二烯丙基二硫在RORα抑制人胃癌细胞上皮间质转化中的作用*
刘芳,苏坚,曾颖,夏红,苏波,凌晖,曾希,苏琦
[1.南华大学肿瘤研究所(湖南省胃癌研究中心 湖南省高校肿瘤细胞与分子病理学重点实验室),湖南 衡阳 421001;2.南华大学附属第二医院 病理科,湖南 衡阳 421001]
二烯丙基二硫在RORα抑制人胃癌细胞上皮间质转化中的作用*
刘芳1,苏坚2,曾颖1,夏红1,苏波1,凌晖1,曾希1,苏琦1
[1.南华大学肿瘤研究所(湖南省胃癌研究中心 湖南省高校肿瘤细胞与分子病理学重点实验室),湖南 衡阳 421001;2.南华大学附属第二医院 病理科,湖南 衡阳 421001]
目的探讨二烯丙基二硫(DADS)是否上调维甲酸相关孤核受体α(RORα)抑制人胃癌细胞株MGC803细胞上皮-间质转化(EMT)。方法相差显微镜观察MGC803细胞形态的改变。逆转录PCR(RTPCR)、蛋白免疫印迹法(Western blot)、免疫荧光与免疫组织化学检测EMT的相关分子表达。裸鼠实验检测DADS与沉默RORα对移植瘤生长的影响。结果相差显微镜显示,沉默RORα细胞较MGC803细胞大小与形状差异更明显。DADS作用后,细胞大小较一致,呈圆形或椭圆形,梭形细胞减少,异型性下降。RT-PCR与Western blot显示,DADS处理后较处理前各组RORα mRNA与蛋白表达上调(P<0.05)。DADS作用对照组与空载体组较沉默组效果更为明显(P<0.05)。RORα沉默较对照组和空载体组细胞锌指转录因子(Snail)和波形蛋白(Vimentin)mRNA与蛋白表达上调,而E-钙黏蛋白(E-cadherin)mRNA与蛋白下调(P<0.05)。DADS处理后,各组Snail蛋白下调、Vimentin mRNA与蛋白下调和E-cadherin mRNA与蛋白上调(P<0.05)。免疫荧光显示,沉默组细胞Snail与Vimentin阳性表达较对照组增强,而E-cadherin阳性表达减弱。DADS作用后,结果相反。裸鼠移植瘤实验显示,RORα沉默组移植瘤较对照组生长加快和体重增加(P<0.05),增殖细胞核抗原(Ki-67)、Snail、CD34及Vimentin阳性表达较对照组增加,而E-cadherin阳性降低。DADS处理各组移植瘤生长与体积减慢与减小,Ki-67、Snail、CD34与Vimentin阳性表达较对照组减弱,而E-cadherin阳性表达增强。结论DADS通过上调RORα可体内外抑制人胃癌MGC803细胞EMT。
二烯丙基二硫;维甲酸相关孤核受体α;人胃癌MGC803细胞;上皮-间质转化
胃癌是我国最常见的恶性肿瘤之一,发生率与死亡率居第2位。由于患者就诊时大多已发生侵袭转移,疗效不佳,5年生存率<10%[1-2]。因此,筛选有效药物和寻找治疗靶点对防治胃癌具有重要的意义。二烯丙基二硫(diallyl disulfide,DADS)是大蒜中的一种脂溶性的有效成分,对多种肿瘤均有抑制作用,是一种具有开发潜力的抗肿瘤药物[3]。笔者发现,DADS处理人胃癌MGC803细胞后,维甲酸相关孤核受体α(retinoid acid receptor related orphan receptor α,RORα)蛋白上调[4]。目前认为,RORα是候选抑癌基因,在肿瘤表达下调,与肿瘤的发生密切相关,可能是肿瘤治疗的靶点[5-6]。RORα在胃癌中低表达,与胃癌发生和分化程度有关[7]。本研究进一步探讨DADS上调RORα表达对胃癌细胞上皮间质转化(epithelial-mesenchymal transformation,EMT)的影响及其相关机制。
1 材料与方法
1.1 细胞培养
人胃癌MGC803细胞由本实验室保存,RORα沉默MGC803细胞由本实验室构建[8],置于含10%胎牛血清的RPMI 1640培养基中,37℃、5%二氧化碳CO2、饱和湿度的培养箱内传代培养。取对数生长期的细胞用于实验。
1.2 主要试剂
DADS(购自美国Fluka公司),RNA提取试剂盒(购自美国Omega公司),逆转录试剂盒与二喹啉甲酸(bicinchoninic acid,BCA)蛋白定量试剂盒(购自美国Promega公司),E-cadherin、Vimentin与βactin抗体(购自英国Abcam公司),Snail、Ki-67与CD34等抗体及增强化学发光(enhanced chemiluminescence,ECL)试剂盒(购自美国Santa Cruz公司),胎牛血清(购自浙江省杭州四季青生物工程公司),引物经Primer Premier 5.0软件设计,由上海生物工程(股份)有限公司合成。羊抗兔IgG-HR和羊抗小鼠IgG-HRP(购自江苏省南京凯基生物科技发展有限公司),羊抗小鼠IgG(H+L)(购自美国Protech生物公司),荧光染料DAPI和正常山羊血清(购自湖北省博士德生物公司),Max VisionTM试剂盒(购自福建省福州迈新生物技术开发公司)。
1.3 相差显微镜观察
人胃癌MGC803细胞与RORα沉默MGC803细胞培养24 h后,倒置相差显微镜观察DADS处理前后细胞形态学变化。
1.4 逆转录PCR(RT-PCR)分析
总RNA提取试剂盒(Total RNA Kit)提取细胞总RNA,AMV逆转录酶作用下逆转录合成cDNA。设计并合成PCR引物序列。RORα正向引物:5'-G TCAGCAGCTTCTACCTGGAC-3';反向引物:5'-CAG TTGGGGAAGTCTCGCCG-3',产物长度151 bp。Vimentin正向引物:5'-ACACCCTGCAATCTTTCAGAC A-3';反向引物:5'-AGAAATCCTGCTCTCCTCGCCT-3',产物长度635 bp。E-cadherin正向引物:5'-CTC CCAATACATCTCCCTTCAC-3';反向引物:5'-CGCC TCCTTCTTCATCATAGTAA-3',产物长度423 bp。β-actin正向引物:5'-TCTACAATGAGCTGCGTGTG G-3';反向引物:5'-GGAACCGCTCATTGCCAATG-3',产物长度498bp。PCR反应条件:94℃预变性5min,94℃变性40s,(RORα、Vimentin和E-cadherin退火温度分别为:53℃、52℃和54℃)45 s退火,72℃延伸80 s,28个循环,72℃继续延伸10min。5μl的PCR产物经1%的琼脂糖电泳,溴化乙啶染色,IS1000图像分析软件读取灰度值,相对值以目的基因与βactin灰度值之比表示。
1.5 Western blot检测
收集细胞,提取细胞总蛋白,BCA法测定蛋白浓度,每组取等量样本进行十二烷基磺酸钠-聚丙烯酰胺凝胶电泳(SDS-PAGE)凝胶电泳,电泳后转膜,封闭1h,加一抗,4℃过夜,三羟甲基氨基甲烷缓冲溶洗膜,加二抗孵育1h,洗膜,ECL发光,X片曝光、显影、定影。
1.6 细胞免疫荧光实验
先在6孔板中滴加培养基,然后将消毒的盖玻片放入6孔板。将对数期的MGC803细胞制成悬液,每孔接种5×105个细胞,待细胞贴壁融合至80%时,弃旧培养基。取出盖玻片,PBS洗5min×3,4%多聚甲醛固定细胞,常温下静置15min,PBS洗5min×3;将0.5%Triton覆盖细胞后,常温下静置30min,后用PBS洗5min×3,吸干净残存PBS液;山羊血清封闭,37℃孵育1h;滤纸吸净封闭液,加入一抗,湿盒内4℃孵育过夜;第2天移置37℃复温1h,PBS洗5min×3次;在暗室中加异硫氰酸荧光素的二抗,37℃避光湿盒中孵育1h,PBS洗5min×3。染核:将0.4 μg/μl的DAPI覆盖细胞,常温下避光静置2~5min,PBS洗5min×3,甘油封片在荧光显微镜下观察。
1.7 裸鼠成瘤实验
裸鼠(购自北京维通利华实验动物技术有限公司),4周龄,雄性,分为MGC803细胞组(对照组)、(MGC803+DADS)组、RORα沉默组和(RORα沉默+DADS)组,每组5只。将各组处于对数生长期的细胞密度调至1×107个/ml,分别取0.2ml细胞悬液接种于裸鼠腋下。观察接种后裸鼠进食、饮水、毛发、精神及活动等情况。每隔7d测量移植瘤大小(长径和短径),肿瘤体积:V=a×b2/2。移植瘤组织固定于10%的中性甲醛。
1.8 免疫组织化学法检测
采用MaxVisionTM法,分别滴加一抗室温60min,4℃过夜,PBS洗3min×3,滴加即用型Max VisionTM试剂,室温下15min,PBS洗5min×3。二氨基联苯胺显色,自来水冲洗,苏木素复染,脱水、透明、封固及镜检。
1.9 统计学方法
数据分析采用SPSS 13.0统计软件,计量资料以均值±标准差(±s)表示,多组间均值比较用重复测量设计的方差分析或单因素方差分析,两两比较用t检验,P<0.05为差异有统计学意义。
2 结果
2.1 DADS与沉默RORα对MGC803细胞RORα表达的影响
RT-PCR与Western blot显示,RORα沉默组较对照组MGC803细胞RORα mRNA(F=1 885.149,P=0.000)与蛋白表达下调(F=190.822,P=0.000)。DADS处理后较处理前各组RORα mRNA(F=1 885.149,P=0.000)与蛋白表达上调(F=190.822,P=0.000)。见图1。
2.2 DADS与沉默RORα对MGC803细胞形态与EMT相关分子的影响
相差显微镜显示,MGC803细胞大小不一,大部分呈长梭形,纤维母细胞样,细胞膜可见突起,核浆比值增大,异型性明显。RORα沉默细胞较MGC803细胞大小与形状差异更明显,纤维母细胞样梭形细胞增多,异型性更为明显。DADS作用后,对照组与沉默组较处理前细胞大小一致,大部分呈圆形或椭圆形,核浆比值下降,异型性降低(见图2)。表明DADS可上调RORα抑制MGC803细胞向间质细胞转化。
RT-PCR与 Western blot显示,RORα 沉默MGC803细胞较对照组的Snail蛋白表达上调(F=545.652,P=0.000)。并且沉默RORα可上调Vimentin和下调E-cadherin mRNA(F=1 250.268和67.561,P=0.000和0.016)与蛋白表达(F=155.246和35.624,P=0.000)。DADS处理后,各组Snail蛋白(F=545.652,P=0.000)、Vimentin mRNA(F=1 250.268,P=0.000)与蛋白明显下调(F=155.246,P=0.000)和E-cadherin mRNA(F=67.561,P=0.000)与蛋白上调(F=35.624,P=0.000)。表明DADS通过上调RORα下调Snail与Vimentin和上调E-cadherin抑制MGC803细胞EMT。见图3~5。

图1DADS与沉默RORα对RORα表达的影响

图2DADS与沉默RORα对MGC803细胞形态的影响(×40)
2.3 DADS与沉默RORα对EMT相关蛋白表达的影响
免疫荧光检测显示,Snail蛋白定位胞核,Vimentin与E-cadherin蛋白主要定位胞浆。RORα沉默组细胞Snail与Vimentin阳性信号较对照组增强,而E-cadherin阳性信号减弱,然而,DADS作用后,Snail与Vimentin阳性信号较对照组降低,而E-cadherin阳性升高,与Western blot检测结果一致。见图6。
2.4 DADS与沉默RORα对MGC803细胞裸鼠移植瘤生长的影响
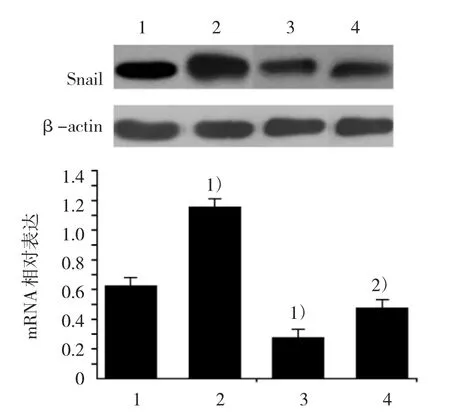
图3DADS与沉默RORα对MGC803细胞Snail蛋白表达的影响
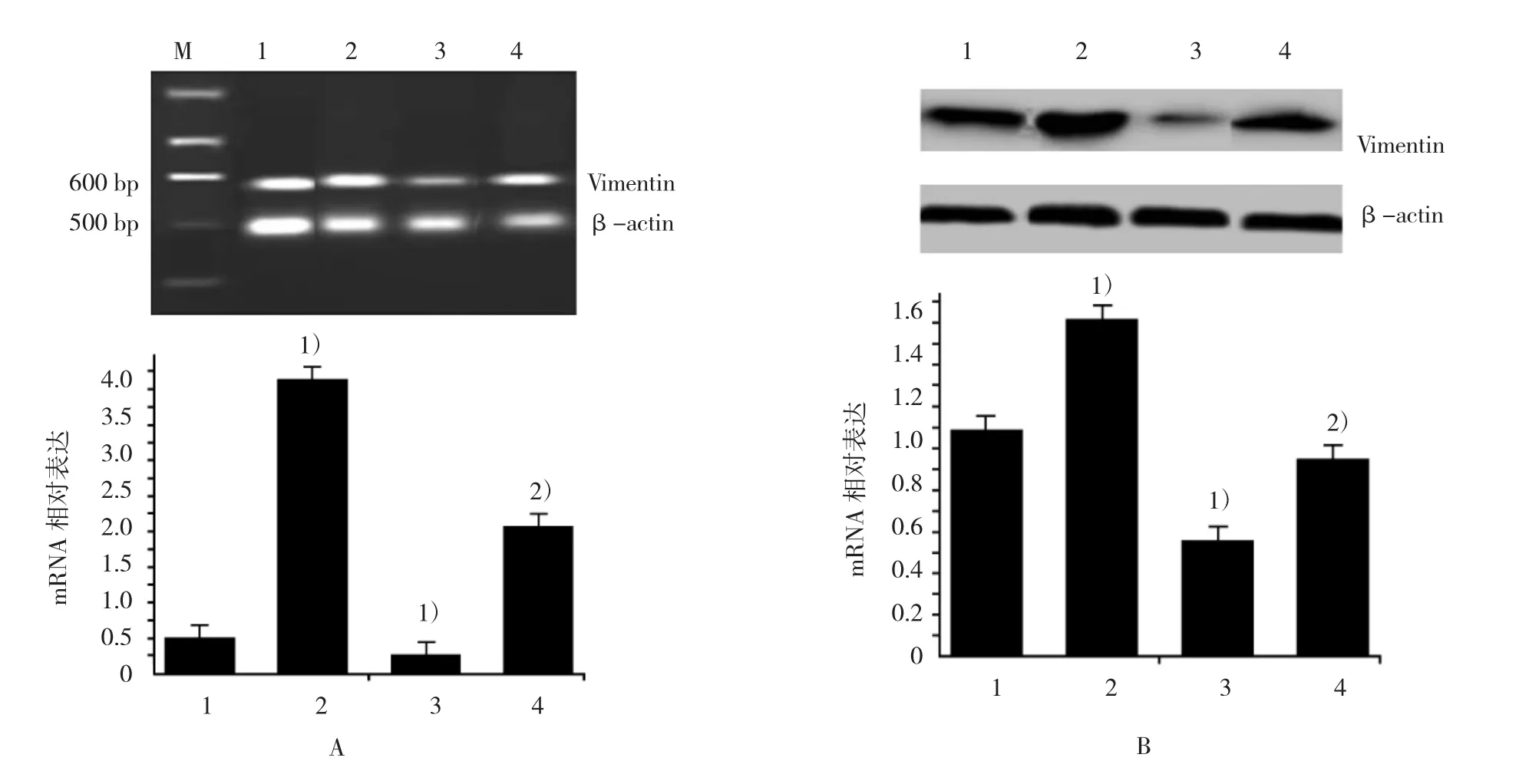
图4DADS与沉默RORα对MGC803细胞Vimentin表达的影响
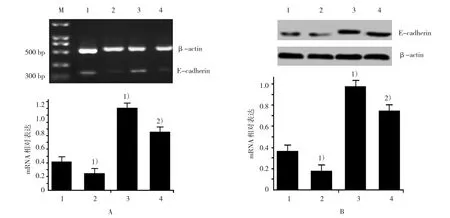
图5 DADS与沉默RORα对MGC803细胞E-cadherin表达的影响
采用重复测量设计的方差分析,结果显示:①各组组内裸鼠移植瘤体积随着时间延长,肿瘤体积变大,差异有统计学意义(F=69.921,P=0.000)。②各组与对照组比较生长变慢瘤体变小,差异有统计学意义(F=90.148,P=0.000),miR-RORα与对照组比较生长加快瘤体变大,差异有统计学意义(F=72.324, P=0.000),而(miR-RORα+DADS)组与miR-RORα组比较生长又变慢,瘤体变小,差异有统计学意义(F=86.356,P=0.000);③(MGC803+DADS)组和miRRORα组分别与对照组肿瘤体积变化趋势比较,差异有统计学意义(F=109.278和122.437,均P=0.000),而(miR-RORα+DADS)组与miR-RORα组肿瘤体积变化趋势比较,差异有统计学意义(F=78.905,P=0.000)。见附表。
2.5 DADS与沉默RORα对裸鼠移植瘤组织EMT相关蛋白表达的影响
免疫组织化学显示,RORα沉默组较MGC803细胞对照组的Ki-67、Snail及Vimentin阳性表达均增强,E-cadherin阳性表达减弱。但是MGC803+DADS组与(RORα沉默组+DADS组)结果相反,Ki-67、Snail及Vimentin阳性表达均减弱,E-cadherin阳性增强。见图7。
附表 各组移植瘤不同时间平均体积 (n=5,cm3,±s)
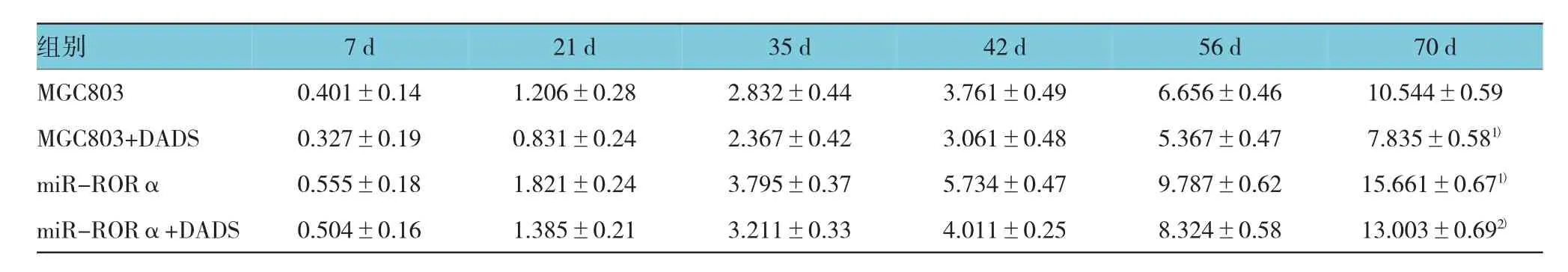
附表 各组移植瘤不同时间平均体积 (n=5,cm3,±s)
注:1)与MGC803比较,P<0.05;2)与miR-RORα比较,P<0.05
组别70 d MGC803 0.401±0.14 1.206±0.28 2.832±0.44 3.761±0.49 6.656±0.46 10.544±0.59 MGC803+DADS 0.327±0.19 0.831±0.24 2.367±0.42 3.061±0.48 5.367±0.47 7.835±0.581)miR-RORα 0.555±0.18 1.821±0.24 3.795±0.37 5.734±0.47 9.787±0.62 15.661±0.671)miR-RORα+DADS 0.504±0.16 1.385±0.21 3.211±0.33 4.011±0.25 8.324±0.58 13.003±0.692)7d21 d35 d42 d56 d
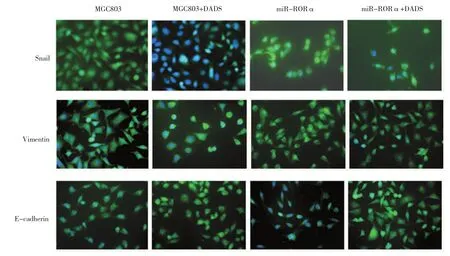
图6DADS与沉默RORα对EMT相关蛋白表达的影响 (×40)
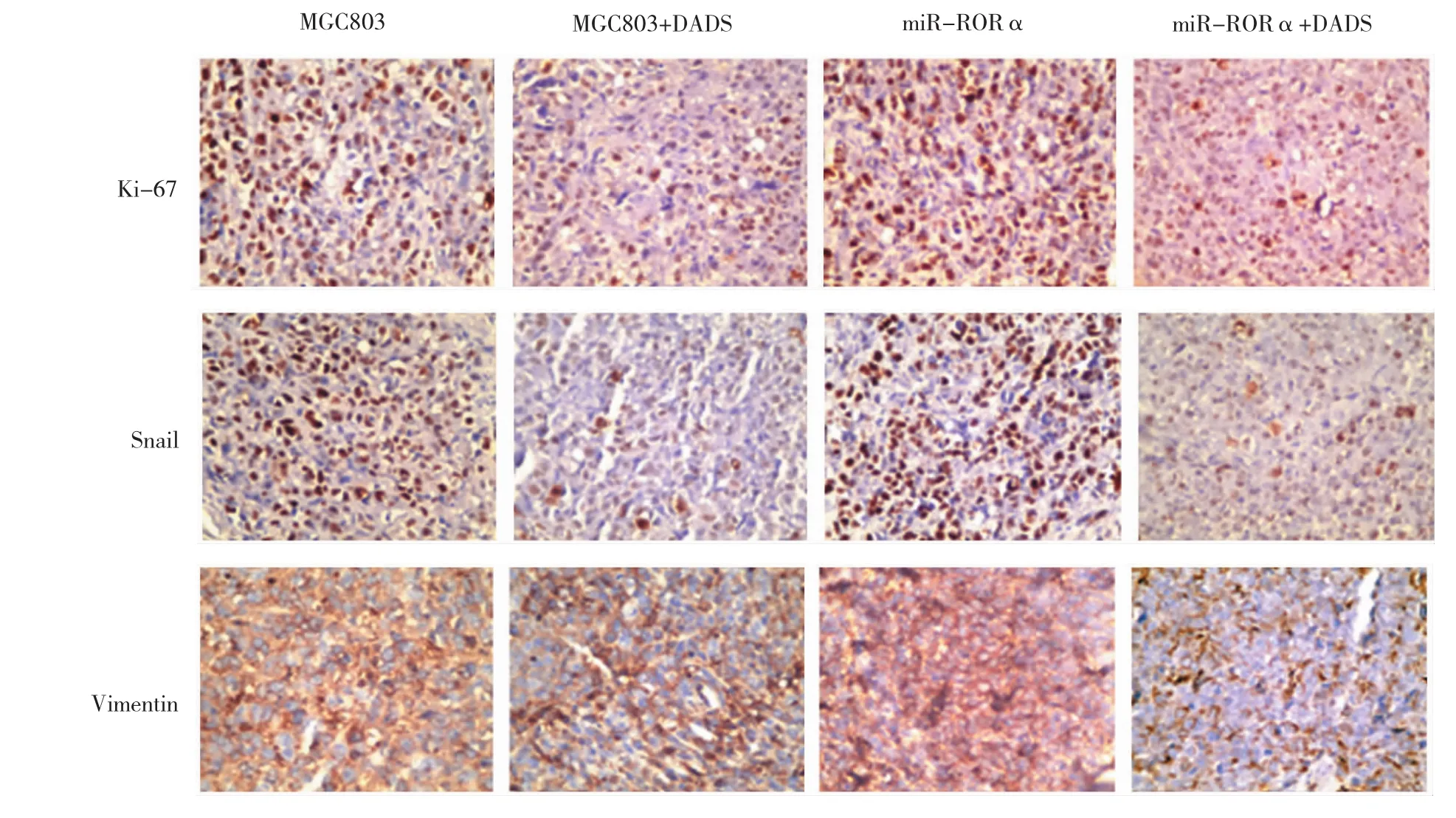
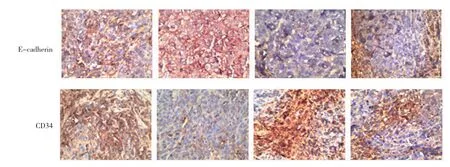
图7 DADS与RORα沉默对移植瘤EMT相关蛋白表达影响 (SP×40)
3 讨论
众所周知,肿瘤细胞迁移和侵袭是肿瘤转移起始的重要步骤,而上皮-间质转化(epithelial-mesenchymal transformation,EMT)是肿瘤细胞获得迁移和侵袭能力的关键。上皮源性肿瘤细胞发生EMT,除形态学改变外,还具有较高的迁移与侵袭、抗凋亡和降解细胞外基质的能力等间质表型,如上皮标志物E-cadherin、紧密连接蛋白(ZO-1)等表达下调,间质标志物vimentin、α-SMA及N-cadherin等上调以及Snail、Slug和Twist等转录因子活性增强。因此,阻止EMT发生已成为抑制恶性肿瘤转移的治疗策略[9-11]。ZHAN等[12]研究显示,泛素样蛋白(NEDD8)活化酶抑制因子MLN4924可上调RORα,抑制骨肉瘤细胞增殖,阻滞细胞周期与诱导凋亡。近年来,研究天然植物来源的抗肿瘤药物抑制肿瘤EMT已成为研究热点。有人发现,姜黄通过Wnt信号通路下调β-catenin,TCF4与vimentin和上调E-cadherin抑制EMT[13]。川陈皮素可对抗TGF-β1/Smad3信号通路抑制非小细胞肺癌细胞EMT[14]。
笔者前期研究证明,DADS可抑制MGC803细胞的增殖,增加细胞骨架蛋白合成,恢复细胞缝隙连接通讯功能,上调组蛋白乙酰化与细胞周期调控因子(p21WAF1),激活p38、抑制细胞外信号调节激酶(ERK)通路,调节ATR/Chk1/Cdc25C/Cyclin β1,阻滞G2/M[15-19]。笔者采用蛋白质组学技术鉴定DADS处理人胃癌细胞的差异蛋白质中,发现LIMK1下调和RORα表达上调[4]。并证实DADS通过Rac1-Pak1/ Rock1通路下调单丝氨酸蛋白激酶1(LIMK1)、基质金属蛋白酶9(MMP-9)和上调基质金属蛋白酶抑制剂 3(TIMP-3),抑制人胃癌细胞EMT与侵袭[20]。研究表明,RORα在胃癌、结肠癌、食管癌、胰腺癌、肝癌、乳腺癌、子宫颈癌、卵巢癌、前列腺癌、膀胱癌、头颈部癌及白血病等多种肿瘤中表达下调,上调RORα可体内外抑制乳腺癌细胞增殖与侵袭等恶性表型,提示RORα可能是肿瘤治疗靶点[6]。RORα在乳腺癌组织与细胞中表达下调,恢复RORα表达可抑制乳腺癌细胞侵袭能力与裸鼠移植瘤生长,表明RORα是潜在的抑癌基因[21]。LEE等报告,RORα通过Wnt5a与蛋白激酶C(PKC)依赖方式负调控Wnt通路,抑制其靶基因表达[22]。笔者证明,DADS可阻断Wnt通路上调miR-200b和miR-22,抑制胃癌细胞增殖与侵袭和诱导凋亡[23]。但是,DADS是否上调RORα抑制胃癌细胞EMT,尚不清楚。
本研究RT-PCR与Western blot显示,DADS处理后,各组RORα mRNA与蛋白上调,且对照组与空载体组较沉默组效果更为显著。相差显微镜显示,RORα沉默组较MGC803细胞大小与形状差异更明显,纤维母细胞样梭形细胞增多,异型性更为显著。DADS处理对照组与沉默组较处理前细胞大小一致,大部分呈圆形或椭圆形,异型性降低,表明DADS可上调RORα抑制MGC803细胞向间质细胞转化。基于Snail、E-cadherin与Vimentin是肿瘤EMT的关键因子[11,24],Ki-67是肿瘤增殖能力的重要指标[25],CD34是血管形成的标志[26]。本研究进一步检测表明,RORα沉默较对照组与空载体组的Snail与Vimentin上调和E-cadherin下调。DADS处理后,各组Snail与Vimentin下调和E-cadherin上调。且DADS作用对照组与空载体组较沉默组效果更为明显。免疫荧光结果与Western blot检测结果一致。裸鼠实验显示,沉默组移植瘤较对照组生长增快,体重明显增加,Ki-67、Snail、CD34与Vimentin阳性表达均增高,E-cadherin阳性减弱。而DADS作用对照组与RORα沉默组后,移植瘤体重低于处理前,Ki-67、Snail、Vimentin阳性均减弱,E-cadherin阳性增强。上述结果表明,DADS可上调RORα通过下调Snail与Vimentin和上调E-cadherin体内外抑制MGC803细胞EMT。
[1]CHEN W,ZHENG R,BAADE P D,et al.Cancer statistics in China,2015[J].Ca A Cancer Journal for Clinicians,2016,66(2): 115-132.
[2]HAREWOOD G C.Treatment of gastric cancer[J].New England Journal of Medicine,2006,355(13):1386.
[3]YI L,SU Q.Molecular mechanisms for the anti-cancer effects of diallyl disulfide[J].Food& Chemical Toxicology,2013,57(7): 362-370.
[4]SU B,SU J,HE H,et al.Identification of potential targets for diallyl disulfide in human gastric cancer MGC-803 cells using proteomics approaches[J].Oncology Reports,2015,3(5):2484-2494.
[5]赵晓红,苏琦.维甲酸相关孤核受体α与Wn1t信号途径及肿瘤的关系[J].国际病理科学与临床杂志,2011,31(3):234-237.
[6]DU J,XU R.RORα,a potential tumor suppressor and therapeutic target of breast cancer[J].International Journal of Molecular Sciences,2012,13(12):15755-15766.
[7]石莺,黄建军,苏坚,等.RORα蛋白在胃癌中的表达及临床病理意义[J].实验与病理学杂志,2012,28(3):270-273.
[8]凌晖,陈真伟,曾铁兵,等.RORα miRNA真核表达载体构建及对人胃癌细胞增殖的影响[J].中国现代医药杂志,2011,13(2):1-4.
[9]朱理辉,罗勇,廖文秋,等.MicroRNA-219-5p靶向E-钙黏蛋白调控上皮间质转化抑制肝癌细胞侵袭转移[J].中国现代医学杂志, 2016,26(18):22-29.
[10]MENG F,WU G.The rejuvenated scenario of epithelial-mesenchymal transition(EMT)and cancer metastasis[J].Cancer and Metastasis Reviews,2012,31(3):455-467.
[11]ABOUHASHEM N S,IBRAHIM D A,MOHAMED A M.Prognostic implications of epithelial to mesenchymal transitionrelated proteins(E-cadherin,Snail)and hypoxia inducible factor 1α in endometrioid endometrialcarcinoma[J].AnnalsofDiagnostic Pathology,2016(22):1-11.
[12]ZHANG S,ZHANG J,DENG Z,et al.Circadian clock components RORα and Bmal1 mediate the anti-proliferative effect of MLN4924 in osteosarcoma c ells[J].Oncotarget,2016,7(40): 66087-66099.
[13]ZHANG Z,CHEN H,XU C,et al.Curcumin inhibits tumortransition by downregulating the Wnt signaling pathway and upregulating NKD2 expression in colon cancer cells[J].Oncology Reports,2016,35(5):2615-2623.
[14]DA C,LIU Y,ZHAN Y,et al.Nobiletin inhibits epithelialmesenchymal transition of human non-small cell lung cancer cells by antagonizing the TGF-β1/Smad3 signaling pathway[J]. Oncology Reports,2016,35(5):2767-2774.
[15]YUAN J P,ZHANG M X,LING H,et al.Diallyl disulfide-induced G2/M arrest of human gastric cancer MGC803 cells involves activation of p38 MAP kinase pathways[J].World Journal of Gastroenterology,2004,10(18):2731-2374.
[16]LING H,ZHANG L Y,SU Q,et al.Erk is involved in the differentiation induced by diallyl disulfide in the human gastric cancer cell line MGC803[J].Cellular&Molecular Biology Letters,2006,11(3):408-423.
[17]SU B,XIANG S L,SU J,et al.Diallyl disulfide increased histone acetylation and p21WAF1 expression in human gastric cancer cells in vivo and in vitro[J].Biochem Pharmacol,2012, 1(7):1-10.
[18]LING H,WEN L,JI X X,et al.Growth inhibitory effect and Chk1-dependent signaling involved in G2/M arrest on human gastric cancer cells induced by diallyl disulfide[J].Braz J Med Biol Res,2010,43(3):271-278.
[19]LING H,LU L F,HE J,et al.Diallyl disulfide selectively causes checkpoint kinase-1 mediated G2/M arrest in human MGC803 gastric cancer cell line[J].Oncology Reports,2014,32(5):2274-2282.
[20]SU B,SU J,ZENG Y,et al.Diallyl disulfide suppresses epithelial-mesenchymaltransition,invasion and proliferation by downregulation of LIMK1 in gastric cancer[J].Oncotarget,2016, 7(9):10498-10512.
[21]XIONG G,WANG C,EVERS B M,et al.RORα suppresses breast tumor invasion by inducing SEMA3F expression[J].Cancer Research,2012,72(7):1728-1739.
[22]LEE J M,KIM I S,KIM H,et al.ROR alpha attenuates Wnt/ beta-catenin signaling by PKCalpha-dependent phosphorylation in colon cancer[J].Molecular Cell,2010,37(2):183-195.
[23]TANG H,KONG Y,GUO J,et al.Diallyl disulfide suppresses proliferation and inducesapoptosisin human gastric cancer through Wnt-1 signaling pathway by up-regulation of miR-200b and miR-22[J].Cancer Letters,2013,340(1):72-81.
[24]LAZAROVA D L,BORDONARO M.Vimentin,colon cancer progression and resistance to butyrate and other HDACis[J]. Journal of Cellular&Molecular Medicine,2016,20(6):989-993.
[25]LEE W S,PARK Y L,KIM N,et al.Myeloid cell leukemia-1 regulates the cell growth and predicts prognosis in gastric can cer[J].International Journal of Oncology,2015,46(5):2154-2162.
[26]LEE O,CHOI M R,CHRISTOV K,et al.Progesterone receptor antagonism inhibits progestogen-related carcinogenesis and suppresses tumor cell proliferation[J].Cancer Letters,2016,376(2): 310-317.
Up-regulation of RORα inhibits EMT in human gastric MGC803 cells induced by diallyl disulfide*
Fang Liu1,Jian Su2,Ying Zeng1,Hong Xia1,Bo Su1,Hui Ling1,Xi Zeng1,Qi Su1
[1.Cancer Institute of University of South China(Center for Gastric Cancer Research of Hunan Province/Key Laboratory of Cancer Cellular and Molecular Pathology of Hunan Provincial University),Hengyang,Hunan 421001,China;2.Department of Pathology,the Second Affiliated Hospital,University of South China,Hengyang,Hunan 421001,China]
ObjectiveTo investigate whether diallyl disulfide (DADS)inhibits epithelial mesenchymal trasition(EMT)in human gastric cancer MGC803 cells by up-regulation of retinoid acid receptor related orphan receptor α (RORα).MethodsThe morphological effect of MGC803 cells was observed by phase contrast microscope.The expressions of molecules correlated to EMT were detected by RT-PCR,Western blot, immunofluorescence and immunohistochemistry.The influence of DADS and silencing RORα on the growth of xenograft tumor in athymic mice was observed.ResultsPhase contrast microscopy showed that MGC803 cellsof RORα silence were discordancy in size and shape and the number of spindle cells increased.Besides,the MGC803 cells treated by DADS were found with characteristics such as size unification,round or ellipse shape,decreased spindle cells and less atypia.RT-PCR and Western blot revealed up-regulation of RORα mRNA and protein in each group treated by DADS (P<0.05).Moreover,the effect in control group and the vector group increased compared to the RORα silence group treated by DADS (P<0.05).RT-PCR and Western blot showed the up-regulation of Snail protein and Vimentin mRNA and protein,and down-regulation of E-cadherin mRNA and protein in the RORα silence cells (P<0.05).However,in the MGC803 cells treated by DADS Snail and Vimentin were down-regulated and E-cadherin was up-regulated(P<0.05). Immunofluorescence showed that the positive expressions of Snail and Vimentin in the RORα silence cells were strengthened,but E-cadherin was attenuated.Nevertheless,the positive expressions of Snail and Vimentin were attenuated,and E-cadherin was strengthened in the MGC803 cells treated by DADS.The growth of the xenograft tumor was accelerated,and the weight of the transplantation tumor increased in the RORα silence group compred to the control group (P<0.05).The positive expressions of Ki-67,Snail,CD34 and Vimentin were obviously increased,while the positive rate of E-cadherin also increased in the RORα silence group. Inversely,the rate of the xenograft tumor growth slowed down and the volume of tumor was significantly diminished.The expressions of Ki-67,Snail,CD34 and Vimentin decreased and the positive expression of E-cadherin increased in the MGC803 cells treated by DADS.ConclusionsDADS can inhibit EMT in MGC803 cellsin vivoandin vitrothrough up-regulation of RORα.
diallyl disulfide;RORα;human gastric cancer MGC803 cell;EMT
R735.2
A
10.3969/j.issn.1005-8982.2017.18.002
1005-8982(2017)18-0007-08
2016-12-15
国家自然科学基金(No:81374013);湖南省卫计委资助项目(No:B2015-182);湖南省教育厅资助项目(No:17K081)
苏琦,E-mail:suqi1945@163.com;Tel:0734-8281547

