Neuroprotective potential of Quercetin in combination with piperine against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity
Shamsher Singh, Sumit Jamwal, Puneet Kumar
1 Department of Pharmacology, I.S.F College of Pharmacy, Ferozepur Road, Moga, Punjab, India
2 I.K. Gujral Punjab Technical University, Jalandhar, Punjab, India
Neuroprotective potential of Quercetin in combination with piperine against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity
Shamsher Singh1,2, Sumit Jamwal1,2, Puneet Kumar1,*
1 Department of Pharmacology, I.S.F College of Pharmacy, Ferozepur Road, Moga, Punjab, India
2 I.K. Gujral Punjab Technical University, Jalandhar, Punjab, India
How to cite this article:Singh S, Jamwal S, Kumar P (2017) Neuroprotective potential of Quercetin in combination with piperine against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. Neural Regen Res 12(7):1137-1144.
1-Methy-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a neurotoxin that selectively damages dopaminergic neurons in the substantia nigra pars compacta and induces Parkinson’s like symptoms in rodents. Quercetin (QC) is a natural polyphenolic bioflavonoid with potent antioxidant and anti-inflammatory properties but lacks of clinical attraction due to low oral bioavailability. Piperine is a well established bioavailability enhancer used pre-clinically to improve the bioavailability of antioxidants (e.g., Quercetin).erefore, the present study was designed to evaluate the neuroprotective potential of QC together with piperine against MPTP-induced neurotoxicity in rats. MPTP (100 μg/μL/rat, bilaterally) was injected intranigrally on days 1, 4 and 7 using a digital stereotaxic apparatus. QC (25 and 50 mg/kg, intragastrically) and QC (25 mg/kg, intragastrically) in combination with piperine (2.5 mg/kg, intragastrically) were administered daily for 14 days starting from day 8 aer the 3rdinjection of MPTP. On day 22, animals were sacrificed and the striatum was isolated for oxidative stress parameter (thiobarbituric acid reactive substances, nitrite and glutathione), neuroin fl ammatory cytokine (interleukin-1β, interleukin-6, and tumor necrosis factor-α) and neurotransmitter (dopamine, norepinephrine, serotonin, gamma-aminobutyric acid, glutamate, 3,4-dihydroxyphenylacetic acid, homovanillic acid, and 5-hydroxyindoleacetic acid) evaluations. Bilateral infusion of MPTP into substantia nigra pars compacta led to significant motor deficits as evidenced by impairments in locomotor activity and rotarod performance in open fi eld test and grip strength and narrow beam walk performance. Both QC (25 and 50 mg/kg) and QC (25 mg/kg) in combination with piperine (2.5 mg/kg), in particular the combination therapy, significantly improved MPTP-induced behavioral abnormalities in rats, reversed the abnormal alterations of neurotransmitters in the striatum, and alleviated oxidative stress and in fl ammatory response in the striatum.ese fi ndings indicate that piperine can enhance the antioxidant and anti-in fl ammatory properties of QC, and QC in combination with piperine exhibits strong neuroprotective e ff ects against MPTP-induced neurotoxicity.
nerve regeneration; 1-methy-4-phenyl-1,2,3,6-tetrahydropyridine; Quercetin; Piperine; Parkinson’s disease; excitotoxicity; oxido-nitrosative stress; neurotransmitters; neural regeneration
Accepted: 2017-06-05
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative hypokinetic movement disorder which results from the selective degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc) accompanied by behavioral and motor abnormalities like resting tremor, rigidity, bradykinesia, and postural instability (Fu et al., 2015).e exact etiology of PD is unknown and it is believed to be caused by increased oxidative stress, long term toxin exposure, mitochondrial dysfunction and neurotransmitter imbalance. Numerous bodies of evidences have indicated that neurotransmitter imbalance (i.e., dopamine, norepinephrine (NE), serotonin, gamma-aminobutyric acid (GABA) and glutamate), excitotoxicity, increased cytokine level and advanced age are directly linked to the severity of PD (di Michele et al., 2013). Excessive degeneration of dopaminergic neurons is well correlated with increased reactive oxygen species (ROS) concentration and glutamate hyperactivity (Singh et al., 2016).e treatment of PD is currently a major problem worldwide so there is a need to determine e ff ective compounds that have fewer side e ff ects in order to treat PD either alone or as adjuvant with available therapy. Moreover, a large number of neurotoxins like 1-methy-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), 6-hydroxy dopamine (6-OHDA), and rotenone are used in preclinical practice to investigate the pathological background of PD.
MPTP is a widely used neurotoxin to mimic PD in animal models. It can directly inhibit mitochondrial complex I activity, which leads to the death of striatal dopaminergic neurons (Meredith and Rademacher, 2011). Previous reports have demonstrated that MPTP results in striatal dopaminergic destruction and alters GABA and glutamate levels and therefore it is usually used to mimic the clinical practice in idiopathic PD patients (Hajj et al., 2015; Caravaggio et al., 2016).
Quercetin (QC) is a polyphenolic bio fl avonoid abundantly found in vegetables, fruits, red wines, and black berries. QC possesses potent antioxidant, anti-inflammatory and neu-roprotective properties (Bournival et al., 2009). Structurally, QC contains two pharmacophores in its chain providing optimal capacity to scavenge free radicals (Sandhir and Mehrotra, 2013) and has been shown to protect nerve cells that die in Parkinson’s disease (PD) (Ahn and Jeon, 2015). Besides its numerous therapeutic applications, QC has low plasma bioavailability and high tissue distribution as its aglycone moiety immediately undergoes a series of biotransformation reactions and converts into di ff erent types of active metabolites upon oral administration (Gonzales et al., 2016). Previous reports have shown the limited penetrability of QC across blood-brain barrier and interpecies variation in bioavailability (Reinboth et al., 2010; Ishisaka et al., 2011).e other drug used in the present study is piperine, a nitrogenous and commercially available compound of plant origin obtained from Piper nigrum and Piper longum. Piperine has been reported to enhance the bioavailability of numerous compounds through diverse mechanisms like reducing biotransformation rate in gut by inhibiting cytochrome P3A (CYP3A) and UDP-GDH and enhancing the blood supply in the enteric vessel due to its local vasodilatory e ff ect (Bhardwaj et al., 2002). Therefore, the present study was designed to investigate the neuroprotective, anti-inflammatory and antioxidant e ff ect of QC in combination with piperine against MPTP-induced neurotoxicity in rats.
Material and Methods
Experimental animals
Forty- fi ve male Wistar rats weighing 250–280 g were housed under standard laboratory conditions (room temperature 22 ± 1°C and relative humidity of 60%) with 12-hour light/dark cycles. These animals were fed with standard diet in accordance with Institutional Animal Ethics Committee (IAEC) guidelines.e experimental protocol was reviewed and approved by the Institutional Animal Ethics Committee (ISFCP/ IAEC/CPCSCE/M15/P276/2015) and performed according to Indian National Science Academy (INSA) for the use and care of experimental animals. Rats were randomly divided into fi ve groups containing nine animals in each (n= 9) to avoid variability: control group (normal control, intranigral injection of normal saline), MPTP group (intranigral injection of MPTP), QC 25 mg/kg and 50 mg/kg groups (intranigral injection of MPTP followed by intragastric (i.g.) administration of QC 25 mg/kg and 50 mg/kg), and QC + piperine group (intranigral injection of MPTP followed by i.g. administration of QC 25 mg/kg and i.g. administration of piperine 2.5 mg/kg).
Drugs and treatment schedule
The following drugs were used: MPTP (Sigma-Aldrich Corporation, St. Louis, MO, USA) (100 μg/μL, intranigral administration on days 1, 4 and 7) after dissolving in 0.9% normal saline solution. QC (low dose 25 mg/kg, i.g. and high dose 50 mg/kg, i.g. (Hi Media, Mumbai, India)) and piperine [2.5 mg/kg, i.g. (Sigma-Aldrich Corporation)] were dissolved in sodium carboxy methyl cellulose solution (0.5% v/w). QC alone and in combination with piperine were administered once daily for 14 days in two separate groups, starting from day 8 aer the 3rdinjection of MPTP. Behavioral parameters were assessed on days 1, 7, 14 and 21, respectively. On day 22, the animals were sacri fi ced, the striatum was isolated for oxidative stress parameter [thiobarbituric acid reactive substances (TBARS), nitrite, reduced glutathione], pro-inflammatory cytokine [interleukin (IL)-1β, IL-6 and tumor necrosis factor-alpha (TNF-α)] and neurotransmitter [dopamine (DA), NE, 5-hydroxytryptamine (5-HT), gamma-amino butyric acid (GABA), glutamate, 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), 5-hydroxyindoleacetic acid (5-HIAA)] evaluations (Figure 1).
MPTP administration
Brain surgery was performed using a stereotaxic apparatus (Stoelting, Wood Dale, IL, USA). Animals were anesthetized with ketamine [80 mg/kg, intraperitoneally (i.p.)] and diazepam (5 mg/kg, i.p.). Aer exposure of skull surface, cannulas were implanted 2 mm above the SNpc using coordinates (Paxinos and Watson, 2007): anteroposterior (AP): −5.0 mm from bregma; mediolateral (ML): ± 2.1 mm from midline; dorsoventral (DV): −7.7 mm from skull as described by Paxinos and Watson (2007). All groups were intranigrally administered MPTP (100 μg/μL) except the control group in which normal saline (0.9%) was used. All rats aer surgery were treated with antibiotic gentamicin (5 mg/kg, i.p.) and were placed back to their home cages.
Measurement of body weight
The percentage change in body weight was calculated according to the formula: Body weight change = (weight of animal on day 1–weight of animal on day 22)/ weight of animal on day 1× 100%.
Behavioral assessments
Spontaneous locomotor performance
Spontaneous locomotor activity was assessed by the open field test (Kumar et al., 2011) in an apparatus made up of wooden, rectangular box measuring 100 × 100 × 40 cm3. All animals were provided with 3 minute habituation period before initiating the actual motor activity tasks.e rat’s horizontal locomotor activity was recorded as the total number of squares crossed during a 5-minute period as described earlier (Kumar et al., 2011).
Rotarod performance
Grip strength measurement
Forelimb grip strength of rats was measured by holding the tail and then the tail was lowered toward the apparatus untilit grabbed a mesh with the frontal paws. Aer holding the platform, the rats were immediately pulled backward with the tail in a horizontal plane. Total beam crossing time and the number of foot errors were recorded in KgF (Karunanithi et al., 2011).
Beam crossing task
Beam crossing task (Karunanithi et al., 2011) was used to measure the gait abnormalities and foot slip counts.e apparatus consisted of a fl at wooden beam (length 130 cm and width 1 cm) suspended at a height of 100 cm from the fl oor to avoid the intentional fall. A black box containing nesting material was placed at the end of beam as a target platform. All animals were previously habituated (trained) with the apparatus for 5 minutes prior to experimentation.e time taken to cross the beam from one point to other end and the number of foot slips were recorded in each trial.
Dissection and homogenization
On day 22, nine animals in each group were further divided into three sub-groups for biochemical estimations, neuroinflammatory cytokine and neurotransmitter analysis immediately after behavioral assessment. All the tested samples were run in duplicate to minimize the statistical errors. Aer sacrificing the animals, the brains were isolated and preserved at –80°C until analysis. On the experimental day, the brains were removed from the deep freezer and the striatum was isolated, weighed and homogenized in 0.1 M phosphate bu ff er (pH 7.4).e homogenized striatal suspension solution was then used for oxidative stress, neuroin fl ammatory cytokine and neurotransmitter estimations.
Measurement of striatal oxidative stress parameters
TBARS values
Nitrite level
Reduced glutathione level
Reduced glutathione (GSH) in the striatal tissue was quantifi ed according to the method of Ellman (1959). Yellow color formed in the tested sample was measured at 412 nm using a Shimadzu spectrophotometer.e results were calculated and expressed as percentage of the normal control group.
Protein level
Striatal proin fl ammatory cytokines
Striatal neurotransmitter analysis
Catecholamines
Estimation of striatal catecholamines was performed by high performance liquid chromatography using an electrochemical (EC) detector. Striatal levels of catecholamines (DA, norepinephrine, 5-HT and their metabolites DOPAC, HVA and 5-HIAA) were determined according to the method of Patel et al. (2005).
GABA and glutamate estimation
For the quantification of amino acids, striatal homogenate solution was derivatized with o-phthalaldehyde/β-mercaptoethanol (OPA/β-ME) following the method reported earlier (Reinhoud et al., 2013).e quantitative analysis of GABA and glutamate in the striatal tissue sample was performed in accordance with the method of Lasley and Gilbert (2002).
Statistical analysis
All data are presented as the mean ± SEM and analyzed via GraphPad Prism 5.0 software for Windows (GraphPad Software, San Diego, CA, USA). Values were expressed as the mean ± SEM. Two-way analysis of variance (ANOVA) followed by Bonferroni’spost hoctest was applied to check the level of significance for behavior results, whereas the biochemical results were analyzed using one-way analysis of variance followed by Tukey’spost hoctest. A value ofP< 0.05 was considered statistically signi fi cant.
Results
Protective e ff ect of QC alone and in combination with piperine on body weight and behavioral changes in MPTP-treated rats
MPTP administered rats showed gradual declines in body weight, locomotor activity, motor coordination and balance, and grip strength at the end of the study (day 21) (P< 0.001) as compared with normal control rats. QC (25 and 50 mg/kg) treatment signi fi cantly restored rat’s body weight, locomotor activity, motor coordination, and grip strength as compared with administration of MPTP alone (P< 0.05). Further, piperine treatment (2.5 mg/kg) together with low dose QC (25 mg/kg) signi fi cantly enhanced its protective e ff ect thantreatment with QC alone (P< 0.05) (Figures 2 and 3).
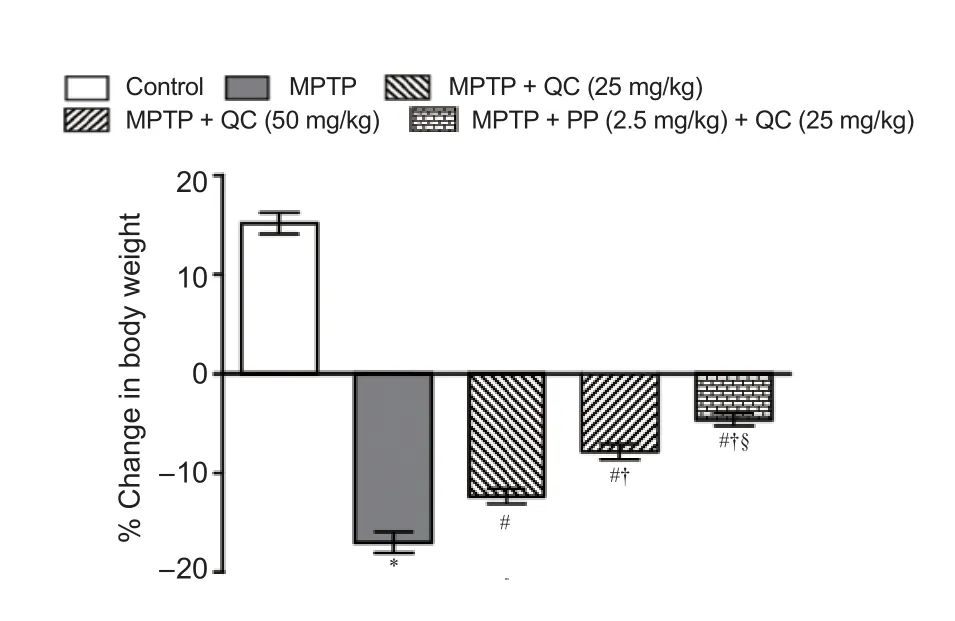
Figure 2 Protective e ff ect of QC alone and its combination with piperine on body weight of MPTP-treated rats.
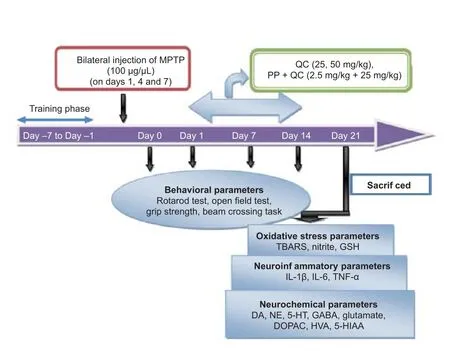
Figure 1 Experimental procedure.
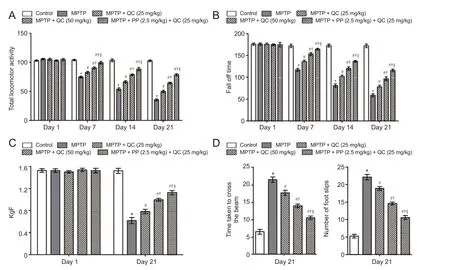
Figure 3 Protective e ff ect of QC alone and its combination with piperine (PP) on behavioral changes in MPTP-treated rats.
Protective e ff ect of QC alone and its combination with piperine on oxidative stress in MPTP-treated rats
MPTP-treated rats showed raised oxido-nitrosative stress as con fi rmed by high TBARS and nitrite levels and decreased striatal reduced glutathione level on day 22 (P< 0.001) as compared with normal control rats. QC (25 and 50 mg/kg) treatment signi fi cantly and dose-dependently attenuated the increase in TBARS and nitrite levels with decrease in gluta-thione level as compared with the control group (P< 0.05). Piperine (2.5 mg/kg) together with low dose QC (25 mg/kg) signi fi cantly ameliorated the oxido-nitrosative stress as compared with QC alone (25 and 50 mg/kg) due to its enhanced bioavailability (P< 0.05) (Figure 4).
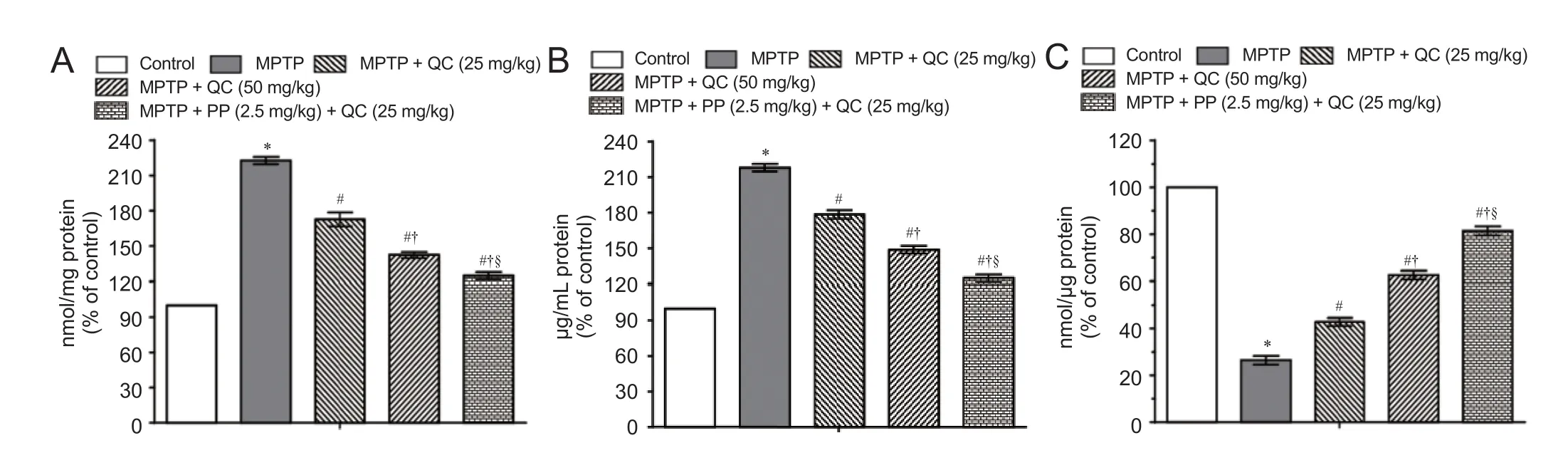
Figure 4 Protective e ff ect of QC alone and its combination with piperine on oxidative stress in MPTP-treated rats on day 22.
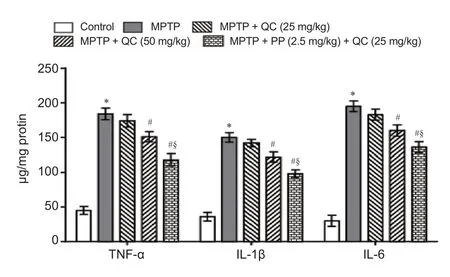
Figure 5 Protective e ff ects of QC alone and its combination with piperine on neuroin fl ammatory cytokines in MPTP-treated rats on day 22.
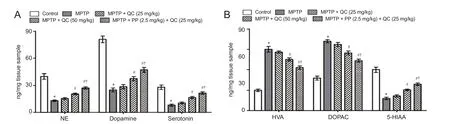
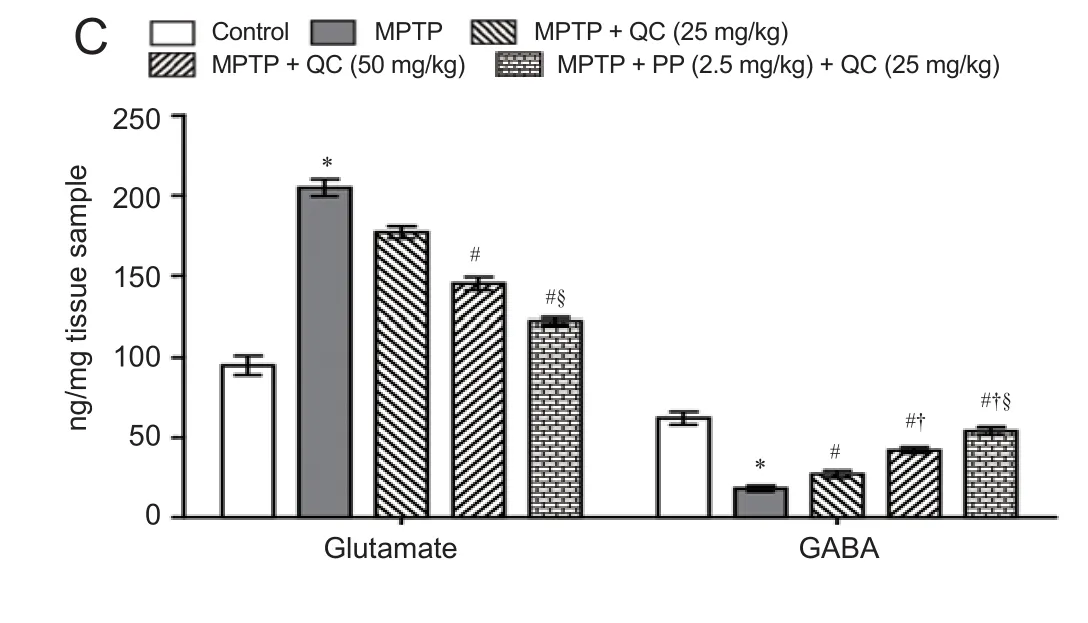
Figure 6 Protective e ff ect of QC alone and its combination with piperine on striatal levels of neurotransmitters in MPTP-treated rats on day 22.
Protective e ff ect of QC alone and its combination with piperine on neuroin fl ammatory cytokines in MPTP-treated rats
MPTP-treated rats showed gradually increased striatal proinfl ammatory cytokines level on day 22 (P< 0.001) as compared with normal control rats. QC (25 and 50 mg/kg) treatment for 14 days substantially and dose-dependently reversed the increases in the levels of TNF-α, IL-1β and IL-6 as compared with MPTP group (P< 0.05). Piperine (2.5 mg/kg) together with QC (25 mg/kg) further improved its protective e ff ect as compared with QC alone (P< 0.05) (Figure 5).
Protective e ff ect of QC alone and its combination with piperine on neurotransmitters in MPTP-treated rats
Bilateral infusion of MPTP into SNpc showed significant reduction in the levels of striatal catecholamines (norepinephrine, dopamine and serotonin) at the end of the study (on day 22) (P< 0.01) as compared with the control group. QC (25 and 50 mg/kg) treatment significantly increased catecholamine levels as compared with MPTP control (P<0.05). Piperine (2.5 mg/kg) together with QC (25 mg/kg) signi fi cantly increased striatal catecholamine levels as compared with QC alone (P< 0.01). MPTP treatment resulted in elevated DOPAC and HVA levels as well as decreased striatal 5-HIAA levels. QC (25 and 50 mg/kg) administration signi fi cantly attenuated the increased DOPAC and HVA levels and decreased 5-HIAA levels as compared with MPTP control (P< 0.05). Piperine (2.5 mg/kg) together with QC (25 mg/kg) signi fi cantly enhanced the protective e ff ect of QC as compared with QC alone (P< 0.05) (Figure 6).
MPTP-treated rats showed gradually decreased striatal GABA level and increased glutamate level at the end of the study (on day 22) (P< 0.001) as compared with normal control rats. QC (25 and 50 mg/kg) signi fi cantly and dose-dependently reversed the alteration in striatal GABA and glutamate levels as compared with MPTP control (P< 0.05). Co-administration of piperine (2.5 mg/kg) together with QC (25 mg/kg) dose-dependently increased GABA level and decreased glutamate level than administratin of QC alone (P< 0.05) (Figure 6).
Discussion
An increasing number of evidence has demonstrated that increased oxidative stress, mitochondrial dysfunction, and neuroinflammation further exacerbate the damage to macromolecules including DNA, proteins, and lipids (Guo et al., 2013). However, degeneration of the membrane lipid yields high intracellular ROS level eventually leading to the loss of membrane integrity and finally dopaminergic neurodegeneration. Moreover, dopaminergic neuron loss is not only due to decreased mitochondrial functionin but also increased level of reactive oxygen species (ROS) and proin fl ammatory cytokines also play a crucial role. Dopaminergic neurons of nigrostriatal area are more sensitive to oxidative damage as this area is associated with high energy consumption and low antioxidant (GSH) makes them more prone to oxidative damage (Yamaguchi and Shen, 2007). In the present study, administration of QC alone and in combination with piperine significantly decreased ROS as evidenced by elevated GSH level indicating the antioxidant potential of QC. Structurally GSH contains thiol moiety, which tends to plays a pivotal role in preventing oxidative damage and hence acts as a biomarker of oxidative stress in biological systems. It is well implicated that high iron content in the brain makes the substantia nigra more susceptible to PD and is also evidenced by postmortem reports. QC prevents against iron induced striatal toxicity by acting as ion chelator (Lee and Andersen, 2010).
In recent years, the involvement of neuroinflammatory mediators in the nigrostriatal degeneration of dopaminergic neurons in PD has gained increasing attention. Moreover, consistent increase in oxidative stress due to mitochondrial failure after MPTP challenge activates the microglial cells which initiate the inflammatory responses responsible for neuronal dysfunction particularly in the SNpc as evidenced by various experimental models of PD. A postmortem study has also demonstrated that detection of microglial expression in the nigrostriatal region and cerebrospinal fl uid particularly including TNF-α, IL-1β and IL-6 suggested the involvement of immune components in PD pathogenesis (Chao et al., 2014). Similarly in the present study, the levels of in fl ammatory mediators were significantly increased after MPTP treatment, indicating that neuroinflammation plays a crucial role in the neurodegenerative disorders particularly in PD. These results were consistent with clinical fi ndings from a reported study (Wang et al., 2015). In the present study, quercetin signi fi cantly attenuated MPTP-induced neuroin fl ammation.
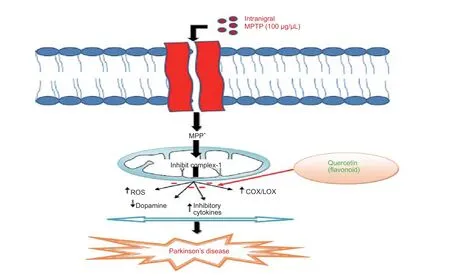
Figure 7 Quercetin acts as a potential neuroprotective agent through antioxidant and anti-in fl ammatory mechanisms.
QC, a powerful neuroprotective agent, attenuates the death of striatal neuronal cells and thereby improves motor dysfunction (Karuppagounder et al., 2013). QC is also preclinically reported as a neuroprotective agent in Alzheimer’s disease and other neurological disorders due to retention of antioxidant and anti-in fl ammatory activities (Sabogal et al., 2015). QC was reported to act as a pro-antioxidant and thus curb the oxidative stress dependent release of neuroin fl ammatory mediators like interleukin (IL-6, IL-1β) and tumor necrosis factor (i.e. TNF-α) (Figure 7) (Sun et al., 2015). In addition to this, QC was reported to decrease the expression of inducible cyclooxygenase-2 and thus protect the dopaminergic neurons in SNpc (Pany et al., 2014). Piperine is a natural component reported to have antioxidant and anti-in fl ammatory activities inin vitrostudies (Shrivastava et al., 2013). It has been evaluated that piperine at low dose reduces neuronal loss and doubles the plasma concentration of some herbal antioxidants (Wadhwa et al., 2014).
In summary, the present fi ndings con fi rmed the neuroprotective e ff ect of combined administration of QC and piperine against MPTP-induced motor deficits, oxidative stress, neuroinflammatory and neurotransmitter alterations. The present findings suggest that QC prevents MPTP-induced degeneration of dopaminergic neurons through its powerful antioxidant and anti-inflammatory mechanisms supported by its metal ion scavenging properties.erefore, the use of these antioxidants alone or in combination with available drugs may serve as potential therapeutic targets for the treatment of neurodegenerative disorders like PD.
Acknowledgments:
Author contributions:PK designed this study. SS and SJ performed the research. SS was responsible for data acquisition. All authors approved the fi nal version of this paper.
Con fl icts of interest:None declared.
Research ethics:the guidelines of the Indian National Science Academy (INSA) for the Use and Care of Experimental Animals and “Consensus Author Guidelines on Animal Ethics and Welfare” produced by the International Association of Veterinary Editors (IAVE).e article was prepared in accordance with the “Animal Research: Reporting of In Vivo Experiments Guidelines” (ARRIVE Guidelines).
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and signi fi cance of this paper.
Ahn TB, Jeon BS (2015)e role of quercetin on the survival of neuron-like PC12 cells and the expression of α-synuclein. Neural Regen Res 10:1113-1119.
Bhardwaj RK, Glaeser H, Becquemont L, Klotz U, Gupta SK, Fromm MF (2002) Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4. J Pharmacol Exper 302:645-650.
Bournival J, Quessy P, Martinoli MG (2009) Protective e ff ects of resveratrol and quercetin against MPP+-induced oxidative stress act by modulating markers of apoptotic death in dopaminergic neurons. Cell Mol Neurobiol 29:1169-1180.
Caravaggio F, Nakajima S, Plitman E, Gerretsen P, Chung JK, Iwata Y, Gra ff -Guerrero A (2016)e e ff ect of striatal dopamine depletion on striatal and cortical glutamate: A mini-review. Prog Neuropsychopharmacol Biol Psychiatry 65:49-53.
Chakraborty J, Singh R, Dutta D, Naskar A, Rajamma U, Mohanakumar KP (2014) Quercetin improves behavioral de fi ciencies, restores astrocytes and microglia, and reduces serotonin metabolism in 3-nitropropionic acid-induced rat model of Huntington’s disease. CNS Neuroscier 20:10-19.
Chao Y, Wong SC, Tan EK (2014) Evidence of inflammatory system involvement in Parkinson’s disease. Biomed Res Int 2014:308654.
di Michele F, Luchetti S, Bernardi G, Romeo E, Longone P (2013) Neurosteroid and neurotransmitter alterations in Parkinson’s disease. Front Neuroendocrinol 34:132-142.
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70-77.
Fu SP, Wang JF, Xue WJ, Liu HM, Liu BR, Zeng YL Liu JX (2015) Anti-in fl ammatory e ff ects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J Neuroin fl ammation 12:1- 14.
Gonzales GB, Smagghe G, Wittevrongel J, Huynh NT, Van Camp J, Raes K (2016) Metabolism of Quercetin and Naringenin by Food-Grade Fungal Inoculum, Rhizopus azygosporus Yuan et Jong (ATCC 48108). J Agric Food Chem 64:9263-9267.
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fl uids. Anal Biochem 126:131-138.
Grosch J, Winkler J, Kohl Z (2016) Early degeneration of both dopaminergic and serotonergic axons–A common mechanism in Parkinson’s disease. Front Cell Neurosci 10:293.
Guo C, Sun L, Chen X, Zhang D (2013) Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res 8:2003-2014.
Hajj R, Milet A, Toulorge D, Cholet N, La ff aire J, Foucquier J, Robelet S, Mitry R, Guedj M, Nabirotchkin S, Chumakov I, Cohen D (2015) Combination of acamprosate and baclofen as a promising therapeutic approach for Parkinson’s disease. Sci Rep 5:16084.
Hutter-Saunders JA, Gendelman HE, Mosley RL (2012) Murine motor and behavior functional evaluations for acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) intoxication. J Neuroimmune Pharmacol 7:279-288.
Ishisaka A, Ichikawa S, Sakakibara H, Piskula MK, Nakamura T, Kato Y, Ito M, Miyamoto K, Tsuji A, Kawai Y, Terao J (2011) Accumulation of orally administered quercetin in brain tissue and its antioxidative e ff ects in rats. Free Radic Biol Med 51:1329-1336.
Karunanithi K, Annadurai A, Krishnamoorthy M, Elumalai P, Manivasagam T (2011) 1-Methyl 4-phenyl 1, 2, 3, 6-tetrahydropyridine is a potent neurotoxin: Gamma-tocopherol recuperate behavior, dopamine, and oxidative stress on Parkinsonic mice. Int J Nutr Pharmacol Neurol Dise 1:139.
Karuppagounder SS, Madathil SK, Pandey M, Haobam R, Rajamma U, Mohanakumar KP (2013) Quercetin up-regulates mitochondrial complex-I activity to protect against programmed cell death in rotenone model of Parkinson’s disease in rats. Neuroscience 236:136-148.
Kumar P, Kalonia H, Kumar A (2011) Role of LOX/COX pathways in 3-nitropropionic acid-induced Huntington’s disease-like symptoms in rats: protective e ff ect of licofelone. Br J Pharmacol 164(2b):644-654.
Kumar P, Kalonia H, Kumar A (2012) Possible GABAergic mechanism in the neuroprotective e ff ect of gabapentin and lamotrigine against 3-nitropropionic acid induced neurotoxicity. Eur J Pharmacol 674(2-3):265-274.
Lasley SM, Gilbert ME (2002) Rat hippocampal glutamate and GABA release exhibit biphasic e ff ects as a function of chronic lead exposure level. Toxicol Sci 66:139-147.
Lee DW, Andersen JK (2010) Iron elevations in the aging Parkinsonian brain: a consequence of impaired iron homeostasis? J Neurochem 112:332-339.
Liu J, Huang D, Xu J, Tong J, Wang Z, Huang L, Yang Y, Bai X, Wang P, Suo H, Ma Y, Yu M, Fei J, Huang F (2015) Tiagabine protects dopaminergic neurons against neurotoxins by inhibiting microglial activation. Sci Rep 5:15720.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265-275.
Meredith GE, Rademacher DJ (2011) MPTP mouse models of Parkinson’s disease: an update. J Parkinsons Dis 1:19-33.
Pany SU, Pal AB, Sahu PK (2014) Neuroprotective e ff ect of quercetin in neurotoxicity induced rats: role of neuroin fl ammation in neurodegeneration. Asian J Pharm Clin Res 7:152-156.
Patel AB, de Graaf RA, Mason GF, Rothman DL, Shulman RG, Behar KL (2005)e contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proc Natl Acad Sci U S A 102: 5588-5593.
Paxinos G, Watson C (2007)e rat brain in stereotaxic coordinates. 6thedition. Academic Press, San Diego.
Reinboth M, Wol ff ram S, Abraham G, Ungemach FR, Cermak R (2010) Oral bioavailability of quercetin from di ff erent quercetin glycosides in dogs. Br J Nutr 104:198-203.
Sabogal-Guáqueta AM, Muñoz-Manco JI, Ramírez-Pineda JR, Lamprea-RM, Osorio E and Cardona-Gómez GP (2015) The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 93:134-145.
Sandhir R, Mehrotra A (2013) Quercetin supplementation is e ff ective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: implications in Huntington’s disease. Biochim Biophys Acta 1832:421-430.
Shrivastava P, Vaibhav K, Tabassum R, Khan A, Ishrat T, Khan MM, Ahmad A, Islam F, Sai MM, Islam F (2013) Anti-apoptotic and anti-in fl ammatory e ff ect of Piperine on 6-OHDA induced Parkinson’s rat model. J Nutr Biochem 24:680-687.
Singh S, Kumar P (2016) Neuroprotective activity of curcumin in combination with piperine against quinolinic acid induced neurodegeneration in rats. Pharmacology 97(3-4):151-160.
Sun GY, Chen Z, Jasmer KJ, Chuang DY, Gu Z, Hannink M, Simonyi A (2015) Quercetin attenuates in fl ammatory responses in BV-2 microglial cells: Role of MAPKs on the Nrf2 pathway and induction of heme oxygenase-1. PLoS One 10:e0141509.
Wadhwa S, Singhal S, Rawal S (2014) Bioavailability enhancement by piperine: a review. Asian J Biomed Pharm Sci 4:1-8.
Wang Q, Liu Y, Zhou J (2015) Neuroin fl ammation in Parkinson’s disease and its potential as therapeutic target. Transl Neurodegener 4:19.
Yamaguchi H, Shen J (2007) Absence of dopaminergic neuronal degeneration and oxidative damage in aged DJ-1-de fi cient mice. Mol Neurodegener 2:10.
Copyedited by Li CH, Song LP, Zhao M
Puneet Kumar, M. Pharm., P.D.C.R., Ph.D., F.L.S. London, punnubansal79@gmail.com.
10.4103/1673-5374.211194
*< class="emphasis_italic">Correspondence to: Puneet Kumar, M. Pharm., P.D.C.R., Ph.D., F.L.S. London, punnubansal79@gmail.com.
orcid: 0000-0002-7978-1043 (Puneet Kumar)
- 中国神经再生研究(英文版)的其它文章
- SoxC transcription factors in retinal development and regeneration
- Umbilical cord: an unlimited source of cells di ff erentiable towards dopaminergic neurons
- Targeting 14-3-3 adaptor protein-protein interactions to stimulate central nervous system repair
- RACK1 regulates neural development
- Schwann cell development, maturation and regeneration: a focus on classic and emerging intracellular signaling pathways
- BDNF pro-peptide: a novel synaptic modulator generated as an N-terminal fragment from the BDNF precursor by proteolytic processing

