SoxC transcription factors in retinal development and regeneration
Kun-Che Chang, Jonathan Hertz
Department of Ophthalmology, School of Medicine, Stanford University, Palo Alto, CA, USA
SoxC transcription factors in retinal development and regeneration
Kun-Che Chang*, Jonathan Hertz*
Department of Ophthalmology, School of Medicine, Stanford University, Palo Alto, CA, USA
How to cite this article:Chang KC, Hertz J (2017) SoxC transcription factors in retinal development and regeneration. Neural Regen Res 12(7):1048-1051.
Glaucoma and other optic neuropathies result in optic nerve degeneration and the loss of retinal ganglion cells (RGCs) through complex signaling pathways. Although the mechanisms that regulate RGC development remain unclear, uncovering novel developmental pathways may support new strategies to regenerate the optic nerve or replace RGCs. Here we review recent studies that provide strong evidence that the Sry-related high-mobility-group C (SoxC) subfamily of transcription factors (TFs) are necessary and suffi cient for axon guidance and RGC fate speci fi cation.ese fi ndings also uncover novel SoxC-dependent mechanisms that serve as master regulators during important steps of RGC development. For example, we review work showing that SoxC TFs regulate RGC axon guidance and direction through the optic chiasm towards their appropriate targets in the brain. We also review work demonstrating that Sox11 subcellular localization is, in part, controlled through small ubiquitin-like post-translational modi fi er (SUMO) and suggest compensatory cross-talk between Sox4 and Sox11. Furthermore, Sox4 overexpression is shown to positively drive RGC di ff erentiation in human induced pluripotent stem cells (hiPSCs). Finally, we discuss how these fi ndings may contribute to the advancement of regenerative and cell-based therapies to treat glaucoma and other optic nerve neuropathies.
Sox4; Sox11; retinal ganglion cell; optic nerve; regeneration; SUMOylation; cell transplantation; stem cell
Kun-Che Chang, Ph.D.,
kunche@stanford.edu or
Jonathan Hertz, Ph.D.,
jhertz32@gmail.com.
orcid:
0000-0002-0871-5612
(Kun-Che Chang)
Accepted: 2017-06-28
Glaucoma: Neurodegenerative Disease in the Eye
What are the molecular mechanisms that control retinal ganglion cell (RGC) development? During development, RGCs di ff erentiate from multipotent retinal progenitor cells (RPCs) but little is known about the intrinsic and extrinsic mechanisms that control RGG fate speci fi cation and axon growth. In neurodegenerative diseases such as glaucoma and other optic neuropathies, RGCs’ failure to survive results in the deterioration of the optic nerve. The optic nerve, composed of RGC axons, connects the eye to targets in the brain that are required for visual processing. Unfortunately, the loss of RGCs is irreversible and leads to vision impairment and blindness. Glaucoma affects roughly 60 million people worldwide and is among the leading causes of blindness. Currently, the only modifiable risk factor to treat glaucoma is lowering intraocular pressure (IOP); however, IOP reduction is not always su ffi cient to stop the underlying progression of RGC death. Therefore, neuroprotection and cell-based therapies are critical therapeutic objectives for mitigating and ultimately eliminating vision loss from glaucoma and other optic neuropathies. Stimulating developmental signaling pathways in adult animal models of injury and disease have demonstrated progress towards these goals; however, many important questions remain unknown.ese questions must be addressed in order to develop e ff ective therapies. For example, can signaling pathways controlling developmental RGC fate and axon growth be used to stimulate stem cells to generate a source of transplantable donor RGCs to replace or protect RGC loss due to disease or injury? Here, we review recently published works that establish (1) SoxC transcription factors (TFs) as fundamental for RGC fate speci fi cation and axon growth during development and (2) how SoxC-dependent mechanisms may advance neuroprotective and cell-based therapies for glaucoma and other optic neuropathies.
SoxC Transcription Factors: Molecular Basis for RGC Fate Speci fi cation
The Sry-related high mobility box (Sox) superfamily genes encode families of TFs that all share the high mobility group (HMG) DNA binding domain. Sox TFs promote and repress the expression of complex networks of downstream genes that control mechanisms required for development including pluripotent stem cell properties and cell fate specification (Schepers et al., 2002). For example, the SoxC family of TFs, which include Sox4, Sox11 and Sox12, has been shown to play essential roles in skeletal and neural development (Schepers et al., 2002). More speci fi cally SoxC TFs regulate important steps in retinal development. In an amphibian model, it was discovered that interference of Sox4 and Sox11 expression results in signi fi cant defects during eye development (Cizelsky et al., 2013). These studies firmly establish the importance of SoxC TFs during retinal development andhave encouraged further study into more speci fi c functions controlled by SoxC TFs.

Figure 1 Functions of SoxC TFs in contralateral retina.
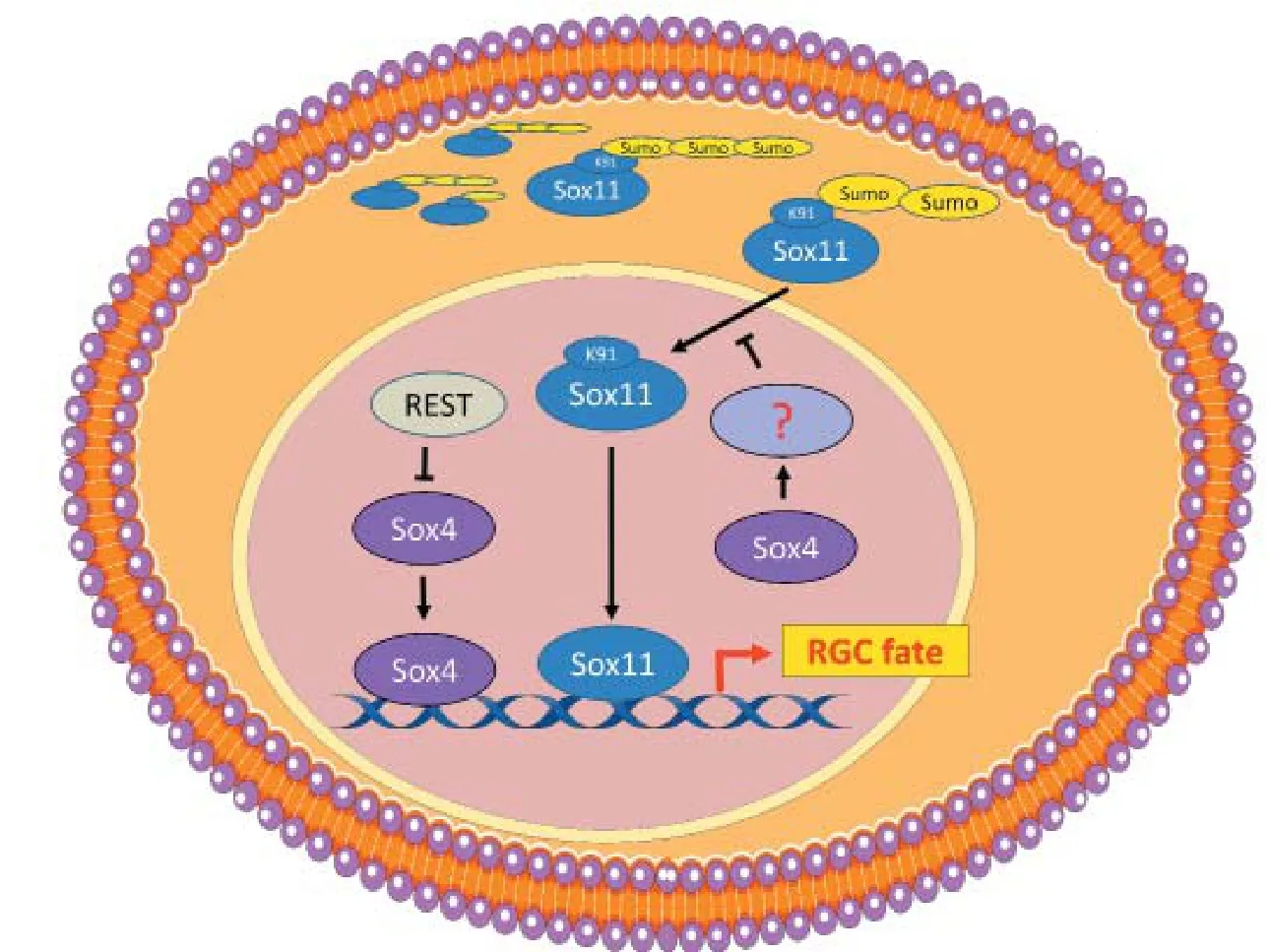
Figure 2 Regulatory mechanism of Sox4 and Sox11 in RGC fate speci fi cation.
What factors control RGC di ff erentiation and axon growth during development? Recently, studies by three di ff erent labs uncovered novel SoxC-dependent functions that are both necessary and sufficient for RGC differentiation, including how RGC cell fate is specified and how RGCs regulate the direction of axon growth. In 2013, one study fi rst reported that the deletion of Sox4 and Sox11 during retinal development results in the complete absence of RGCs and optic nerve in a mouse model (Jiang et al., 2013). In early 2017, another rodent study uncovered that SoxC TFs control the di ff erentiation of RGC axon guidance as well as further confi rmed the importance of SoxC TFs in RGC fate speci fi cation (Kuwajima et al., 2017). More speci fi cally, they demonstrated that SoxC TFs regulate contralateral RGC di ff erentiation by antagonizing hes family bHLH transcription factor 5 (Hes5) previously shown to suppress RGC di ff erentiation (Figure 1).ey also found that SoxC TFs control contralateral but not ipsilateral RGC axon guidanceviathe regulation of encoding plexin-A1 (Plexna1) and encoding neural cell adhesion molecule (Nrcam) expression. Both zic family member 2 and EPH receptor B1 (EphB1) have been previously shown to be important for ipsilateral RGC axon projections (Figure 1) (Kuwajima et al., 2017). Here, they discovered that the transcription factor Islet 2 suppresses ipsilateral RGC axon growth by repressing zic family member 2 (Zic2) and EPH receptor B1 (EphB1) gene expression. Taken together, these studies provide strong evidence that Sox4 and Sox11 are essential regulators of both RGC differentiation and axon growth.
Consistent with these studies, we observed that the doubleknockout of Sox4 and Sox11 during retinal development results in a near complete loss of RGCs and the total loss of ON in mice (Chang et al., 2017). We also found that Sox4 expression is repressed by RE1-silencing transcription factor (REST) and results in decreased RGC differentiation. Furthermore, we uncovered a novel mechanism between Sox4 and Sox11 that helps explain compensatory effects observed between these two TFs in RGCs. Sox4 and Sox11 are expressed in an overlapping but sequential pattern in the mouse retina, and at least Sox4 expression is suppressed by Notch signaling early in retinal development (Usui et al., 2013). Interestingly, we found that small ubiquitin-like modifier (SUMO) modification of Sox11 proteins controls Sox11’s localization between the cytoplasm and the nucleus of RGCs (Chang et al., 2017).rough cytoplasmic and nuclear protein fractionation we found that cytoplasmic Sox11 proteins are SUMOylated while nuclear Sox11 proteins are not. In Sox4 KO mice, we found SUMOylated cytoplasmic Sox11 proteins become deSUMOylated and translocate into the nucleus.ese data suggest a relationship between Sox4 and Sox11 in which the absence of Sox4 protein triggers the deSUMOylation of Sox11.is then results in translocation of Sox11 into the nucleus to potentially target Sox4-dependent DNA binding sites and regulate the expression of downstream genes (Figure 2) (Chang et al., 2017). Prior to our work, one study showed that ubiquitin-conjugating enzyme 9 (Ubc9), a protein important for SUMOylation, interacts with Sox4 and represses its transcriptional activity through a SUMOylation-independent interaction (Pan et al., 2006). Whether Ubc9 regulates Sox11 SUMOylation remains unknown but is an interesting topic for future study. Taken together, these data elucidate a complex transcriptional regulatory network that controls RGC fate speci fi cation.
Control of RGC Di ff erentiation for Cell-Basederapies
Can RGCs be replaced in diseased retina or following injury? In glaucoma and other optic neuropathies, RGC loss is irreversible because the adult retina is unable to endogenously generate new RGCs. Cell-replacement therapies whereby RGCs are generatedin vitroand transplanted into injured or glaucomatous retina may provide a source of ‘replacement RGCs’. Pluripotent stem cells are capable of differentiating into specific cell types including RGCs (Tanaka et al., 2015; Gill et al., 2016; Chang et al., 2017; Teotia et al., 2017).e discovery that adult human fi broblasts can be reprogrammed into induced pluripotent stem cells (iPSCs)in vitroand subsequently differentiated into speci fi c cell types, including RGCs, is a paradigm shifor the advancement of cell-based therapies (Ohlemacher et al., 2016). Although numerous methods for di ff erentiating and transplanting RGCsin vitrofrom stem cells have been published, integration of these cells into theretina following transplantation has been insu ffi cient for measureable retinal repair (Chamling et al., 2016). In addition, the e ffi ciencies or qualities of RGC-like progeny might be too variable or unreliable in the hiPSCs. For example, immunoselection of RGC-like progeny by Thy1 expression dose not yield adequate cell numbers ify1 expression is not turned on during differentiation. Work from our lab demonstrated signi fi cant improvements in the integration of transplanted RGCs in the retina. We fi rst found that the transplantation of acutely puri fi ed RGCs from developmental ages but not adult were able to survive, migrate and extend neurites following transplantation in retinal explants and to a lesser degreein vivofollowing intravitreal injection (Hertz et al., 2014). In a subsequent study, signi fi cant improvements in the integration of transplanted primary RGCsin vivowere observed. RGCs extended neurites towards the optic nerve head and into the optic nerve. Furthermore, using electrophysiological techniques, transplanted RGCs demonstrate electrical excitability and are responsive to light similarly to their host RGCs (Venugopalan et al., 2016). Both studies provide significant and encouraging evidence for the potential of RGC replacement therapies; however, generating RGCs with similar properties to those foundin vivoremains an area for study.
Can RGCs, capable of functional integration into the adult retina, be generatedin vitrofrom stem cells? Although methods for generating iPSC-derived RGCs have been uncovered, the efficiency of RGC differentiation remains low, and it remains unknown to what extent these cells are RGC-like. One hypothesis for these de fi ciencies is that these cells are missing signals necessary for generating RGC-like cells with more complete RGC properties. In our recent study, we found that Sox4 overexpression in human iPSCs results in a significant increase in RGC differentiation. Furthermore, these cells were capable of fi ring action potentials similarly to acutely purified RGCs (Chang et al., 2017).erefore, RGCs generated by Sox4 overexpression may target developmental signals missing in previous methods of stem cell derived RGC di ff erentiation. Does the combined overexpression of Sox4 and Sox11 work together synergistically to generate RGCs that have more RGC-like properties? And, do these cells exhibit an enhanced capacity for integration following transplantation? Both of these questions remain unknown but are of great interest for future study.
Conclusions and Perspectives
The work reviewed here demonstrates that SoxC TFs are master regulators of RGC development by controlling multiple facets of RGC development including RGC fate speci fi cation and axon guidance. While a majority of these studies were conducted in animal models and many of these questions remain unknown for human cells, these recent data from mouse development and human iPSCs suggest that these signals are indeed critical. Advances in human iPScell biology, combined with progress of our understanding of RGC development and integration into adult retina, gives hope for the possibility of stem cell derived RGC-replacement therapies to repair or restore vision lost in glaucoma and other optic neuropathies.
Author contributions:KCC and JH wrote the text and KCC drew the fi gures. Both authors participated in revising the manuscript.
Con fl icts of interest:None declared.
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and signi fi cance of this paper.
Open peer review report:
Reviewer: Muriel Perron, CNRS - Unviersite Paris Sud, France.
Comments to author: In this research highlight, the authors have reviewed recently published work on the implication of the transcription factors of the SOXC family during retinal ganglion cell fate speci fi cation and axon growth during development, and how this knowledge on SOXC functions could contribute to the development of cell-based therapies for glaucoma and other optic neuropathies.e review is very well-written and the fi gures are relevant. See additional fi le for more details.
Additional fi le:Open peer report 1 on “SoxC transcription factors in retinal development and regeneration”.
Chamling X, Sluch VM, Zack DJ (2016) The potential of human stem cells for the study and treatment of glaucoma. Invest Ophthalmol Vis Sci 57:ORSFi1-6.
Chang KC, Hertz J, Zhang X, Jin XL, Shaw P, Derosa BA, Li JY, Venugopalan P, Valenzuela DA, Patel RD, Russano KR, Alshamekh SA, Sun C, Tenerelli K, Li C, Velmeshev D, Cheng Y, Boyce TM, Dreyfuss A, Uddin MS, et al. (2017) Novel regulatory mechanisms for the SoxC transcriptional network required for visual pathway development. J Neurosci 37:4967-4981.
Cizelsky W, Hempel A, Metzig M, Tao S, Hollemann T, Kuhl M, Kuhl SJ (2013) sox4 and sox11 function during Xenopus laevis eye development. PLoS One 8:e69372.
Gill KP, Hung SS, Sharov A, Lo CY, Needham K, Lidgerwood GE, Jackson S, Crombie DE, Nayagam BA, Cook AL, Hewitt AW, Pebay A, Wong RC (2016) Enriched retinal ganglion cells derived from human embryonic stem cells. Sci Rep 6:30552.
Hertz J, Qu B, Hu Y, Patel RD, Valenzuela DA, Goldberg JL (2014) Survival and integration of developing and progenitor-derived retinal ganglion cells following transplantation. Cell Transplant 23:855-872.
Jiang Y, Ding Q, Xie X, Libby RT, Lefebvre V, Gan L (2013) Transcription factors SOX4 and SOX11 function redundantly to regulate the development of mouse retinal ganglion cells. J Biol Chem 288:18429-18438.
Kuwajima T, Soares CA, Sitko AA, Lefebvre V, Mason C (2017) SoxC transcription factors promote contralateral retinal ganglion cell differentiation and axon guidance in the mouse visual system. Neuron 93:1110-1125 e1115.
Ohlemacher SK, Sridhar A, Xiao Y, Hochstetler AE, Sarfarazi M, Cummins TR, Meyer JS (2016) Stepwise di ff erentiation of retinal ganglion cells from human pluripotent stem cells enables analysis of glaucomatous neurodegeneration. Stem Cells 34:1553-1562.
Pan X, Li H, Zhang P, Jin B, Man J, Tian L, Su G, Zhao J, Li W, Liu H, Gong W, Zhou T, Zhang X (2006) Ubc9 interacts with SOX4 and represses its transcriptional activity. Biochem Biophys Res Commun 344:727-734.
Schepers GE, Teasdale RD, Koopman P (2002) Twenty pairs of sox: extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev Cell 3:167-170.
Tanaka T, Yokoi T, Tamalu F, Watanabe S, Nishina S, Azuma N (2015) Generation of retinal ganglion cells with functional axons from human induced pluripotent stem cells. Sci Rep 5:8344.
Teotia P, Chopra DA, Dravid SM, Van Hook MJ, Qiu F, Morrison J, Rizzino A, Ahmad I (2017) Generation of functional human retinal ganglion cells with target specificity from pluripotent stem cells by chemically de fi ned recapitulation of developmental mechanism. Stem Cells 35:572-585.
Usui A, Mochizuki Y, Iida A, Miyauchi E, Satoh S, Sock E, Nakauchi H, Aburatani H, Murakami A, Wegner M, Watanabe S (2013)e early retinal progenitor-expressed gene Sox11 regulates the timing of the di ff erentiation of retinal cells. Development 140:740-750.
10.4103/1673-5374.211178
*Correspondence to:
- 中国神经再生研究(英文版)的其它文章
- Mitochondrial quality control in amyotrophic lateral sclerosis: towards a common pathway?
- Umbilical cord: an unlimited source of cells di ff erentiable towards dopaminergic neurons
- Targeting 14-3-3 adaptor protein-protein interactions to stimulate central nervous system repair
- RACK1 regulates neural development
- Schwann cell development, maturation and regeneration: a focus on classic and emerging intracellular signaling pathways
- BDNF pro-peptide: a novel synaptic modulator generated as an N-terminal fragment from the BDNF precursor by proteolytic processing

