内皮祖细胞培养上清对高氧暴露新生大鼠肺结构的改善作用*
李 志, 陆爱珍, 张小媚, 钱莉玲
(复旦大学附属儿科医院, 上海 201102)
内皮祖细胞培养上清对高氧暴露新生大鼠肺结构的改善作用*
李 志, 陆爱珍, 张小媚, 钱莉玲△
(复旦大学附属儿科医院, 上海 201102)
目的: 研究内皮祖细胞培养上清(endothelial progenitor cell-conditioned medium,EPC-CM)对高氧暴露新生大鼠肺损伤时肺泡结构的改善作用及其机制。方法: 从新生SD大鼠骨髓中获取内皮祖细胞并鉴定,收集第3代细胞的培养上清备用。另取新生SD大鼠40只随机分为4组,即空气组:仔鼠在空气(21% O2)中喂养21天;高氧组:仔鼠在85% O2中喂养21天;内皮细胞基础培养基 (endothelial cell basal medium, EBM)干预组:仔鼠在85% O2中喂养至第14天时,经气道给予100 μL EBM,然后喂养至第21天;EPC-CM干预组:仔鼠在85% O2中喂养至第14天,经气道给予100 μL EPC-CM,喂养至第21天。第21天处死小鼠,左肺用4%多聚甲醛固定,留作石蜡切片,随后HE染色进行肺组织病理形态学观察,并做辐射状肺泡计数(radical alveolar count,RAC)及肺泡平均线性截距(mean linear intercept,MLI)测量;免疫组织化学方法对血管内皮细胞FVIII染色,计数肺组织微血管密度;右肺留作实时荧光定量PCR检测肺组织KGF、VEGF、SP-A和SP-C的mRNA表达。结果: 培养所得细胞具有典型的EPCs形态改变,能结合异硫氰酸荧光素标记的荆豆凝集素1并摄取DiI荧光标记的乙酰化低密度脂蛋白。高氧组及EBM干预组的仔鼠体重、RAC、MLI和微血管密度较空气组显著降低(P<0.05),EPC-CM干预组的RAC和微血管密度较高氧组和EBM干预组明显增加(P<0.05),而体重和MLI的变化无明显差异,但有增高的趋势。高氧组和EBM干预组肺组织KGF、VEGF、SP-A和SP-C的mRNA表达较空气组显著降低(P<0.05),EPC-CM干预组的表达显著高于高氧组和EBM干预组(P<0.05)。结论: EPC-CM可改善高氧暴露新生大鼠的肺泡化和肺血管发育,可能与促进肺内KGF和VEGF mRNA的表达相关。
高氧; 内皮祖细胞; 肺损伤; 旁分泌
支气管肺发育不良(bronchopulmonary dysplasia,BPD)是一种好发于早产儿的常见慢性肺部疾病,主要是由于囊泡期肺组织发育异常所致[1],病理表现为肺血管异常和肺泡简化。目前对于BPD的治疗仍无有效的措施。BPD发病机制的研究显示在肺组织发育过程中,肺泡管的发生与肺组织血管网的形成是同步的[2],抑制肺组织内血管形成可导致肺泡发育简化[3-4],因此基于通过促进肺组织内血管网的形成来同步改善肺组织的肺泡化成为治疗BPD的新思路。内皮祖细胞(endothelial progenitor cells,EPCs)是内皮细胞的前体细胞,具有很强的自我更新和高度增殖能力,并能分化为血管内皮细胞参与血管生成。临床研究和动物实验均发现EPCs的数量和功能与BPD发生相关[5-7]。然而,有实验表明内皮细胞前体祖细胞并未直接通过分化为内皮细胞参与血管的形成[8],动物研究显示移植EPCs定植于损伤的肺组织数量极少,EPCs旁分泌因子能促进血管形成,维持血管内皮结构的完整,促进肺泡发育[9]。因此,本实验主要研究EPCs培养上清对新生鼠高氧肺损伤的治疗作用及其可能的机制。
材 料 和 方 法
1 EPCs的分离、培养与鉴定[10-11]
用10%水合氯醛麻醉出生5~7 d清洁级SD大鼠,75%酒精消毒四肢,分离出股骨、胫骨和肱骨,在超净台中用D-PBS冲洗骨髓腔,收集骨髓冲洗液,应用Ficoll淋巴细胞分离液(Sigma-Aldrich)密度梯度离心分离出骨髓中的单个核细胞,用添加hEGF、Hydrocortisone、VEGF、hFGF-B、R3-IGF-1、Ascorbic acid、FBS等成分的EBM培养基,即EGM-2MV完全培养基(LONZA)重悬,以每孔2×106的密度接种于纤连蛋白(Sigma-Aldrich)包被过夜的6孔板中培养3 d,去除未贴壁细胞,每3 d换液,待细胞融合度达70%时进行传代。倒置显微镜下观察记录细胞的生长特点。
培养所得的爬片细胞与DiI-ac-LDL(Biomedical Technologie, 终浓度 10 mg/L) 37 ℃避光孵育4 h,D-PBS洗3遍,4%多聚甲醛固定20 min,D-PBS洗3遍,再与FITC-UEA-1(Sigma;终浓度为10 mg/L)孵育1 h,荧光显微镜下观察双染细胞即为EPCs。
2 实验方法
2.1 EPCs培养上清的收集 培养的第3代EPCs用PBS清洗后,加入不含生长因子的内皮细胞基础培养基(endothelial cell basal medium, EBM;购自LONZA),培养24 h后收集上清,移入Amicon® ultra-4,10 kD超滤管(Millipore)内,4 ℃ 3 000×g离心35 min,收集浓缩后上清备用。Bradford法测蛋白浓度,调整蛋白浓度至50 μg/100 μL, -80 ℃保存备用。
2.2 实验对象分组 清洁级健康SD孕鼠(复旦大学上海医学院动物中心),待其自然分娩,选择1日龄新生鼠作为研究对象,雌雄不限。随机分为空气组(control组)、高氧(hyperoxia,H)组、EBM干预组和内皮祖细胞培养上清(endothelial progenitor cell-conditioned medium, EPC-CM)干预组,每组10只。各组处理如下:空气组:仔鼠在空气(21% O2)中饲养21 d;高氧组:仔鼠在85% O2中饲养21 d;EBM干预组:仔鼠在85% O2中饲养21 d,在第14天时经气管给予每只仔鼠100 μL EBM 1次;EPC-CM干预组:仔鼠在85% O2中饲养21 d,在第14天时经气管给予每只仔鼠100 μL EPC-CM(含50 μg 蛋白)。
2.3 高氧BPD模型的建立[11]订制塑料高氧箱52 cm×40 cm×31 cm,在容器上设立3个不同的孔区,一个作为进气孔,一个用作出气孔,第3孔区与测氧仪相连,箱内放温度湿度计监测箱内温度和湿度,使箱内温度控制在25 ℃~26 ℃,同时湿度控制在60%~70%。高氧组将1日龄SD新生鼠置于氧箱中,不间断输入氧气,测氧仪每日3次监测箱内氧气浓度,使氧浓度维持在85%左右,每天定时开箱30 min添加水及饲料,更换垫料和干燥剂,并与正常对照组交换母鼠以避免母鼠因氧中毒致喂养能力下降。正常对照组置于同一室内空气中。EBM干预组和EPC-CM干预组在第14天,用异氟烷麻醉仔鼠,在颈部切开一小口,分离寻找到气管,用100 μL微量注射器分别给予100 μL EBM或100 μL EPC-CM。第21天腹腔注射10%水合氯醛(8 mL/kg)处死小鼠,打开胸腔,结扎右侧肺门,取出右肺,用于mRNA的检测; 0.9%生理盐水持续灌流冲洗肺循环以去除循环细胞,经气管注入4%多聚甲醛固定左肺组织,完整取出后置于4%多聚甲醛中固定24 h,石蜡包埋,制备常规5 μm切片,留做病理检测。所有动物实验均通过我院动物实验伦理委员会批准。
2.4 检测指标及方法 (1)肺发育形态学观察: 常规制备5 μm切片,经HE染色后,于光镜下(×100)观察肺组织形态变化;(2)肺组织辐射状肺泡计数(radical alveolar count,RAC)测量:从呼吸性细支气管中心至最近纤维隔(或胸膜)作垂线,计数垂线经过的肺泡数,每只仔鼠取4张切片,每张切片随机选取10个非重复视野计数,取平均值;(3)肺组织肺泡平均线性截距(mean linear intercept,MLI)测量:利用Image-Pro Plus软件,每只仔鼠取5张切片,每张切片在光镜(×200)下选取至少10个视野,十字交叉法测量经十字线的肺泡间隔(NS)数目,同时测量十字线的总长(L),根据公式MLI=L/NS计算;(4)微血管密度(microvascular density)测定:FVIII存在于血管内皮细胞胞浆内,免疫组织化学方法检测FVIII在肺组织的表达,参考Weidner血管计数法[12],在低倍镜(×100)下寻找血管密度较高的区域,然后在高倍镜(×200)下计数血管密度,每只仔鼠取3张切片,每张切片取10个非重复视野,计数VIII因子染色阳性的血管数;(5)肺组织角质细胞生长因子(keratinocyte growth factor,KGF)、血管内皮生长因子(vascular endothelial growth factor,VEGF)、表面活性蛋白A(surfactant protein A,SP-A)和表面活性蛋白C(surfactant protein C,SP-C) mRNA表达的检测:利用Trizol (Invitrogen)提取肺组织总RNA,将1 mg的RNA用PrimeScript® RT试剂盒(TaKaRa)逆转录成cDNA。每个生物学指标用2 μL 的cDNA 采用SYBR® Premix Ex TaqTMII试剂盒(TaKaRa)进行实时荧光PCR分析。反应条件为: 95 ℃ 30 s; 95 ℃ 5 s, 60 ℃ 30 s, 40个循环;生成熔解曲线。GAPDH 作为内参照。各生物学指标的引物见表1。检验目的基因和内参基因扩增效率是否一致,如果一致,采用2-ΔΔCt方法进行相对定量分析。
3 统计学处理

表1 引物序列
应用SPSS 19.0统计软件进行统计学处理。数据均以均数±标准差(mean±SD)表示。多组间比较采用单因素方差分析,两两比较采用Bonferroni法,以P<0.05为差异有统计学意义。
结 果
1 EPCs的培养与鉴定
倒置显微镜下观察收集的骨髓单个核细胞,第3天可见贴壁细胞大部分为小圆形,部分细胞呈短梭形,培养至第5~7天可见细胞呈典型铺路石样集落生长(图1)。贴壁细胞用DiI-acLDL和FITC-UEA-1行荧光化学染色鉴定,EPCs胞浆摄取DiI-acLDL呈红色;细胞膜结合FITC-UEA-1呈绿色,双染色阳性为橙色,表明是正在分化的EPCs,见图2。

Figure 1.The morphological observation of endothelial progenitor cells (×100). On the 3rd day, the cells were small and round; on the 6th day, typical cobblestone-like colony appeared.
图1 内皮祖细胞形态学特点
2 动物体重的变化
空气组、高氧组、EBM干预组及EPC-CM干预组新生大鼠在第 1天体重间差异无统计学显著性,至第21天,4组体重的差异具有统计学意义(P<0.05),高氧组和EBM干预组较空气组体重显著下降(P<0.05),EPC-CM干预组与空气组体重间的差异无统计学显著性,见表2。

Figure 2.The abilities of EPCs to uptake DiI-ac-LDL and bind to FITC-UEA-1 (×100). EPCs uptaking DiI-ac-LDL appeared red under fluorescence microscope, and EPCs binding to FITC-UEA-1 appeared green. The double staining was orange, suggesting the differentiating EPCs. The nucleus was blue with DAPI.
图2 EPC摄取DiI-ac-LDL和结合FITC-UEA-1的荧光图像
表2 各组大鼠体重的变化
Table 2.The changes of the body weight of the rats with different treatments (g.Mean±SD.n=10)

Age(d)AirgroupHyperoxiagroupHyperoxia+EBMgroupHyperoxia+EPC⁃CMgroup16.44±0.666.34±0.816.31±0.906.45±0.872142.79±5.5631.68±7.42∗30.93±7.68∗35.34±7.93
*P<0.05vsair group.
3 肺泡化的观察
光镜下观察HE染色肺组织显示,空气组肺泡结构规整,肺泡大小均一,肺泡间隔较薄;高氧组与EBM干预组肺泡数量减少,肺泡腔增大出现肺泡简化,同时可见局部肺泡间隔增厚;EPC-CM干预组肺泡腔增大程度较高氧组和EBM干预组改善,见图3。
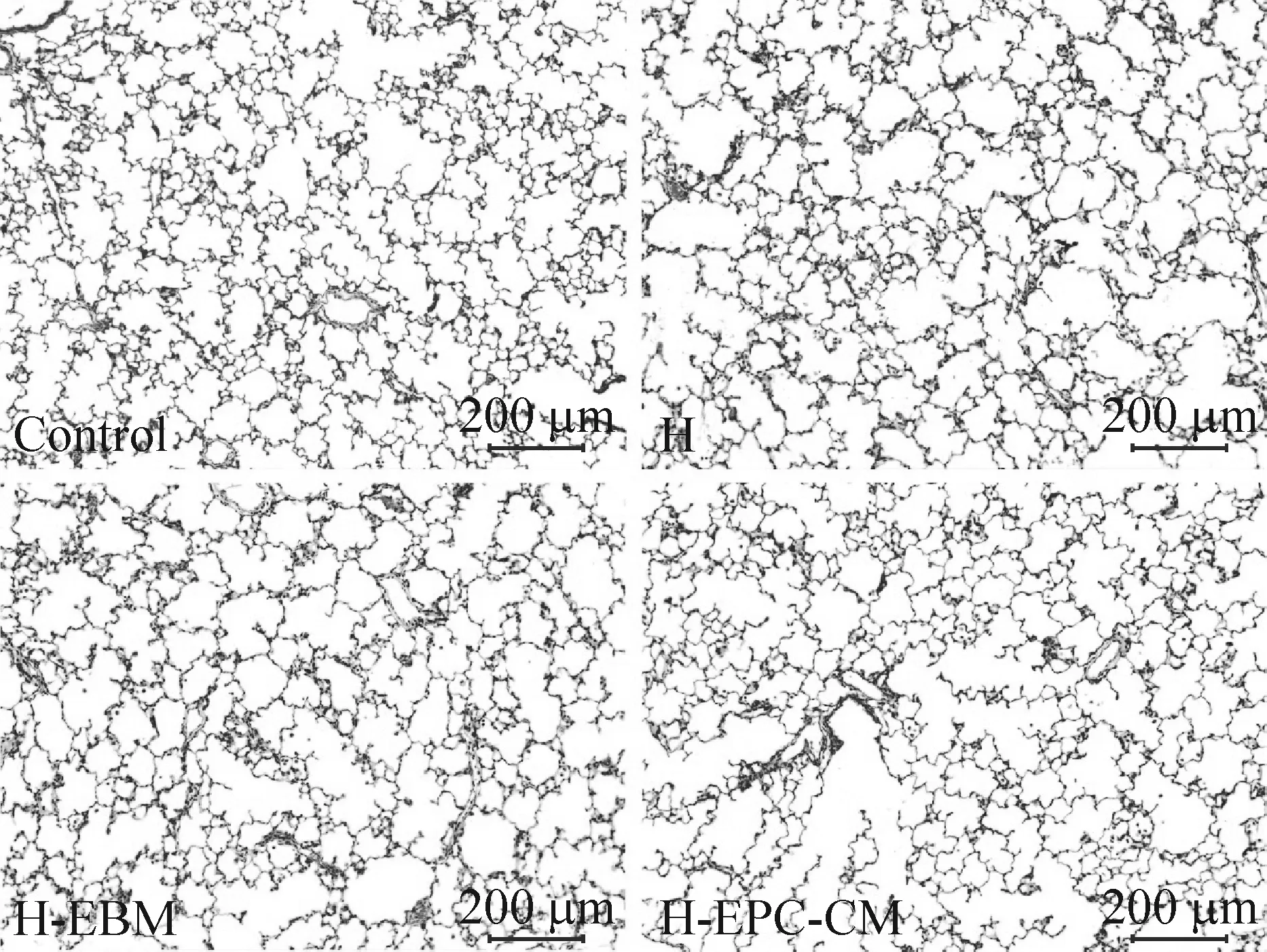
Figure 3.HE staining of lung tissue under light microscope. The scale bar=200 μm. Control: air group; H: hyperoxia group; H-EBM: hyperoxia+EBM group; H-EPC-CM: hyperoxia+EPC-CM group.
图3 光镜下肺组织HE染色观察
4组仔鼠肺组织RAC计数分别为:空气组(9.02±0.30)个,高氧组(3.67±0.51)个,EBM干预组(3.81±0.11)个,EPC-CM干预组(6.97±0.36)个。4组间RAC计数差异有统计学意义(P<0.05),高氧组、EBM干预组及EPC-CM干预组的RAC计数较空气组明显降低(P<0.05),高氧组及EBM干预组较EPC-CM干预组明显降低(P<0.05),见图4。
4组仔鼠肺组织MLI测量分别为:空气组为(37.18±3.44) μm,高氧组为(43.31±3.33) μm,EBM干预组为(42.81±2.66) μm,EPC-CM干预组为(40.02±3.86) μm。4组间MLI的差异有统计学意义(P<0.05),高氧组、EBM干预组的MLI较空气组显著增宽(P<0.05),EPC-CM干预组与空气组相比MLI的差异无统计学显著性,EPC-CM干预组和高氧组、EBM干预组的MLI比较差异无统计学显著性,但可见降低的趋势,见图4。

Figure 4.The radical alveolar count (RAC; A) and the alveolar mean linear intercept (MLI; B) in each group. Control: air group; H: hyperoxia group; H-EBM: hyperoxia+EBM group; H-EPC-CM: hyperoxia+EPC-CM group. Mean±SD.n=10.*P<0.05vscontrol;#P<0.05vsH-EPC-CM.
图4 各组间辐射状肺泡计数和肺泡平均线性截距
4 肺微血管密度的测量
各组肺组织VIII因子的免疫组化染色显示肺组织血管密度,胞浆黄棕色颗粒为阳性染色,阳性分布为围绕血管内皮细胞的棕黄色染色(图5)。4组间微血管密度的差异存在统计学显著性(P<0.05),高氧组和EBM干预组的微血管密度较空气组显著降低(P<0.05),高氧组和EBM干预组的微血管密度较EPC-CM干预组显著降低(P<0.05),而EPC-CM干预组的微血管密度与空气组相比差异无统计学显著性,见图6。
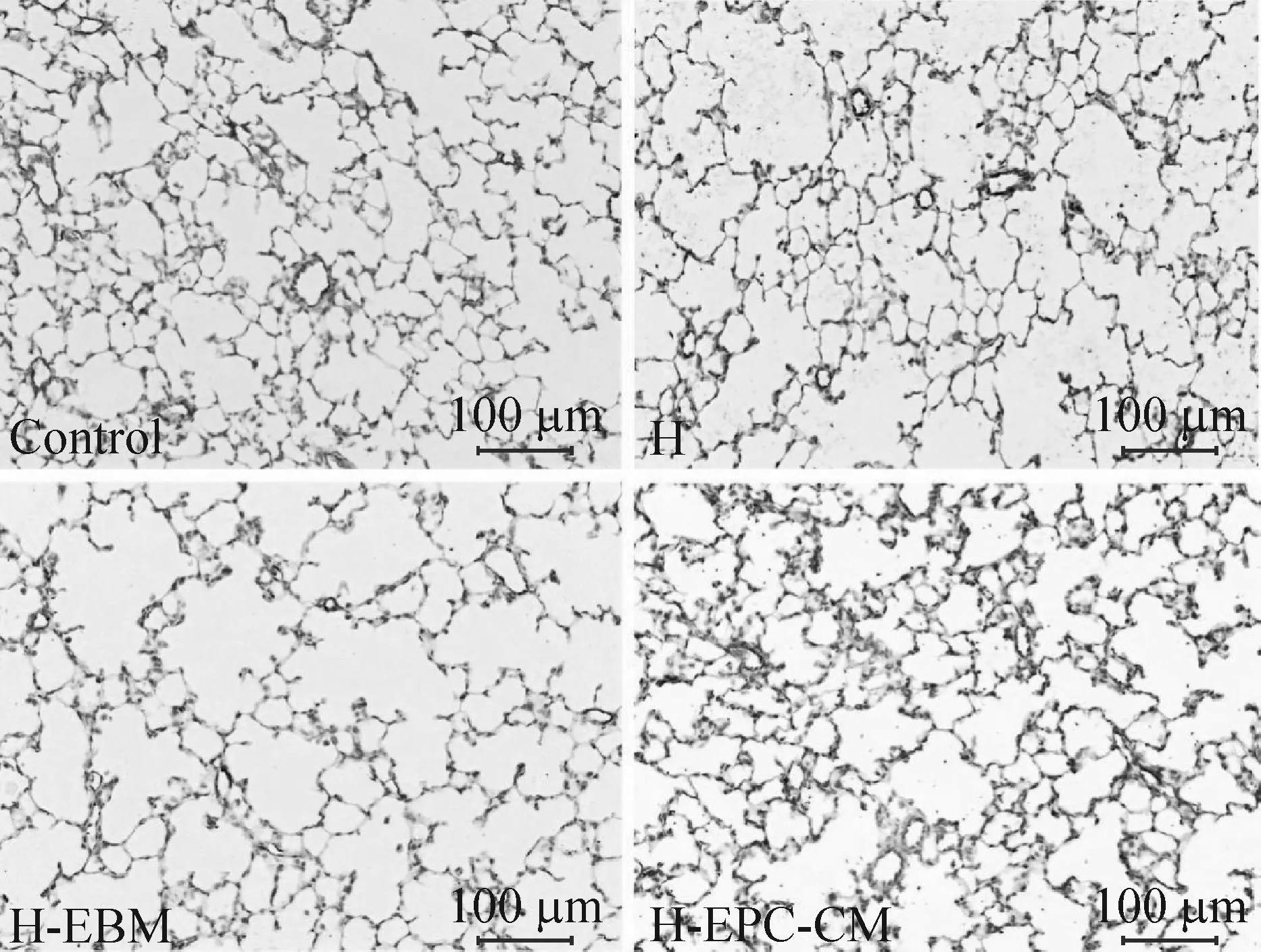
Figure 5.Factor VIII staining for microvascular density (×200). The scale bar=100 μm. Control: air group; H: hyperoxia group; H-EBM: hyperoxia+EBM group; H-EPC-CM: hyperoxia+EPC-CM group.
图5 FVIII染色显示微血管密度
Figure 6.The microvascular density in each group. Control: air group; H: hyperoxia group; H-EBM: hyperoxia+EBM group; H-EPC-CM: hyperoxia+EPC-CM group. Mean±SD.n=10.*P<0.05vscontrol;#P<0.05vsH-EPC-CM.
图6 各组微血管密度计数
5 肺组织KGF和VEGF的mRNA表达
4组肺组织KGF和VEGF的mRNA表达水平差异有统计学意义(P<0.05)。高氧组、EBM干预组及EPC-CM干预组较空气组显著降低(P<0.05),高氧组与EBM干预组较EPC-CM干预组显著降低(P<0.05),见图7。
6 肺组织SP-A和SP-C的mRNA表达
4组肺组织SP-A和SP-C的mRNA表达水平的比较见图8。高氧组、EBM干预组和EPC-CM干预组较空气组明显降低(P<0.05),并且高氧组和EBM干预组较EPC-CM干预组明显降低(P<0.05)。
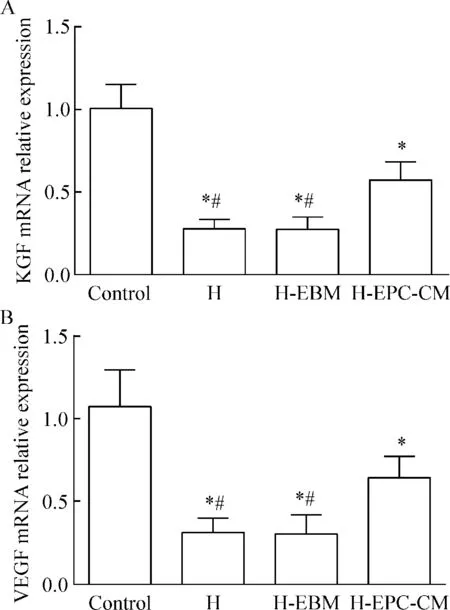
Figure 7.The relative mRNA expression of KGF (A) and VEGF (B) in each group. Control: air group; H: the hyperoxia group; H-EBM: hyperoxia+EBM group; H-EPC-CM: hyperoxia+EPC-CM group. Mean±SD.n=10.*P<0.05vscontrol;#P<0.05vsH-EPC-CM.
图7 各组间 KGF和VEGF mRNA的相对表达
讨 论
本实验表明在85% O2的环境下暴露21 d可抑制终末肺泡腔的形成,造成肺泡腔变大,发生肺泡简化,并抑制肺组织内血管的形成,使新生鼠肺组织发生类似BPD的病理变化,本课题组的前期研究[11,13]已成功地建立此高氧BPD模型。
我们的研究发现经气管滴入EPC-CM可改善高氧对肺组织造成的伤害,增加肺泡数量,降低MLI,增加肺组织内血管密度,提示EPC培养上清对高氧暴露大鼠肺结构有改善作用。Rehman等[14]早在2003年就证实EPC能通过旁分泌方式表达和释放许多信号分子,如VEGF、肝细胞生长因子(hepatocyte growth factor,HGF)、粒细胞集落刺激因子(gra-nulocyte colony-stimulating factor,G-CSF)、粒-巨噬细胞集落刺激因子(granulocyte-macrophage colony-stimulating factor,GM-CSF)等。Kim等[15]的研究也表明具有内皮祖细胞特性的人脐带血来源的干细胞(human cord blood-derived stem cells,hCB-SCs)能够分泌多种细胞因子及趋化因子,如转化生长因子β(transforming growth factor-β,TGF-β)、血小板源性生长因子(platelet-derived growth factor,PDGF)、碱性成纤维细胞生长因子(basic fibroblast growth factor,bFGF)、表皮生长因子(epidermal growth factor,EGF)、KGF和VEGF,并且能分泌具有募集干细胞功能的细胞因子G-CSF和GM-CSF,hCB-SCs可通过这些分泌因子促进内皮细胞或内皮祖细胞向伤口处聚集,加速伤口愈合。本课题组前期的研究也发现,EPCs可分泌VEGF、FGF10等肺发育相关生长因子,其旁分泌因子可促进高氧暴露下的肺泡II型上皮细胞增殖,抑制其分化为肺泡 I 型上皮细胞[10]。有研究发现EPC-CM 可抑制肺微血管内皮细胞凋亡,促进其增殖[16]。Alphonse 等[9]研究发现内皮细胞集落形成细胞培养上清(endothelial colony-forming cells-derived conditioned media,ECFC-CdM) 可促进体外培养的ECFC 网状结构的形成,避免高氧所造成的损伤;将ECFC-CdM 经腹腔注入高氧暴露的新生小鼠也可促进肺泡发育,肺血管形成,降低肺动脉高压的发生。Baker 等[17]的研究也证明了内皮祖细胞来源的旁分泌因子可促进肺动脉内皮细胞及肺泡II型细胞的增殖,促进高氧暴露下肺动脉内皮管样结构的形成。Ikutomi等[18]的研究表明晚期集落形成EPCs(late-outgrowth EPCs,LOC)不单是通过直接分化为内皮细胞,还通过旁分泌因子来改善血管内皮的损伤,抑制血管内膜的增生。
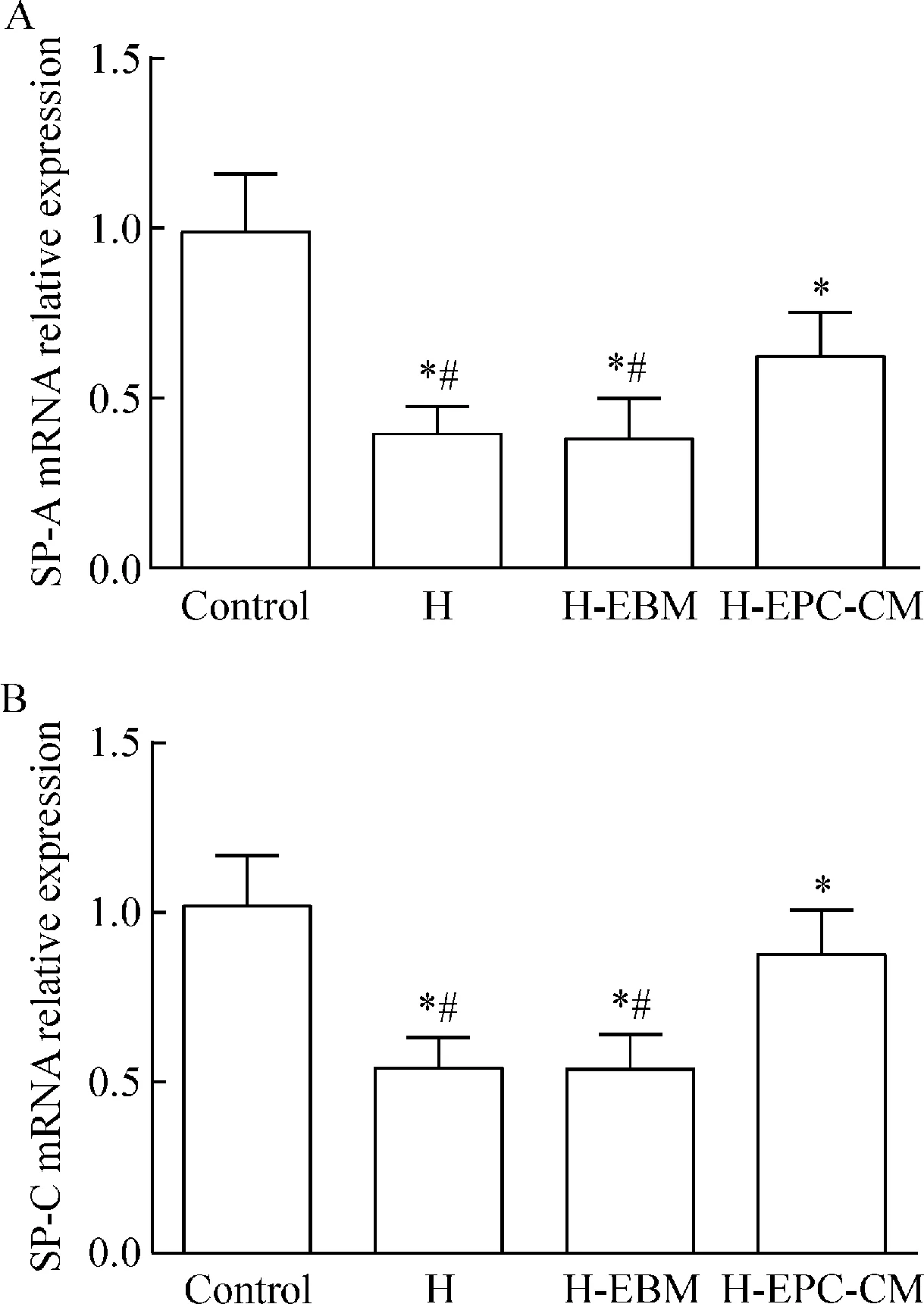
Figure 8.The relative mRNA expression of SP-A (A) and SP-C (B) in each group. Control:air group; H: hyperoxia group; H-EBM: hyperoxia+EBM group; H-EPC-CM: hyperoxia+EPC-CM group. Mean±SD.n=10.*P<0.05vscontrol;#P<0.05vsH-EPC-CM.
图8 各组间SP-A和SP-C mRNA的相对表达
我们进一步通过RT-PCR检测肺组织内KGF、VEGF、SP-A和SP-C在mRNA水平上的表达,发现KGF、VEGF、SP-A和SP-C的表达在EPC-CM治疗组较单纯高氧组显著增高。在肺组织内KGF主要由间质细胞产生[19],是一种具有促进AECII增殖,抑制炎症反应的生长因子[20-22]。Fons 等[23]的研究发现成纤细胞生长因子(fibroblast growth factor,FGF)/成纤维细胞生长因子受体(fibroblast growth factor receptor,FGFR)通路激活可募集循环EPCs至血管形成部位,促进血管的生成,KGF作为FGF的家族成员,EPCs分泌蛋白在促进肺组织KGF生成的同时,也许会促进FGF家族其它成员的增加,参与高氧肺组织的损伤修复。另外,Frank 等[24]的研究发现KGF也可调控肺泡上皮间质性改变,降低肺组织纤维化,改善肺组织形态结构。结合我们的研究结果,EPC-CM改善肺结构可能与促进肺内KGF基因高表达相关。VEGF是一种重要的血管内皮生长因子,其高表达可明显增加血管的形成,促进肺组织肺泡化[25],并对不成熟肺组织的发育有促进作用[26]。我们的研究中,EPC-CM组肺内VEGF mRNA的表达显著增高,提示EPC-CM能够促进肺内VEGF高表达,发挥促血管生成和修复血管内皮的作用。SP-A和SP-C是重要的肺表面活性物质,由II型肺泡上皮细胞合成,SP-A主要参与肺组织免疫应答,保护肺组织上皮细胞免受炎症因子的损伤[27-29]。SP-C主要降低肺表面张力的增加,充足的SP-C有助于维持肺泡扩张,避免发生肺不张。EPC-CM干预组,SP-A和SP-C mRNA的表达增加提示,EPC-CM 可能直接或间接地作用于肺泡II型上皮细胞,促进、维持和修复肺泡II型上皮细胞的活性与功能。
本研究采用新生大鼠骨髓来源EPCs,更好地保留了EPCs的干细胞特性,其增殖分泌能力较成年大鼠骨髓来源EPCs强。首次将EPCs培养上清经气道干预高氧暴露所致BPD大鼠模型,使EPC-CM直接作用于肺组织,避免了对其它部位造成的影响,更有利于最大限度地发挥在肺内的作用。EPCs移植是目前血管修复和再生领域有前景的治疗策略,也是BPD 防治的重要方法。但移植EPCs的功能受体内环境的影响,治疗效果并不理想。本研究表明,应用EPC-CM可促进高氧暴露肺损伤大鼠肺泡化和肺血管发育,这可能与其促进肺内KGF和VEGF mRNA的表达相关。该结果提示优化单纯的细胞移植方法,如应用EPCs分泌蛋白或相当的合成制剂模拟生理性EPCs旁分泌功能,或应用某一基因修饰的EPCs,可为BPD 防治策略提供新思路。
[1] Shahzad T, Radajewski S, Chao CM, et al. Pathogenesis of bronchopulmonary dysplasia: when inflammation meets organ development[J]. Mol Cell Pediatr, 2016, 3(1):23.
[2] Cardoso WV. Molecular regulation of lung development[J]. Annu Rev Physiol, 2001, 63:471-494.
[3] Thebaud B, Ladha F, Michelakis ED, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization[J]. Circulation, 2005, 112(16):2477-2486.
[4] Stenmark KR, Balasubramaniam V. Angiogenic therapy for bronchopulmonary dysplasia: rationale and promise[J]. Circulation, 2005, 112(16):2383-2385.
[5] Qi Y, Jiang Q, Chen C, et al. Circulating endothelial progenitor cells decrease in infants with bronchopulmonary dysplasia and increase after inhaled nitric oxide[J]. PLoS One, 2013, 8(11):e79060.
[6] Borghesi A, Massa M, Campanelli R, et al. Circulating endothelial progenitor cells in preterm infants with bronchopulmonary dysplasia[J]. Am J Respir Crit Care Med, 2009, 180(6):540-546.
[7] Bertagnolli M, Nuyt AM, Thebaud B, et al. Endothelial progenitor cells as prognostic markers of preterm birth-associated complications[J]. Stem Cells Transl Med, 2017, 6(1):7-13.
[8] Purhonen S, Palm J, Rossi D, et al. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth[J]. Proc Natl Acad Sci U S A, 2008, 105(18):6620-6625.
[9] Alphonse RS, Vadivel A, Fung M, et al. Existence, functional impairment, and lung repair potential of endothelial colony-forming cells in oxygen-induced arrested alveolar growth[J]. Circulation, 2014, 129(21):2144-2157.
[10]王传凯, 陆爱珍, 祁媛媛, 等. 内皮祖细胞培养上清对高氧暴露下Ⅱ型肺泡上皮细胞增殖和分化的影响[J]. 中国病理生理杂志, 2016, 32(1):8-14.
[11]Lu A, Sun B, Qian L. Combined iNO and endothelial progenitor cells improve lung alveolar and vascular structure in neonatal rats exposed to prolonged hyperoxia[J]. Pediatr Res, 2015, 77(6):784-792.
[12]Weidner N. Intratumor microvessel density as a prognostic factor in cancer[J]. Am J Pathol, 1995, 147(1):9-19.
[13]Chang LW, Qian LL, Rong ZH, et al. Pathogenetic role of matrix metalloproteinases and its tissue inhibitors in preterm rat lungs exposed to hyperoxia[J]. Acta Pharmacol Sin, 2002, 23(Suppl):59-63.
[14]Rehman J, Li J, Orschell CM, et al. Peripheral blood "endothelial progenitor cells" are derived from monocyte/macrophages and secrete angiogenic growth factors[J]. Circulation, 2003, 107(8):1164-1169.
[15]Kim J, Lee JH, Yeo SM, et al. Stem cell recruitment factors secreted from cord blood-derived stem cells that are not secreted from mature endothelial cells enhance wound healing[J]. In Vitro Cell Dev Biol Anim, 2014, 50(2):146-154.
[16]Xia L, Fu GS, Yang JX, et al. Endothelial progenitor cells may inhibit apoptosis of pulmonary microvascular endothelial cells: new insights into cell therapy for pulmonary arterial hypertension[J]. Cytotherapy, 2009, 11(4):492-502.
[17]Baker CD, Seedorf GJ, Wisniewski BL, et al. Endothelial colony-forming cell conditioned media promote angiogenesisinvitroand prevent pulmonary hypertension in experimental bronchopulmonary dysplasia[J]. Am J Physiol Lung Cell Mol Physiol, 2013, 305(1):L73-L81.
[18]Ikutomi M, Sahara M, Nakajima T, et al. Diverse contribution of bone marrow-derived late-outgrowth endothelial progenitor cells to vascular repair under pulmonary arterial hypertension and arterial neointimal formation[J]. J Mol Cell Cardiol, 2015, 86:121-135.
[19]Rubin JS, Osada H, Finch PW, et al. Purification and characterization of a newly identified growth factor specific for epithelial cells[J]. Proc Natl Acad Sci U S A, 1989, 86(3):802-806.
[20]Sakamoto S, Yazawa T, Baba Y, et al. Keratinocyte growth factor gene transduction ameliorates pulmonary fibrosis induced by bleomycin in mice[J]. Am J Respir Cell Mol Biol, 2011, 45(3):489-497.
[21]Shyamsundar M, McAuley DF, Ingram RJ, et al. Keratinocyte growth factor promotes epithelial survival and resolution in a human model of lung injury[J]. Am J Respir Crit Care Med, 2014, 189(12):1520-1529.
[22]Fehrenbach H, Kasper M, Tschernig T, et al. Keratinocyte growth factor-induced hyperplasia of rat alveolar type II cellsinvivois resolved by differentiation into type I cells and by apoptosis[J]. Eur Respir J, 1999, 14(3):534-544.
[23]Fons P, Gueguen-Dorbes G, Herault J, et al. Tumor vasculature is regulated by FGF/FGFR signaling-mediated angiogenesis and bone marrow-derived cell recruitment: this mechanism is inhibited by SSR128129E, the first allosteric antagonist of FGFRs[J]. J Cell Physiol, 2015, 230(1):43-51.
[24]Frank L. Protective effect of keratinocyte growth factor against lung abnormalities associated with hyperoxia in prematurely born rats[J]. Neonatology, 2003, 83(4):263-272.
[25]Thebaud B, Ladha F, Michelakis ED, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization[J]. Circulation, 2005, 112(16):2477-2486.
[26]Compernolle V, Brusselmans K, Acker T, et al. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice[J]. Nat Med, 2002, 8(7):702-710.
[27]Carreto-Binaghi LE, Aliouat EM, Taylor ML. Surfactant proteins, SP-A and SP-D, in respiratory fungal infections: their role in the inflammatory response[J]. Respir Res, 2016, 17(1):66.
[28]Saito A, Ariki S, Sohma H, et al. Pulmonary surfactant protein A protects lung epithelium from cytotoxicity of human beta-defensin 3[J]. J Biol Chem, 2012, 287(18):15034-15043.
[29]Goto H, Mitsuhashi A, Nishioka Y. Role of surfactant protein A in non-infectious lung diseases[J]. J Med Invest, 2014, 61(1-2):1-6.
(责任编辑: 林白霜, 罗 森)
TGF-β/Smad2/3信号在肌成纤维细胞增殖期间需要Wnt/β-catenin信号的激活
动物模型与人类疾病中的纤维化与Wnt/β-连环蛋白(β-catenin)通路的异常活化有关。尽管经过广泛的研究努力,目前科学家仍未发现有效治疗纤维化的方法。肌成纤维细胞(myofibroblasts)是纤维化的主要效应细胞,负责细胞外基质沉积。抑制肌成纤维细胞增殖对于纤维化的治疗至关重要。肌成纤维细胞的增殖可引发一系列效应,从而导致纤维化。近年来,Wnt通路被认为是纤维化疾病的主要影响因素,但其介导的促纤维化的具体机制仍不甚清楚的。转化生长因子β(transforming growth factor-β, TGF-β)和肌成纤维细胞活性在纤维化发病机制中的核心作用已经被普遍接受,然而这两个过程之间相互作用的细节仍不清楚。Xu等的研究检测了纤维化标志性蛋白[波形蛋白(vimentin)、α-平滑肌肌动蛋白(α-smooth muscle actin, α-SMA)和胶原蛋白I(collagen I)]和TGF-β信号通路分子(包括 Smad2/3 及其磷酸化形式p-Smad2/3)的持续表达水平,并详细分析β-catenin介导的可能分子机制,包括上皮-间充质转化(epithelial-mesenchymal transition)和成纤维细胞向肌成纤维细胞的转变,以及信号网络的调节中β-catenin活性增强,以抵消自分泌的TGF-β/Smad2/3信号。该研究主要提出了对纤维化机制的新认识,即TGFβ1-Smad2/3信号通过Wnt/β-catenin激活上皮和间充质细胞,从而有助于肺纤维化的形成。
J Cell Mol Med, 2017, 21(8):1545-1554(李肖肖)
Endothelial progenitor cell-conditioned medium improves lung structure in neonatal rats exposed to hyperoxia
LI Zhi, LU Ai-zhen, ZHANG Xiao-mei, QIAN Li-ling
(Children’sHospitalofFudanUniversity,Shanghai201102,China.E-mail:llqian@126.com)
AIM: To investigate the therapeutic effect of endothelial progenitor cell-conditioned medium (EPC-CM) on the lung structure of neonatal rat exposed to hyperoxia, and to explore the mechanisms.METHODS: Bone marrow-derived endothelial progenitor cells (EPCs) were collected from new born Sprague-Dawley (SD) rats and the EPCs were identified. The conditioned medium from the passage 3 EPCs was collected. Newborn SD rats (n=40) were randomly divided into 4 groups. The rats in room air group were exposed to the room air (21% O2) for 21 d. The rats in hyperoxia group were exposed to hyperoxia (85% O2) for 21 d. The rats in endothelial cell basal medium (EBM) group were exposed to hyperoxia for 21 d, and
100 μL EBM on postnatal day 14 (P14) in a single intratracheal (IT) injection. The rats in EPC-CM group were exposed to hyperoxia for 21 d, and received 100 μL EPC-CM on P14 in a singlie IT injection. The rats were sacrified on the 21st day. The left lungs were excised, placed in 4% paraformaldehyde, serially dehydrated in ethanol and embedded by paraffin. Serial sectioning of the paraffin-embedded left lung tissues was prepared for 5 μm thickness, and stained with hematoxylin and eosin. The pulmonary radical alveolar count (RAC) and alveolar mean linear intercept (MLI) were then calculated. The microvascular density was determined by FVIII immunostaining. The mRNA expression of KGF, VEGF, SP-A and SP-C in the right lung tissues was detected by real-time fluorescence quantitative PCR. RESULTS: The cultured cells had typical EPC morphological characteristics, and had the abilities to bind to FITC-UEA-1 and uptake DiI-ac-LDL. The body weight of the rats on day 21, RAC, MLI and microvascular density were significantly lower in hyperoxia group and EBM group than those in room air group (P<0.05). The EPC-CM group had significantly higher RAC and microvascular density than those in hyperoxia group and EBM group (P<0.05), but the body weight and MLI had no significant difference. The mRNA expression levels of KGF, VEGF, SP-A and SP-C in hyperoxia group and EBM group were significantly lower than those in room air group (P<0.05). The mRNA expression levels of KGF, VEGF, SP-A and SP-C in EPC-CM group were significantly higher than those in hyperoxia group and EBM group (P<0.05). CONCLUSION: EPC-CM promotes the lung alveolarization and microvascular formation in neonatal rats exposed to hyperoxia. These benefits may be correlated with the increased KGF and VEGF mRNA expression.
Hyperoxia; Endothelial progenitor cells; Lung injury; Paracrine
1000- 4718(2017)08- 1467- 08
2017- 03- 07
2017- 04- 17
国家自然科学基金资助项目(No. 81270727)
R363; R722.1
A
10.3969/j.issn.1000- 4718.2017.08.020
杂志网址: http://www.cjpp.net
△通讯作者 Tel: 021-64931913; E-mail: llqian@126.com

