Song Y,et al.Inorg.Chem.,2011, 50:7284-7294
[32](a)Kostakis G E,Mondal K C,Abbas G,et al.CrystEngComm, 2009,11:2084-2088 (b)Su Q J,Li S H,Wang L,et al.Inorg.Chem.Commun., 2010,13:1210-1212 (c)Sengupta O,Gole B,Mukherjee S,et al.Dalton Trans., 2010,39:7451-7465 (d)Li X B,Ma Y,Zhang X M,et al.Eur.J.Inorg.Chem., 2011,30:4738-4744 (e)Mukherjee S,Patil Y P,Mukherjee P S.Dalton Trans., 2012,41:54-64 (f)Mukherjee S,Mukherjee P S.Dalton Trans.,2013,42:4019-4030
[33](a)Zeng Y F,Liu F C,Zhao J P,et al.Chem.Commun., 2006,21:2227-2229 (b)Gu Z G,Song Y,Zuo J L,et al.Inorg.Chem.,2007,46: 9522-9524 (c)Liu F C,Zeng Y F,Zhao J P,et al.Inorg.Chem.,2007, 46:7698-7700 (d)Han Y F,Wang T W,Song Y,et al.Inorg.Chem.Commun., 2008,11:207-209
[34](a)Sun W W,Qian X B,Tian C Y,et al.Inorg.Chim.Acta, 2009,362:2744-2748 (b)He Z,Wang Z M,Gao S,et al.Inorg.Chem.,2006,45: 6694-6705 (c)Zeng Y F,Zhao J P,Hu B W,et al.Chem.-Eur.J.,2007, 13:9924-9930 (d)Liu X Y,Chen S P,Grancha T,et al.Dalton Trans.,2014, 43:15359-15366 (e)Chakrabarty P P,Giri S,Schollmeyer D,et al.Polyhedron, 2015,89:49-54 (f)Setifi Z,Ghazzali M,Glidewell C,et al.Polyhedron,2016, 117:244-248 (g)Liu X Y,Li F F,Ma X H,et al.Dalton Trans.,2017,46: 1207-1217
One-Dimensional CuChain Compound with Simultaneous EO-Azido and Carboxylato Bridges Displaying Strong Ferromagnetic Coupling: Synthesis,Crystal Structure,Magnetic Properties with DFT Calculations
MA Xiao-Hui1LI Fei-Fei1CEN Pei-Pei1LIU Xiang-Yu*,1ZHOU Hui-Liang1LUO Shu-Chang*,2WU Yue-Wei1ZHANG Cheng-Cheng1SONG Wei-Ming*,1
(1College of Chemistry and Chemical Engineering,State Key Laboratory of High-efficiency Coal Utilization and Green Chemical Engineering,Ningxia University,Yinchuan 750021,China)
(2School of Chemical Engineering,Guizhou University of Engineering Science,Bijie,Guizhou 551700,China)
An azido-Cucompound with substituted benzoate derivative,[Cu(4-Fb)(N3)(H2O)]n(1)(4-Fb=4-formylbenzoate),has been successfully synthesized,and then structurally and magnetically characterized.Single crystal structure analysis demonstrates that the asymmetric unit of compound 1 possesses one crystallographically independent Cuion that exhibits distorted tetragonal pyramid geometry.Adjacent Cuions are linked by alternating mixed-bridges of μ-1,1(end-on,EO)azido and syn,syn-carboxylate,forming a linear 1D Cuchainlike motif.Magnetic measurements reveal that the dominant ferromagnetic coupling between adjacent Cuions within each chain due to the counter-complementarity of the dual superexchange pathway is observed in the resulting compounds.However,the interesting plots of magnetic ordering and slow magnetic relaxation are absentin the compound.The critical structural parameter,Cu-N-Cu angle of 113.34°,is corresponding to that of known ferromangetic copper systems containing mixed carboxylate/EO-azido connectors.Magneto-structural correlations are also investigated.Moreover,density functional theory(DFT)calculations(using different methods and basis sets)have been performed on title compound to offer qualitatively theoretical explanation for the ferromagnetic coupling between two Cucenters.CCDC:1496426.
azido-copper;benzoate;crystal structure;magnetic property;DFT calculation
O614.121
A
1001-4861(2017)09-1639-10
10.11862/CJIC.2017.174
2017-04-13。收修改稿日期:2017-05-17。
国家自然科学基金(No.21463020),宁夏自然科学基金(No.NZ16035),宁夏高等学校优秀青年教师培育项目(No.NGY2016063)和国家级大学生创新创业训练计划(No.201710749003)资助。
*通信联系人。E-mail:xiangyuliu432@126.com,songwm@nxu.edu.cn,luosc@gues.edu.cn

