Preclinical and clinical applications of specific molecular imaging for HER2-positive breast cancer
Wei Chen, Xiaofeng Li, Lei Zhu, Jianjing Liu, Wengui Xu, Ping Wang
1Department of Molecular Imaging and Nuclear Medicine;2Department of Radiation Oncology, Tianjin Medical University Cancer Institute and Hospital; National Clinical Research Center for Cancer; Key Laboratory of Cancer Prevention and Therapy, Tianjin; Tianjin's Clinical Research Center for Cancer, Tianjin 300060, China
Preclinical and clinical applications of specific molecular imaging for HER2-positive breast cancer
Wei Chen1, Xiaofeng Li1, Lei Zhu1, Jianjing Liu1, Wengui Xu1, Ping Wang2
1Department of Molecular Imaging and Nuclear Medicine;2Department of Radiation Oncology, Tianjin Medical University Cancer Institute and Hospital; National Clinical Research Center for Cancer; Key Laboratory of Cancer Prevention and Therapy, Tianjin; Tianjin's Clinical Research Center for Cancer, Tianjin 300060, China
Precision medicine and personalized therapy are receiving increased attention, and molecular-subtype classification has become crucial in planning therapeutic schedules in clinical practice for patients with breast cancer. Human epidermal growth factor receptor 2 (HER2) is associated with high-grade breast tumors, high rates of lymph-node involvement, high risk of recurrence, and high resistance to general chemotherapy. Analysis of HER2 expression is highly important for doctors to identify patients who can benefit from trastuzumab therapy and monitor the response and efficacy of treatment. In recent years, significant efforts have been devoted to achieving specific and noninvasive HER2-positive breast cancer imaging in vivo. In this work, we reviewed existing literature on HER2 imaging in the past decade and summarized the studies from different points of view, such as imaging modalities and HER2-specific probes. We aimed to improve the understanding on the translational process in molecular imaging for HER2 breast cancer.
Breast cancer; human epidermal growth factor receptor 2 (HER2); molecular imaging; probes
Introduction
Breast cancer is one of the most common malignant tumors and cause of mortality for women all over the world1. Human epidermal growth factor receptor 2 (HER2) is a member of the epidermal growth factor receptor family of tyrosine kinases. The HER2/neu gene is a proto-oncogene located on the long arm of chromosome 17 and encodes a transmembrane receptor protein2. The HER2 protein comprises an intracellular domain that can regulate important aspects of the physiology and growth of cells, as well as an extracellular domain that facilitates signal transduction as a receptor. Cultured cells can be transformed into a malignant phenotype upon the overexpression of HER22. HER2/neu amplification and/or overexpression is observed in 15%–20% of patients with breast cancer3,4. HER2 is a factor that indicates high-grade breast tumors, high rates of lymph node involvement, high risk of recurrence, and high resistance to general chemotherapy2. Patients with HER2-positive breast cancer present a worse prognosis than those without HER2 overexpression, indicating that HER2 overexpression can be a marker of aggressive malignancy. Moreover, discordance in HER2 status may exist between primary HER2-positive breast cancer and distant metastasis because of heterogeneity5. Trastuzumab is a humanized monoclonal antibody that is FDA-approved for therapeutic use in HER2-positive breast cancer. It is created to specifically bind to HER2 to further inhibit the growth of tumor cells via different mechanisms, including provoking apoptosis, altering downstream signal transduction, and decreasing HER2 expression1.
Currently, immunohistochemistry (IHC) and fluorescence in situ hybridization are the most important pathological techniques applied in the detection of HER2 amplification and/or overexpression. These techniques have also been recommended as guidelines by the American Society of Clinical Oncology/College of American Pathologists. About 20% of HER2/neu tests (including biopsy and IHC) provide false negative or false positive results because of technical problems, sampling bias, heterogeneity of the tumors and other reasons, which further lead to the improper selection of treatments6. Therefore, non-invasive molecular imaging techniques are needed for the accurate assessment of HER2 expression in both primary tumors and distantmetastasis in vivo.
With the development of molecular imaging, one can now monitor the biological and pathophysiological processes of normal and abnormal tissues in vivo. Molecular imaging can thus be a complementary tool in breast cancer imaging for early detection, specific diagnosis, and evaluation of therapeutic responses. Nuclear medicine imaging [including positron emission tomography (PET) and single photon tomography computed tomography (SPECT)], ultrasound, and MRI are the most popular techniques for HER2 breast cancer imaging in both preclinical and clinical practice.
Preclinical HER2-positive breast cancer imaging
Great efforts have been made to image HER2 positive breast cancer with different modalities in the last decade. Probes are the most critical part for the molecular imaging of breast cancer. Probes should be able to easily penetrate the tumor parenchyma and be far from blood and normal organs to increase the tumor-to-tissue contrast. An optimal molecular probe for HER2-positive tumor imaging should have the following characteristics: (1) The targeting component has high affinity and specificity to the extracellular domain of HER2 protein. Various HER2-targeted parts for molecular imaging tracers, such as monoclonal antibodies, antibodybased fragments, diabodies, nanobodies, nonimmunoglobulin scaffolds, affibodies, peptides, and designed ankyrin-repeat proteins, have been explored for HER2-positive breast cancer diagnosis and therapy in the past few years. (2) The signaling component can elevate the contrast difference between tumors and normal tissues to make tumors visible and detectable. Signaling components are designed on the basis of different imaging modalities.
Nuclear medicine imaging
Nuclear imaging, including PET and SPECT, is the first molecular imaging modality to be applied for cancer in clinical practice. It requires the intravenous administration of radio-labeled materials to generate images. Combining PET or SPECT with computed tomography (CT) or MRI can provide both functional and anatomical information with high resolution.18F-fluorodeoxyglucose (FDG) and99mTc are the most common tracers that are used for PET and SPECT imaging, respectively, but they lack specificity to breast cancer. These agents are designed on the basis of imaging equipment. PET
The radionuclides that are usually used for HER2 imaging on PET are18F,64Cu,68Ga,89Zr, and124I. The comparison of the radionuclides of68Ga and111In with the same affibody and chelator shows that68Ga-labeled affibodies are easier to label and are cleared more quickly from the blood and other healthy tissues, except for kidneys, than111In-labeled affibodies.68Ga-labeled affibodies also show good targeting ability and provide good tumor-to-blood and tumor-toorgan ratios within 2 h after injection in favor of clinical imaging7. A recent study conjugated the anti-HER2 affibody with44Sc via a 1, 4, 7, 10-tetraazacylododecane-1, 4, 7, 1-tetraacetic acid (DOTA) chelator for HER2 imaging and compared it with68Ga-labeled affibody, demonstrating the high specificity of the44Sc-labeled affibody. The distributions of radioactivity at 3 h after injection for both affibodies were found to be similar, but the blood clearance and tumor-toblood ratio of the44Sc-labeled affibody were both lower than those of the68Ga-labeled one8.18F, with a half-life of 110 min, exhibits ideal nuclear physical characteristics for PET imaging in both experimental and clinical applications. Compared with other metal radioisotopes,18F is relatively difficult to be introduced into other molecules because its short half-life (<2 h) requires time-efficient synthesis. The direct introduction of18F usually involves harsh conditions. Efforts have been made to identify new ways to insert18F-fluorine into organic molecules. Recent studies employed18F as a radionuclide to label anti-HER2 nanobodies and generated high contrast PET images with high tumor-toblood and tumor-to-muscle ratios9-11. The results demonstrated the feasibility of utilizing18F-labeled anti-HER2 nanobodies to evaluate HER2 expressing cancers, except for cases of high renal retention, which can be a common problem of18F-labeled anti-HER2 nanobodies. Studies even used the short-lived11C (t1/2=20.4 min) to label a HER2 binding affibody molecule with the sel-tagging technique and compared the results with68Ga-labeled affibodies. These studies found that both11C-labeled and68Ga-labeled HER2 affibodies can be cleared rapidly from the blood and that the former shows significantly low kidney retention and overall absorbed dose.11C-labeled affibodies show excellent tumor-targeting capability and can thus successfully be used for rapid and repeated PET imaging for HER2-positive tumors12.
The radio-labeled trastuzumab is the representative of monoclonal antibodies that are widely used for imaging HER2-positive breast cancers in vivo in clinical and preclinical studies13-18. However, some imaging problems are unavoidable because of their large size (MW=150 kDa), andthese problems include long biodistribution time, slow tumor penetration and blood clearance, and low tumor-totissue ratio, all of which limit the application of antibodies in molecular imaging. Affibody molecules are a new class of relatively stable small scaffold proteins (6.5 kDa). They are usually 3-alpha-helice proteins based on a 58-amino-acid Z-domain. As a result of their small size, affibodies have shown high binding affinity to targets, rapid blood clearance, and good tumor penetration, making them ideal candidates for molecular imaging. A study comparing the affibody- and antibody-based PET agents for HER2 imaging revealed that both radio-labeled affibodies and antibodies can specifically bind to HER2 and clearly display HER2 expression in xenografts. The uptake of radio-labeled trastuzumab in tumors is higher than that of affibodies, whereas the latter provides better tumor-to-tissue contrast at an earlier time than trastuzumab due to its quicker clearance of radioactivity from blood and normal organs; such finding illustrates that affibody-based molecules might serve as better tracers than antibodies for the imaging of primary HER2 over-expressing tumors with or without distant metastases19. A probe of a64Cu-labeled affibody was synthesized in both monomeric and dimeric forms, and the result showed higher tumor accumulation and higher tumor-to-blood ratio in the monomer form than in the dimer form20. A18F-labeled affibody was utilized to display HER2 expression tumors and HER2 metastatic tumors to the lung via PET21,22. Researchers also confirmed the feasibility of using18F-labeled affibody molecules for the quantitative assessment and monitoring of HER2 down-regulation after treatment of trastuzumab or an inhibitor of heat-shock protein23,24. The size of affibodies can be further reduced by truncating the third alpha-helix that does not contribute to receptor recognition during synthesis. For this purpose, a68Ga-labeled 2-helix small protein (MUTDS) with a chelator of DOTA was adopted for PET imaging. The result showed high HER2 binding affinity and high tumor accumulation with rapid clearance from normal tissues, indicating the feasibility of a 2-helix protein scaffold serving as a probe to monitor HER2 expression in vivo. The tumor uptake and tumor-to-organ ratio of68Ga-DOTAMUT-DS were approximately 103times lower than those of many 3-helix-affibody molecules. This result is probably due to the relatively weak HER2 binding affinity of the probe, but such characteristic is still acceptable, making68Ga-DOTAMUT-DS an alternative candidate with high imaging contrast25-27. Recently,68Ga-labeled anti-HER2 single-chain variable fragment (scFv) was developed to achieve rapid blood clearance and good tumor-to-blood ratio at an early time point. HER2-positive xenografts were visualized successfully with68Ga-labeled scFv with high accumulation in the tumors, although the tumor-to-blood ratio was relatively low28.
A good chelator should be able to connect the receptorbinding part and the radionuclide for HER2 imaging. Structures of chelators can affect the clearance rate from blood and tumor uptake, as well as the uptake of normal organs. A study conjugated the following macrocyclic chelators with68Ga to the N-terminus of the affibody: DOTA, 1,4,1, 4, 7-7-triazacyclononane-N,N,N-triacetic acid (NOTA), and 1-(1,3-carboxypropyl)-1,4,7-triazacyclononane-4, 7-diacetic acid (NODAGA); their biodistributions and targeting properties were then compared in vivo29. The results showed chelator-dependent differences. The tumor uptake for the68Ga-DOTA-affibody was significantly higher than that for both68Ga-NODAGA-affibody and68Ga-NOTA-affibody at 2 h after injection. This outcome was interpreted as different off-target interactions due to the local charge distribution and conformation of the N-terminus caused by different structures of chelators. The68Ga-NODAGA-affibody had the highest tumor-to-blood ratio and showed a two fold higher tumor-to-liver ratio than the68Ga-NOTA-affibody, which is important for detecting liver metastases. Another study compared64Cu-labeled DOTA and NODAGA antibodies using PET imaging and found that the latter showed favorable performance in vivo with tumorto-tissue ratios of 2-fold and 1.5-fold higher than the former agent at 24 h and 48 h, respectively30.
SPECT
Radionuclides that are usually used for SPECT imaging are86Y,99mTc,111In, and125I.111In-diethylenetriaminepentaacetic acid (DTPA)-trastuzumab was used for HER2-positive tumor imaging and found to be efficiently labeled with high yields, high stability, and good biodistribution in mice. The uptake of the111In-labeled trastuzumab in the liver, spleen, and kidney was expected, and no uptake was observed in the brain due to its size18. In elevating in vivo targeting capabilities, minimal monovalent binding fragments, such as Fab (~55 kDa) and scFv (~28 kDa) with retained binding specificity, are widely used in molecular imaging studies. Trastuzumab Fab labeled with different radionuclides of111In and99mTc are similar in terms of visualizing HER2 xenografts, but111In-trastuzumab-Fab shows higher tumorto-tissue ratios that favor the delayed imaging of tumors. Compared with that of intact trastuzumab, the HER2 binding affinity of trastuzumab Fab fragments shows a 2-fold decrease because of their smaller size, which is acceptable31.In a study, an affibody-based probe labeled with99mTc was developed for SPECT imaging. The result revealed high uptake in HER2-expressing tumors and low uptake in the liver. The moderate- and high-level HER2-expressing xenografts can be clearly visualized 1 h after injection of the
99mTc-labeled affibody, thus proving that it can be a potential agent for SPECT imaging to detect HER2-positive tumors32. Compared with the125I-labeled affibody, the99mTc-labeled agent had higher accumulation in the liver after injection33.
MRI
In recent years, great progress has been made in MRI, especially for soft tissue imaging. Conventional breast MRI can identify tumors with their manifestations, including size, shape, margin, and signal changes. Currently, the contrast agents commonly used for MR imaging are gadolinium DTPA (decreases T1 relaxation time) and supraparamagnetic iron oxide (SPIO, decreases T2 relaxation time), which are non-specific but can increase the signal contrast between tumors and normal tissues. Molecular imaging can be performed upon MRI by using probes that bind to the target protein or molecule specifically. Nanoparticles (NPs) attract great interest in biomedical applications as imaging probes because of their extraordinary properties, including small size, well-modified structure, high binding affinity, long circulating half-life, and high permeability and retention effects that allow an effective accumulation at tumor sites34-36. In the literature, magnetic iron oxide nanoparticles (IONPs) conjugated with trastuzumab was designed to detect HER2 expression in vitro and in vivo using 3T MRI15. A 45% signal drop was found at the tumor site on T2WI after administration of the agent, proving that trastuzumab-NPs are able to distinguish cells and xenografts with different levels of HER2 expression while ensuring low cytotoxicity and good dispersity. On the basis of this work, the researchers developed HER2-targeted IONPs coated with block copolymer poly (PEO-b-PγMPS) and revealed a signal reduction in the tumor but with a low level of uptake by the reticuloendothelial system; PEO-b-PγMPS was found to be superior to most IONPs that require several weeks to be cleared out of the body from the liver and spleen37. Affibodybased SPIO NPs were generated for HER2-specific MR imaging. The agent showed good binding ability, and the tumors were clearly displayed on a gradient-echo sequence on MRI, thus confirming that the affibody-SPIO offers advantageous features that make it a suitable alternative for molecular imaging in comparison with antibody-based MR probes38.
Optical imaging
Optical imaging is a non-invasive technique to detect and analyze signals originating from bioluminescent and fluorescent probes. It uses visible light and the special properties of photons to obtain detailed images of organs and tissues as well as cells and molecules. Optical imaging technologies rely on light-producing optical reporters, such as luciferase and fluorescent proteins, fluorescent dyes, and NPs. Quantum dots (QDs) are fluorescent nanocrystals (2–10 nm), and QD-based imaging can be used in cancer research to localize tumors and monitor possible changes in receptor expression during the treatment process. As an optional probe for determining HER2 expression levels, QD-based nanotechnology has attracted significant interest in the detection of HER2 breast cancer in vivo and in vitro. Studies have demonstrated that QD-based immunofluorescence technology can be used to quantitatively determine HER2 expression levels and serve as a potentially new method for HER2 detection in clinical practice39,40. A study synthesized QD-labeled anti-HER2 antibody and used it for both fixed and live cells. The result showed that the QD bioconjugates can rapidly localize HER2 positive cells, indicating its potential ability for targeted therapy and image-guided surgery41. A QD-based double-color imaging technique was reported to show the HER2 levels on breast cancer cells in the tumor matrix. This technique provided direct visible evidence that HER2 expression levels are directly related to cancer invasion42. QD-labeled anti-HER2 antibody was applied in the single molecular imaging of breast cancer cells with a high-resolution in vivo 3D microscopic system, and the result showed that cancer cells expressing HER2 can be visualized by the NPs in vivo at subcellular resolution43. In animal studies, HER2-xenografts can be visualized with the QD-linked anti-HER2 antibody43,44. A QD-conjugated antibody was also used to evaluate its delivery process to tumors, and valuable information on antibody-conjugated therapeutic NPs was obtained to help increase therapeutic efficacy45.
Multimodal imaging
The development of probes has enhanced the possibility of multimodality imaging of HER2. Sampath et al.17first designed a dual-labeled HER2 imaging agent that conjugated trastuzumab with111In and a fluorescent dye to detect HER2 expression using SPECT and near-infrared (NIR) fluorescence imaging in vivo and in vitro. The outcome indicated the molecular specificity of targeting and the correlationbetween nuclear and optical imaging results, thus confirming the stability of the dual-labeled agent. A few years later, on the basis of their previous work, the authors developed the agent of64Cu-DOTA-trastuzumab-IRDye800, which was dual-labeled for both PET and NIR fluorescence imaging, to detect metastases of HER2-positive breast cancer14. The detection capabilities of the dual-labeled agent and18F-FDGPET were similar for primary tumors, but only the former was able to identify lung metastases . The in vitro NIR fluorescence imaging enabled the visualization of channels between the primary tumor and the axillary lymph nodes, thus providing the ability to track the lymphatic route for cancer cells. Qiao et al.46developed a novel class of multiple modality contrast agents for MRI and NIR imaging. They designed a Gd3+binding site into a stable scaffold protein to increase the relaxivity value of both T1 and T2 so that the sensitivity of disease detection was increased, resulting in 100-fold lower dose usage than the clinically used nontargeting agent DTPA. Gao et al.47designed affibody-based nanoprobes using NIR QDs and IONPs to image HER2-expressing cells and tumors and found that nanoparticleaffibody conjugates may be excellent candidates as targeting probes for HER2 overexpressing tumor diagnosis. Similarly, antibody conjugated multilayered nanoprobes and NIR QDs were successfully developed and used for both NIR and MR imaging of HER2 cells and tumors48. Probes with different HER2-targeting and/or signaling components for HER2 imaging are listed in Table 1.
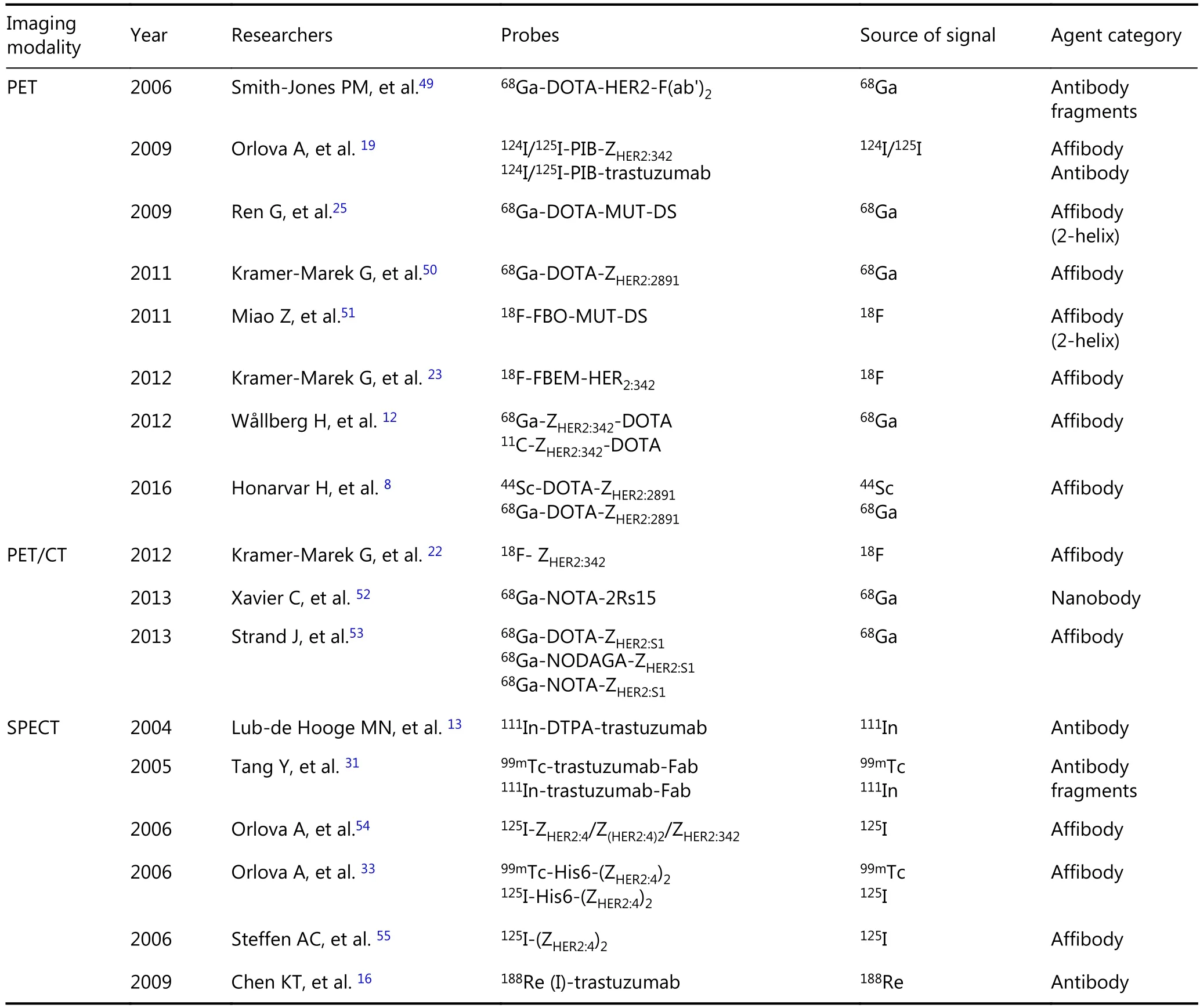
Table 1 Targeting probes for HER2-positive breast cancer imaging

Continued
Clinical application of HER2-specific imaging
Thus far, specific HER2 imaging is not widely performed on patients. Most probes are antibody (trastuzumab)-based agents that are labeled with different radionuclides for PET or SPECT imaging. Perik and his colleagues57developed111In-DTPA-trastuzuma for preclinical study in 2004 and used it to predict the cardiotoxicity of trastuzumab and identify tumors in a clinical observation in 2006. Fifteen HER2-positive breast cancer patients with metastases were enrolled, and they underwent SPECT from 15 min to 7 days after administration of 150 MBq radio-labeled trastuzumab.111In-DTPA-trastuzumab was able to identify HER2-positive tumors, but it cannot predict trastuzumab-associated cardiotoxicity. Dijkers et al.58employed89Zr-trastuzumab as a tracer for PET imaging of patients with HER2-positive metastatic breast cancer and concluded that the best time for tumor visualization is 4–5 days after injection because of the long half-life of89Zr. A similar clinical study applied89Zrtrastuzumab to detect HER2-positive metastases and found no correlation between89Zr-trastuzumab uptake and HER2 expression level; however, almost half of the positive89Zrtrastuzumab foci on PET/CT were false positive59. Both89Zrand111In- labeled trastuzumab were used to assess the clinical response of HER2 positive metastatic breast cancer to the therapy of a heat shock protein 90 inhibitor or trastuzumab, respectively60-62. HER2 imaging can also predict the response to trastuzumab if combined with the traditional18F-FDG PET and if responders can be differentiated from nonresponders62. Probes radio labeled with64Cu have also been applied in clinical practice in some studies. PET or PET/CT with the probe of64Cu-DOTA-trastuzumab has been performed on patients with primary and/or metastatic HER2-positive breast cancer63,64. Both primary breast tumors and metastases in the brain, liver, bone, and lymph nodes were identified with generally high image quality and tumorto-tissue contrast. Furthermore, CT-positive lesions were detected by64Cu-DOTA-trastuzumab but not by18F-FDG in some lesions, and false positivity of64Cu-DOTA-trastuzumab occurred in only one instance. According to the results, tumor uptake and the radiation exposure of64Cu-DOTA-trastuzumab were both comparable to those of18F-FDG, thus demonstrating the feasibility of64Cu-DOTA-trastuzumab as a potentially safe probe to detect the primary and metastatic lesions of HER2-positive breast cancer for clinical use63. Another study, which involved eight patients of HER2-positive metastatic breast cancer, preinjected 45 mg trastuzumab before the introduction of the64Cutrastuzumab, and an approximately 75% reduction in liveruptake of64Cu was found but without significant changes in tumor uptake. This result provided a new strategy for a favorable biodistribution of64Cu and visualization of liver metastases64.
111In- or68Ga-labeled affibody (ABY-002) molecules were first used in a clinical study involving only three patients with recurrent metastatic breast cancer. The blood kinetics analysis showed rapid blood clearance of the radio-labeled agents in humans with the first half-life of 4–11 min for the111In-labeled affibody and 10–14 min for the68Ga-labeled affibody. The images of SPECT or PET/CT were compared with those of18F-FDG PET/CT, and most of the lesions identified by18F-FDG PET/CT were also visualized with the111In- or68Ga-labeled affibody, except for one lesion of liver metastasis and another lesion close to the kidney. Both18F-FDG and68Ga-labeled affibody can improve the diagnosis of metastasis in comparison with CT. They concluded that the affibody is more adaptable for clinical practice with high detection rate compared with antibodies or fragments, although further clinical studies are still needed65. Considering the high liver uptake of radio-labeled affibodies, Sörensen et al.66developed a second-generation affibody with improved biochemical and biophysical characteristics and used it in seven patients of recurrent metastatic HER2-positive or HER2-negative breast cancer. The111In-labeled affibody can discriminate the metastases with high or low HER2 expression. The uptake of HER2-positive metastases increased between 4 and 24 h after injection, whereas it decreased in the negative metastases in the same time duration. Detailed comparisons of sensitivity and/or specificity of radio-labeled trastuzumab vs.111Inlabeled affibody were not permitted due to limited clinical studies. Most recently,68Ga-HER2-nanobody was used for the visualization of both primary and metastatic HER2 positive lesions, and it showed favorable biodistribution and zero toxicity67.
These clinical findings related to HER2 imaging confirm that HER2-targeted imaging can be applied to disease detection, staging, and therapy response monitoring through the determination of the HER2 expression of breast tumors. Probes and trials for clinical HER2-imaging are listed in Table 2.

Table 2 Clinical applications of probes for HER2-positive breast cancer imaging
Conclusions
HER2 plays an important role in cancer progression and prognosis of breast cancer. Increasing attention has been paid to the imaging of HER2 -positive breast cancer. This technology can help to identify patients who would benefit from trastuzumab therapy and monitor the response and efficacy of treatment. In addition, it can detect the discordance of HER2 status between primary breast cancer and distant metastases. Most HER2-targeting probes are still in experimental and preclinical status. Among these techniques, nuclear medicine imaging seems to be the most popular applied imaging modality for HER2 -positive tumors, although technical complexity in probe synthesis and the stability, safety, and toxicity of the probes themselves may limit the clinical applications of HER2 specific probes.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No.81202795) and China Postdoctoral Science Foundation (Grant No.2015M571271). The authors thank Ms. Samantha Calva for polishing the manuscript.
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.Theriault RL, Carlson RW, Allred C, Anderson BO, Burstein HJ, Edge SB, et al. Breast cancer, version 3.2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013; 11: 753-60; quiz 61.
2.Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. NCCN guidelines insights breast cancer, version 1. 2016. J Natl Compr Canc Netw. 2015; 13: 1475-85.
3.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007; 131: 18-43.
4.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007; 25: 118-45.
5.Santinelli A PE, Stramazzotti D, Fabris G. HER-2 status discrepancy between primary breast cancer and metastatic sites. Impact on target therapy. Int J Cancer. 2008; 122: 999-1004.
6.Dendukuri N, Khetani K, McIsaac M, Brophy J. Testing for HER2-positive breast cancer: a systematic review and cost-effectiveness analysis. Cmaj. 2007; 176: 1429-34.
7.Tolmachev V, Velikyan I, Sandstrom M, Orlova A. A HER2-binding Affibody molecule labelled with 68Ga for PET imaging: direct in vivo comparison with the 111In-labelled analogue. Eur J Nucl Med Mol Imaging. 2010; 37: 1356-67.
8.Honarvar H, Muller C, Cohrs S, Haller S, Westerlund K, Karlstrom AE, et al. Evaluation of the first 44Sc-labeled Affibody molecule for imaging of HER2-expressing tumors. Nucl Med Biol. 2016; 45: 15-21.
9.Xavier C, Blykers A, Vaneycken I, D’Huyvetter M, Heemskerk J, Lahoutte T, et al. (18)F-nanobody for PET imaging of HER2 overexpressing tumors. Nucl Med Biol. 2016; 43: 247-52.
10.Vaidyanathan G, McDougald D, Choi J, Koumarianou E, Weitzel D, Osada T, et al. Preclinical evaluation of 18F-labeled anti-HER2 nanobody conjugates for Imaging HER2 receptor expression by immuno-PET. J Nucl Med. 2016; 57: 967-73.
11.Zhou Z, Vaidyanathan G, McDougald D, Kang CM, Balyasnikova I, Devoogdt N, et al. Fluorine-18 Labeling of the HER2-Targeting Single-Domain Antibody 2Rs15d Using a Residualizing Label and Preclinical Evaluation. Mol Imaging Biol. 2017.[please verify!]
12.Wallberg H, Grafstrom J, Cheng Q, Lu L, Martinsson Ahlzen HS, Samen E, et al. HER2-positive tumors imaged within 1 hour using a site-specifically 11C-labeled Sel-tagged affibody molecule. J Nucl Med. 2012; 53: 1446-53.
13.Lub-de Hooge MN, Kosterink JG, Perik PJ, Nijnuis H, Tran L, Bart J, et al. Preclinical characterisation of 111In-DTPA-trastuzumab. Br J Pharmacol. 2004; 143: 99-106.
14.Sampath L, Kwon S, Hall MA, Price RE, Sevick-Muraca EM. Detection of Cancer Metastases with a Dual-labeled Near-Infrared/Positron Emission Tomography Imaging Agent. Transl Oncol. 2010; 3: 307-17.
15.Chen TJ, Cheng TH, Chen CY, Hsu SC, Cheng TL, Liu GC, et al. Targeted Herceptin-dextran iron oxide nanoparticles for noninvasive imaging of HER2/neu receptors using MRI. J Biol Inorg Chem. 2009; 14: 253-60.
16.Chen KT, Lee TW, Lo JM. In vivo examination of (188)Re(I)-tricarbonyl-labeled trastuzumab to target HER2-overexpressing breast cancer. Nucl Med Biol. 2009; 36: 355-61.
17.Sampath L, Kwon S, Ke S, Wang W, Schiff R, Mawad ME, et al. Dual-labeled trastuzumab-based imaging agent for the detection of human epidermal growth factor receptor 2 overexpression in breast cancer. J Nucl Med. 2007; 48: 1501-10.
18.McLarty K, Cornelissen B, Scollard DA, Done SJ, Chun K, Reilly RM. Associations between the uptake of 111In-DTPA-trastuzumab, HER2 density and response to trastuzumab (Herceptin) in athymic mice bearing subcutaneous human tumour xenografts. Eur J Nucl Med Mol Imaging. 2009; 36: 81-93.
19.Orlova A, Wallberg H, Stone-Elander S, Tolmachev V. On the selection of a tracer for PET imaging of HER2-expressing tumors: direct comparison of a 124I-labeled affibody molecule andtrastuzumab in a murine xenograft model. J Nucl Med. 2009; 50: 417-25.
20.Cheng Z, De Jesus OP, Kramer DJ, De A, Webster JM, Gheysens O, et al. 64Cu-labeled affibody molecules for imaging of HER2 expressing tumors. Mol Imaging Biol. 2010; 12: 316-24.
21.Kramer-Marek G, Kiesewetter DO, Martiniova L, Jagoda E, Lee SB, Capala J. [18F]FBEM-Z(HER2:342)-Affibody molecule-a new molecular tracer for in vivo monitoring of HER2 expression by positron emission tomography. Eur J Nucl Med Mol Imaging. 2008; 35: 1008-18.
22.Kramer-Marek G, Bernardo M, Kiesewetter DO, Bagci U, Kuban M, Aras O, et al. PET of HER2-positive pulmonary metastases with 18F-ZHER2:342 affibody in a murine model of breast cancer: comparison with 18F-FDG. J Nucl Med. 2012; 53: 939-46.
23.Kramer-Marek G, Gijsen M, Kiesewetter DO, Bennett R, Roxanis I, Zielinski R, et al. Potential of PET to predict the response to trastuzumab treatment in an ErbB2-positive human xenograft tumor model. J Nucl Med. 2012; 53: 629-37.
24.Kramer-Marek G, Kiesewetter DO, Capala J. Changes in HER2 expression in breast cancer xenografts after therapy can be quantified using PET and (18)F-labeled affibody molecules. J Nucl Med. 2009; 50: 1131-9.
25.Ren G, Zhang R, Liu Z, Webster JM, Miao Z, Gambhir SS, et al. A 2-helix small protein labeled with 68Ga for PET imaging of HER2 expression. J Nucl Med. 2009; 50: 1492-9.
26.Honarvar H, Jokilaakso N, Andersson K, Malmberg J, Rosik D, Orlova A, et al. Evaluation of backbone-cyclized HER2-binding 2-helix Affibody molecule for In Vivo molecular imaging. Nuclear Medicine and Biology. 2013; 40: 378-86.
27.Rosik D, Orlova A, Malmberg J, Altai M, Varasteh Z, Sandstrom M, et al. Direct comparison of 111In-labelled two-helix and three-helix Affibody molecules for in vivo molecular imaging. Eur J Nucl Med Mol Imaging. 2012; 39: 693-702.
28.Ueda M, Hisada H, Temma T, Shimizu Y, Kimura H, Ono M, et al. Gallium-68-labeled anti-HER2 single-chain Fv fragment: development and in vivo monitoring of HER2 expression. Mol Imaging Biol. 2015; 17: 102-10.
29.d'Amico A. Review of clinical practice utility of positron emission tomography with 18F-fluorodeoxyglucose in assessing tumour response to therapy. Radiol Med. 2015; 120: 345-51.
30.Ghosh SC, Pinkston KL, Robinson H, Harvey BR, Wilganowski N, Gore K, et al. Comparison of DOTA and NODAGA as chelators for (64)Cu-labeled immunoconjugates. Nucl Med Biol. 2015; 42: 177-83.
31.Tang Y WJ, Scollard DA, Mondal H, Holloway C, Kahn HJ, Reilly RM. Imaging of HER2/neu-positive BT-474 human breast cancer xenografts in athymic mice using (111)In-trastuzumab (Herceptin) Fab fragments. Nucl Med Biol. 2005; 32: 51-8.
32.Ahlgren S, Wallberg H, Tran TA, Widstrom C, Hjertman M, Abrahmsen L, et al. Targeting of HER2-expressing tumors with a site-specifically 99mTc-labeled recombinant affibody molecule, ZHER2:2395, with C-terminally engineered cysteine. J Nucl Med. 2009; 50: 781-9.
33.Orlova A NF, Wikman M, Widström C, Ståhl S, Carlsson J, Tolmachev V. Comparative in vivo evaluation of technetium and iodine labels on an anti-HER2 affibody for single-photon imaging of HER2 expression in tumors. J Nucl Med. 2006; 47: 512-9.
34.Li X, Zhang XN, Li XD, Chang J. Multimodality imaging in nanomedicine and nanotheranostics. Cancer Biol Med. 2016; 13: 339-48.
35.Dai ZF. Nanomedicine has opened new avenues for cancer diagnosis and therapy. Cancer Biol Med. 2016; 13: 297-8.
36.Cai LT, Sheng ZH. Advances in cancer nanomedicine. Cancer Biol Med. 2015; 12: 141-2.
37.Chen H, Zhen Z, Todd T, Chu PK, Xie J. Nanoparticles for Improving Cancer Diagnosis. Mater Sci Eng R Rep. 2013; 74: 35-69.
38.Kinoshita M, Yoshioka Y, Okita Y, Hashimoto N, Yoshimine T. MR molecular imaging of HER-2 in a murine tumor xenograft by SPIO labeling of anti-HER-2 affibody. Contrast Media Mol Imaging. 2010; 5: 18-22.
39.Chen C, Peng J, Xia HS, Yang GF, Wu QS, Chen LD, et al. Quantum dots-based immunofluorescence technology for the quantitative determination of HER2 expression in breast cancer. Biomaterials. 2009; 30: 2912-8.
40.Chen C, Peng J, Xia H, Wu Q, Zeng L, Xu H, et al. Quantum-dotbased immunofluorescent imaging of HER2 and ER provides new insights into breast cancer heterogeneity. Nanotechnology. 2010; 21: 095101.
41.Rizvi SB, Rouhi S, Taniguchi S, Yang SY, Green M, Keshtgar M, et al. Near-infrared quantum dots for HER2 localization and imaging of cancer cells. Int J Nanomedicine. 2014; 9: 1323-37.
42.Liu XL, Peng CW, Chen C, Yang XQ, Hu MB, Xia HS, et al. Quantum dots-based double-color imaging of HER2 positive breast cancer invasion. Biochem Biophys Res Commun. 2011; 409: 577-82.
43.Takeda M, Tada H, Higuchi H, Kobayashi Y, Kobayashi M, Sakurai Y, et al. In vivo single molecular imaging and sentinel node navigation by nanotechnology for molecular targeting drugdelivery systems and tailor-made medicine. Breast Cancer. 2008; 15: 145-52.
44.Balalaeva IV, Zdobnova TA, Krutova IV, Brilkina AA, Lebedenko EN, Deyev SM. Passive and active targeting of quantum dots for whole-body fluorescence imaging of breast cancer xenografts. J Biophotonics. 2012; 5: 860-7.
45.Tada H, Higuchi H, Wanatabe TM, Ohuchi N. In vivo real-time tracking of single quantum dots conjugated with monoclonal anti-HER2 antibody in tumors of mice. Cancer Res. 2007; 67: 1138-44.
46.Qiao J, Li S, Wei L, Jiang J, Long R, Mao H, et al. HER2 targeted molecular MR imaging using a de novo designed protein contrast agent. PLoS One. 2011; 6: e18103.
47.Gao J, Chen K, Luong R, Bouley DM, Mao H, Qiao T, et al. A novel clinically translatable fluorescent nanoparticle for targeted molecular imaging of tumors in living subjects. Nano Lett. 2012; 12: 281-6.
48.Ma Q, Nakane Y, Mori Y, Hasegawa M, Yoshioka Y, Watanabe TM, et al. Multilayered, core/shell nanoprobes based on magnetic ferricoxide particles and quantum dots for multimodality imaging of breast cancer tumors. Biomaterials. 2012; 33: 8486-94.
49.Smith-Jones PM SD, Afroze F, Rosen N, Larson SM. Early tumor response to Hsp90 therapy using HER2 PET_ comparison with 18F-FDG PET. J Nucl Med. 2006; 47: 793-6.
50.Kramer-Marek G, Shenoy N, Seidel J, Griffiths GL, Choyke P, Capala J. 68Ga-DOTA-affibody molecule for in vivo assessment of HER2/neu expression with PET. Eur J Nucl Med Mol Imaging. 2011; 38: 1967-76.
51.Miao Z, Ren G, Jiang L, Liu H, Webster JM, Zhang R, et al. A novel 18F-labeled two-helix scaffold protein for PET imaging of HER2-positive tumor. Eur J Nucl Med Mol Imaging. 2011; 38: 1977-84.
52.Xavier C, Vaneycken I, D'Huyvetter M, Heemskerk J, Keyaerts M, Vincke C, et al. Synthesis, preclinical validation, dosimetry, and toxicity of 68Ga-NOTA-anti-HER2 Nanobodies for iPET imaging of HER2 receptor expression in cancer. J Nucl Med. 2013; 54: 776-84.
53.Strand J HH, Perols A, Orlova A, Selvaraju RK, Karlström AE, Tolmachev V. Influence of macrocyclic chelators on the targeting properties of (68)Ga-labeled synthetic affibody molecules: comparison with (111)In-labeled counterparts. PLoS One. 2013; 8: e70028.
54.Orlova A, Magnusson M, Eriksson TL, Nilsson M, Larsson B, Hoiden-Guthenberg I, et al. Tumor imaging using a picomolar affinity HER2 binding affibody molecule. Cancer Res. 2006; 66: 4339-48.
55.Steffen AC, Orlova A, Wikman M, Nilsson FY, Stahl S, Adams GP, et al. Affibody-mediated tumour targeting of HER-2 expressing xenografts in mice. Eur J Nucl Med Mol Imaging. 2006; 33: 631-8.
56.Wallberg H, Orlova A, Altai M, Hosseinimehr SJ, Widstrom C, Malmberg J, et al. Molecular design and optimization of 99mTclabeled recombinant affibody molecules improves their biodistribution and imaging properties. J Nucl Med. 2011; 52: 461-9.
57.Perik PJ, Lub-De Hooge MN, Gietema JA, van der Graaf WT, de Korte MA, Jonkman S, et al. Indium-111-labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2006; 24: 2276-82.
58.Dijkers EC, Kosterink JG, Rademaker AP, Perk LR, van Dongen GA, Bart J, et al. Development and characterization of clinicalgrade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J Nucl Med. 2009; 50: 974-81.
59.Ulaner GA, Hyman DM, Ross DS, Corben A, Chandarlapaty S, Goldfarb S, et al. Detection of HER2-Positive Metastases in Patients with HER2-Negative Primary Breast Cancer Using 89Zr-Trastuzumab PET/CT. J Nucl Med. 2016; 57: 2523-8.
60.Gaykema SB, Schroder CP, Vitfell-Rasmussen J, Chua S, Oude Munnink TH, Brouwers AH, et al. 89Zr-trastuzumab and 89Zrbevacizumab PET to evaluate the effect of the HSP90 inhibitor NVP-AUY922 in metastatic breast cancer patients. Clin Cancer Res. 2014; 20: 3945-54.
61.Gaykema SB, de Jong JR, Perik PJ, Brouwers AH, Schroder CP, Oude Munnink TH, et al. (111)In-trastuzumab scintigraphy in HER2-positive metastatic breast cancer patients remains feasible during trastuzumab treatment. Mol Imaging. 2014; 13: 1-6.
62.Gebhart G, Lamberts LE, Wimana Z, Garcia C, Emonts P, Ameye L, et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann Oncol. 2016; 27: 619-24.
63.Tamura K, Kurihara H, Yonemori K, Tsuda H, Suzuki J, Kono Y, et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J Nucl Med. 2013; 54: 1869-75.
64.Mortimer JE, Bading JR, Colcher DM, Conti PS, Frankel PH, Carroll MI, et al. Functional imaging of human epidermal growth factor receptor 2-positive metastatic breast cancer using (64)Cu-DOTA-trastuzumab PET. J Nucl Med. 2014; 55: 23-9.
65.Baum RP, Prasad V, Muller D, Schuchardt C, Orlova A, Wennborg A, et al. Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In- or 68Ga-labeled affibody molecules. J Nucl Med. 2010; 51: 892-7.
66.Sorensen J, Sandberg D, Sandstrom M, Wennborg A, Feldwisch J, Tolmachev V, et al. First-in-human molecular imaging of HER2 expression in breast cancer metastases using the 111In-ABY-025 affibody molecule. J Nucl Med. 2014; 55: 730-5.
67.Keyaerts M, Xavier C, Heemskerk J, Devoogdt N, Everaert H, Ackaert C, et al. Phase I Study of 68Ga-HER2-Nanobody for PET/CT Assessment of HER2 Expression in Breast Carcinoma. J Nucl Med. 2016; 57: 27-33.
Cite this article as: Chen W, Li X, Zhu L, Liu J, Xu W, Wang P, et al. Preclinical and clinical applications of specific molecular imaging for HER2-positive breast cancer. Cancer Biol Med. 2017; 14: 271-80. doi: 10.20892/j.issn.2095-3941.2017.0044
Ping Wang
E-mail: doctorwangp@163.com
April 19, 2017; accepted July 3, 2017.
Available at www.cancerbiomed.org
Copyright © 2017 by Cancer Biology & Medicine
 Cancer Biology & Medicine2017年3期
Cancer Biology & Medicine2017年3期
- Cancer Biology & Medicine的其它文章
- Aberrant control of NF-κB in cancer permits transcriptional and phenotypic plasticity, to curtail dependence on host tissue: molecular mode
- 2016 Chinese expert consensus and guidelines for the diagnosis and treatment of papillary thyroid microcarcinoma
- Nanotechnology-based combination therapy for overcoming multidrug-resistant cancer
- BRAF mutation in colorectal carcinomas with signet ring cell component
- Recent progress on nanoparticle-based drug delivery systems for cancer therapy
- Natural and artificial small RNAs: a promising avenue of nucleic acid therapeutics for cancer
