Schwann cell development, maturation and regeneration: a focus on classic and emerging intracellular signaling pathways
Luca Franco Castelnovo, Veronica Bonalume, Simona Mel fi , Marinella Ballabio, Deborah Colleoni, Valerio Magnaghi
Dipartimento di Scienze Farmacologiche e Biomolecolari, Università degli Studi di Milano, Milan, Italy
Schwann cell development, maturation and regeneration: a focus on classic and emerging intracellular signaling pathways
Luca Franco Castelnovo, Veronica Bonalume, Simona Mel fi , Marinella Ballabio, Deborah Colleoni, Valerio Magnaghi*
Dipartimento di Scienze Farmacologiche e Biomolecolari, Università degli Studi di Milano, Milan, Italy
How to cite this article:Castelnovo LF, Bonalume V, Mel fi S, Ballabio M, Colleoni D, Magnaghi V (2017) Schwann cell development, maturation and regeneration: a focus on classic and emerging intracellular signaling pathways. Neural Regen Res 12(7):1013-1023.
Funding: Financial support by University of Milan, institutional funding “Piano di sostegno per la ricerca 2016 -Linea 2 Azione B”, is gratefully acknowledged.
myelin; neuroactive steroids; electromagnetic fi eld; peripheral nervous system
Accepted: 2017-06-22
Introduction
Schwann cells (SCs) are the main glial cells of the peripheral nervous system (PNS).eir main function is the formation of the myelin sheath, which electrically isolates axons, allowing saltatory conduction of the action potential. Actually, their function is more complex, playing a fundamental role in different processes, from the development of the PNS (Feltri et al., 2016) to the post-injury nerve repair (Jessen and Mirsky, 2016). In this review, we fi rst illustrate the di ff erent phases of SC development and di ff erentiation, describing some of the different signals involved. Then, we discuss in detail some novel receptors and signaling pathways controlling SC biology and functionality during the different developmental, mature and regenerative states.
SCs from Development to Injury
SC development starts from neural crest cells, and leads to the formation of mature myelinating and non-myelinating SCs (Figure 1). The development process goes through different stages that require a dynamic control of SC morphology, with an accurate balance between proliferation and differentiation. These developmental stages include: neural crest cells, SC precursors, immature SCs, pro-myelinating SCs and mature myelinating and/or non-myelinating SCs (Monk et al., 2015). Neural crest cells (Figure 1A) delaminate from the dorsal region of the neural tube.ey possess high proliferative and migratory capacity, originating a wide range of cell types, including the cardiac cells, the skeletal and connective components of the head, the melanocytes, as well as neurons and glial cells of the PNS. Initially, the neural crest induction from the ectoderm requires some signals, such as the bone morphogenic protein (BMP), fi -broblast growth factor (FGF) and the activation of the Wnt signaling pathway (Stuhlmiller and García-Castro, 2012). Successively, other factors seem to be fundamental for the di ff erentiation of neural crest cells into SC precursors, including the transcription factor Sox10 (Woodhoo and Sommer, 2008), the neuregulin-1 (NRG-1) system (Shah et al., 1994), and the histone deacetylases (HDAC) isoforms 1 and 2 (HDAC1, 2) (Jacob et al., 2014). However, the molecular machinery required for these initial stages of SC di ff erentiation has not been completely elucidated.
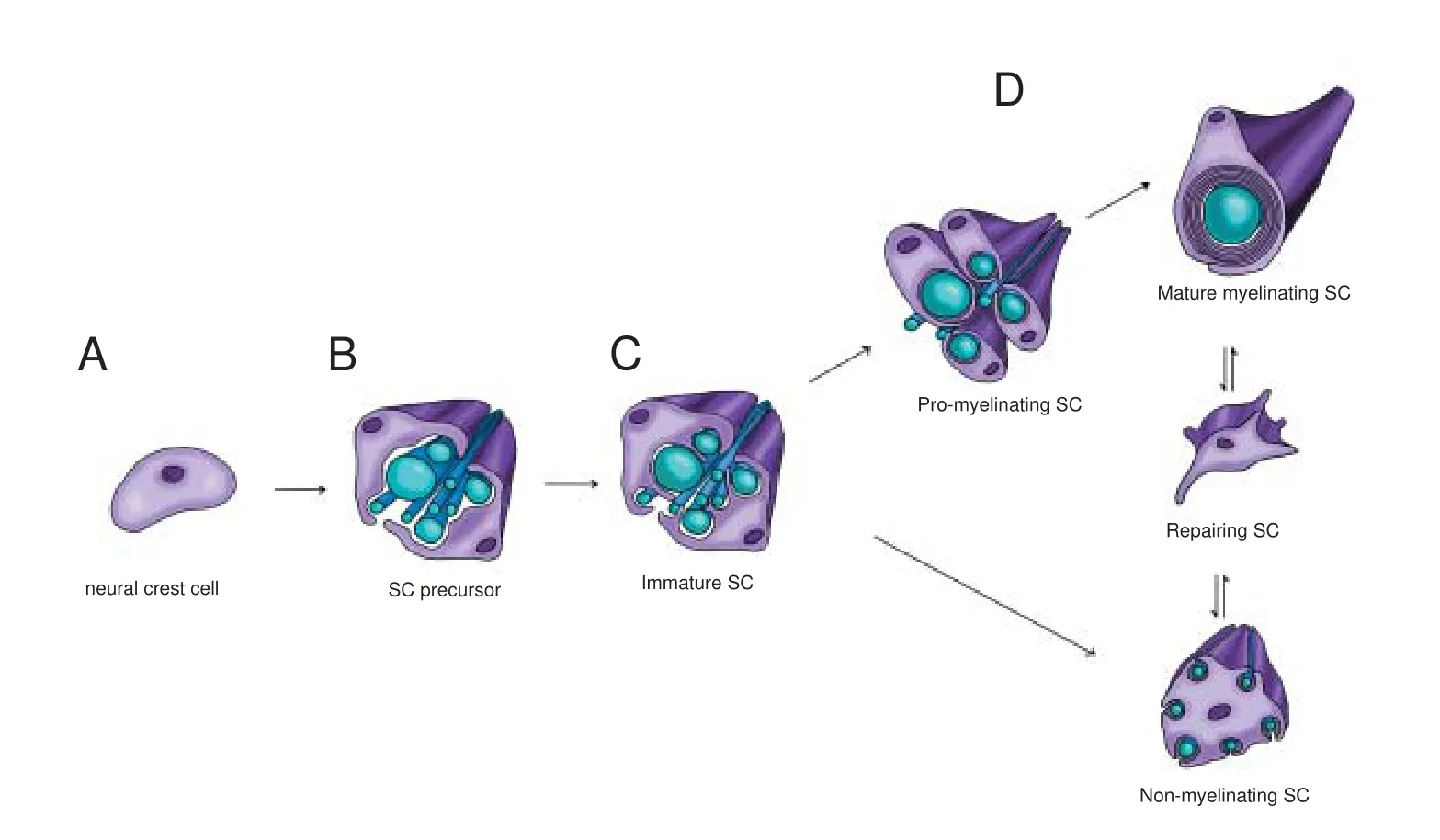
Figure 1 Scheme of development, maturation and repairing of Schwann cells (SCs).
Similarly to neural crest cells, SC precursors (Figure 1B) are proliferative and migratory, and rely on axonal signals for survival. A determinant of SC precursor development is the NRG-1/ErbB2/3 signaling system (Raphael and Talbot, 2011). Successively, SC precursors develop into immature SCs, with a mechanism involving the activation of Notch signaling (Woodhoo et al., 2009).ese cells lose their migratory capacity. Di ff erently from the previous stage, immature SCs are dependent on autocrine signaling for survival (Jessen and Mirsky, 2005). Immature SCs (Figure 1C) are involved in the radial sorting process, during which axons separate based on their caliber. In rodents and humans, this physiologic process starts perinatally and continues during development, determining the morphologic aspect of the mature PNS, in which larger axons are myelinated (Feltri et al., 2016). During this phase, immature SCs undergo cytoskeletal remodelling, leading to the extension of filipodia and lamellipodia. These specialized structures enable SCs to surround axons of mixed calibers and to send cytoplasmic processes between axons, to progressively choose and segregate the larger axons at the periphery of the bundle (Feltri et al., 2016).us, immature SCs which enter in a 1:1 ratio with larger axons, and later proceed towards the pro-myelinating phenotype; SCs that contact small caliber axons, indeed, di ff erentiate towards the non-myelinating phenotype (Figure 1D), leading to the formation of Remak bundles (Salzer, 2015; Feltri et al., 2016). Radial sorting is controlled by several factors, although the main determinants of this process are components of the extracellular matrix (e.g., laminin 211 and 411 or collagen XV), their speci fi c receptors (e.g., integrins α6β1 and α7β1 or dystroglycan) (Monk et al., 2015) and the downstream intracellular-activated pathways, such as the Rho family kinases or merlin (see following paragraphs).
Rac1, Cell Division Control Protein 42 (cdc42), FAK and Src
Cdc42 and FAK are potent regulators of SC proliferation and survival, and they are basic to generate enough cells to engage with axons (Fernandez-Valle et al., 1998; McLean et al., 2004). During the embryonic stages, absence of FAK expression and activation causes a signi fi cant reduction in the number of SC precursors. Further experiments demonstrated no increase in SC apoptosis, confirming that the major process regulated by cdc42 and FAK in SCs was the positive modulation of cell proliferation (Grove et al., 2007).
Moreover, as mentioned above, some of the intracellular molecules involved in axonal sorting are the Rho family kinases, such as the small GTP binding protein Rac1 and the cdc42 protein. Other important factors are FAK, protein kinase A (PKA) and neuro fi bromin 2 (Nf2)/merlin, all downstream the control of β-integrin and laminin (Benninger et al., 2007; Grove et al., 2007; Pereira et al., 2009; Guo et al., 2013a, b). Rho GTPases are expressed by SCs (Terashima et al., 2001).In vitroexperiments using dominant-negative and constitutively active forms of Rac1 and cdc42 indicated that these small GTPases, together with FAK, may be responsible for the growth factor mediated activation of SC motility (Cheng et al., 2000).

Figure 2 Scheme of some intracellular pathways involving FAK, cdc42, rac1, Src and merlin, in Schwann cell (SC) development, radial sorting, myelination and nerve repair.
The interaction between integrin and laminin proteins located at the interface of SCs with the extracellular matrix (Figure 2A), causes FAK phosphorylation and in turn cdc42 activation (Schwartz and Shattil, 2000). Cdc42 then stimulates merlin phosphorylation through p21-activated kinase-dependent (PAK) activation (Thaxton et al., 2008). SCs with high levels of phosphorylated merlin were reported to assume a multipolar phenotype, which is necessary for the radial sorting process (axton et al., 2011). In this light, the interaction of SCs with the extracellular matrix during the PNS development can activate this pathway, determining the SC multipolar morphology and radial sorting process. Later in SC development, at the myelination onset, a sort of negative feedback might control the phosphorylation cascade involving FAK, cdc42, PAK and merlin (Figure 2B). In that case the non-phosphorylated merlin exerts a suppressor function, inhibiting cdc42-mediated activation of PAK (Kissil et al., 2003; Hirokawa et al., 2004; Okada et al., 2005).
Alteration of this intracellular cascade may change the entire axonal sorting process. Indeed, the ablation of FAK, as shownin vivoin mutant SCs, leads to the arrest of large-caliber axon sorting, likely based on the dramatic reduction in SC proliferation during the embryonic stages (Grove et al., 2007). Furthermore, ablation of either cdc42 or Rac1 impaired the radial sorting of axons, suggesting that cdc42 is required for SC proliferation while Rac1 is necessary to correct the SC extension and stabilization (Benninger et al., 2007).
The non-receptor-type tyrosine kinase Src is a key component of different signal transduction pathways that are involved in a wide range of cellular processes, including cell growth, migration, and differentiation. Formerly, Src was shown to be highly enriched in developing axonal tracts in the PNS, decreasing to lower levels during maturation (Bare et al., 1993). However, its expression is enhanced following peripheral nerve injury (Fu and Gordon, 1997). Consistently, SCs distal to the injury site were shown to express high levels of the active dephosphorylated form of Src (Zhao et al., 2003). It has been shown that Src can also modulate FAK. FAK phosphorylation at Tyr395 is an early event, leading to the exposure of a docking site for Src (Schaller et al., 1994; Xing et al., 1994). Under this conditions, Src can further phosphorylate FAK (Figure 2C), promoting its full activation (Calalb et al., 1995) that may occurviacdc42 and merlin signals.
Likely, FAK is required for proliferation, spreading and SC differentiation (Grove et al., 2007). However, in adult SCs FAK seems to be inactive, either for myelin maintenance or for re-myelination after nerve injury (Grove and Brophy, 2014).ese fi ndings suggest a primary role of FAK during SC development but not during SC maturation or regeneration. However, it should be underlined that in mature SCs, Src can be also modulated by other stimuli, such as neuroactive steroids (Mel fi et al., 2017). Indeed, it was demonstrated that Src is directly activated by allopregnanolone (ALLO) through a GABA-A dependent mechanism, involving the modulation of FAK (Figure 2C). ALLO-induced activation of GABA-A receptor determines SC actin remodelling, migration, chemoattractant responsivity and proliferation. Moreover, ALLO treatment causes morphological re-arrangements of SCs, that change into a bipolar shape, extruding lamellipodia necessary for the onset of myelination (Mel fi et al., 2017). In this light, Src and FAK modulate myelination, so that their control may represent a potential approach to-wards the regulation of SCs maturation and the development of novel therapies for the peripheral neuropathies.
Nf2/Merlin
Nf2 gene encodes the tumor suppressor protein neuro fi bromin, also named merlin, a cytoskeleton-associated protein belonging to the ezrin-radixin-moesin (ERM) family, which serves as tumor-suppressor protein in different cells. Mutations in the Nf2 gene are associated with the autosomal dominant multiple syndrome called neuro fi bromatosis type 2 (Had fi eld et al., 2010).
Merlin links the cell membrane to the cytoskeleton, regulating some intracellular signaling pathways, thus leading to cell disorganization when merlin is inactivated. Furthermore, merlin locates to the nucleus and inhibits E3 ubiquitin ligase CRL4DCAF1(Brodhun et al., 2017), thus suppressing the nuclear tumorigenesis (Li et al., 2014). Loss of merlin activates Rac1 and Ras, as well as PAK1, EGFR-Ras-ERK, PI3K-Akt, the mammalian target of rapamycin complex 1 (mTORC1), and Wnt pathways. However, the major e ff ector downstream merlin in regulating growth is the Hippo pathway, a master regulator of proliferation, survival and migration in mammalian cells (Meng et al., 2016). Merlin was proved to suppress tumorigenesis by activating the Hippo pathway (Zhang et al., 2010). Intriguingly, CRL4DCAF1controls an oncogenic program of genes that includes the TEA domain family member (TEAD) target genes, thus suggesting that merlin regulates Hippo signaling by inhibiting CRL4DCAF1(Li and Giancotti, 2010). In any case, all these complex mechanisms are controversial and not completely elucidated, even in SCs.
For instance, during the first stages of myelination (Figure 2B), the activation of β1 integrin by the extracellular matrix on the SC surface, as well as the ErbB2 activation, at the SC-axon interface, can gradually decline the levels of phosphorylated merlin.is allows the SC transition toward a bipolar morphology, establishing a stable 1:1 association with axons that initiates the myelination process (axton et al., 2011).en, merlin stabilizes the bipolar morphology of SCs through inhibition of Rac1 (axton et al., 2011).
Conversely, in Rac1 SC conditional knock-out (Rac1-CKO) mice, it was shown that the phosphorylation of PAK determines a decreased merlin phosphorylation (in agreement with cdc42 functions described above), but in this case the process induces the arrest of SC myelination (Guo et al., 2012). Moreover, it was also shown that, in the absence of Rac1, the non-phosphorylated form of merlin negatively regulates cAMP-mediated myelination. Accordingly, the Nf2/merlin mutation in Rac1-CKO SC restored the cAMP levels, allowing myelin formation (Guo et al., 2012).
Overall, these findings, together with those described above, support a novel pathway in which Rac1 regulates SC myelination through Nf2/merlin and cAMP signaling (Figure 2B).
Merlin is also a negative regulator of mTORC1. In Nf2-related tumors, the functional loss of merlin activates the mTORC1 signaling. However, the mTOR inhibitor rapamycin limited but did not suppress tumorigenesis. Blocking mTORC1 signaling with rapamycin also resulted in elevated phosphorylated Akt levels (Giovannini et al., 2014). Considering the emerging importance of the mTOR signaling in SCs (described below), the interaction between merlin and mTOR pathway deserves deeper analysis.
Furthermore, a recent paper revealed a novel role for merlin and its effector Yes-associated protein (YAP; see below) in the control of SC plasticity and peripheral nerve repair after injury. It was shown that loss of merlin in repairing competent SCs determines a strong failure of axonal regeneration and SC capacity to re-myelinate correctly the large diameter axons (Mindos et al., 2017). Finally, it has been suggested that also some non-coding small RNAs or micro RNAs (miRNAs) may be modulated because of Nf2 gene mutation. miRNAs are master regulators of gene expression, which are oen deregulated in human tumorigenesis. Many miRNAs are changed in different types of tumours (Torres-Martin et al., 2013), suggesting their potential involvement also in neuro fi bromatosis type 2. Torres-Martin and colleagues demonstrated the deregulation of 174 miRNAs, including the upregulation of miR-10b, miR-206, miR-183, miR-133b, and the downregulation of miR-431, miR-221and miR-493, in di ff erent forms of vestibular schwannoma with Nf2 mutations (Torres-Martin et al., 2013). However, as single miRNAs are predicted to target hundreds of transcripts, the role played by miRNAs in SCs is rather complicated. For this reason we refer to other more focused reviews on the argument (Dugas and Notterpek, 2011; Svaren, 2014).
YAP, Transcriptional Co-activator with PDZ-Binding Motif (TAZ), and the Hippo Pathway
YAP and TAZ are two transcriptional coactivators, active in the steps downstream the Hippo pathway (Dupont et al., 2011). They play important roles in organ growth, cell differentiation, proliferation and survival. YAP and TAZ shuttle from the cytoplasm into the nucleus (Zhao et al., 2008; Zhang et al., 2009), where they interact with some DNA-binding transcription factors, such as TEAD1, controlling the gene expression of the peripheral myelin protein of 22 kDa (PMP22) (Lopez-Anido et al., 2016). In the cytosol, YAP and TAZ are phosphorylated and inhibited by LATS1/2 kinases.
The nucleo-cytoplasmic shuttling of YAP and TAZ is critical for regulating cell proliferation during development, a phenomenon ensuring normal tissue growth and organ size (Piccolo et al., 2014). Hence, the nuclear translocation in differentiated adult cells is essential for cancer initiation and for solid tumor growth (Moroishi et al., 2015), making them a promising target for cancer therapies. YAP and TAZ are critical for immature SC development and myelin gene regulation. In particular, these transcriptional regulators are required for SC proliferation and axonal sorting; SCs require YAP and TAZ to enter S-phase and, without them, fail to generate su ffi cient SCs for axonal sorting (Grove et al., 2017).YAP and TAZ are also required for SC di ff erentiation, regulating the transcription of Krox20 (Grove et al., 2017), which is a well-known modulator of the myelination program in SCs (Topilko et al., 1994). However, it has been suggested that the capability of YAP and TAZ to initiate and maintain SC myelination may depend by different pathways (not strictly dependent by Krox20), such as the transcription factor Zeb2; this factor is positively regulated by YAP and TAZ and promotes SC differentiation by inhibiting some differentiation repressors (Quintes et al., 2016). YAP and TAZ can also active the mTORC1 pathway to promote myelination (Kim et al., 2015). Moreover, YAP and TAZ are downstream different regulators of myelination, including NRG-1 type III, integrin α6β1, Gpr126 and Wnt (Quintes et al., 2016; Grove et al., 2017). Indeed, in myelinating SCs these coactivators are regulated by the extracellular matrix (Lopez-Anido et al., 2015; Poitelon et al., 2016). YAP and TAZ are two core factors in the Hippo pathway (Meng et al., 2016), integrating also biochemical and mechanical signals in the cells.
HDAC
HDACs are an important class of epigenetic modulators in SCs. HDACs are chromatin-remodelling proteins, capable of removing acetyl groups from histone tails, favouring chromatin condensation, and making it less accessible for transcription factors (Jacob et al., 2011). HDACs can also act at a non-epigenetic level, de-acetylating di ff erent targets, including some transcription factors (Glozak et al., 2005). HDAC proteins, and in particular the isoforms HDAC1 and HDAC2, were shown to play an important role during development (Jacob et al., 2014) and in postnatal SCs (Jacob et al., 2011).
During the establishment of the SC lineage from the neural crest, an important determinant of SC development is the expression of presence of high Sox10 levels. In these cells, HDAC1 and HDAC2 do not activate directly Sox10, but they induce the expression of transcription factor Pax3, which in turn maintains high Sox10 levels and promoting the expression of other important SC lineage markers, such as proteins P0 and Fabp7 (Jacob et al., 2014).
HDACs have important roles also in SC myelination and postnatal cell survival. Indeed, the double loss of function of HDAC1 and HDAC2 induced partial defects in axonal sorting, blocking myelination (Jacob et al., 2011). Di ff erently from the neural crest cells, in mature SCs, HDACs bind the regulatory regions of Sox10, Krox20 and P0. However, only HDAC2 proved able to promote directly the myelination process, increasing the expression levels of some SC myelination markers, such as Sox10, Krox20, P0, as well as the myelin basic protein (MBP) and the myelin associated glycoprotein (MAG). In this regard, Sox10 plays a critical role by activating its own transcription, in turn increasing Krox20 expression, in synergy with HDAC2 (Jacob et al., 2011). Conversely, HDAC1 is a controller of SC survival during the fi rst postnatal days, through a mechanism that involves the expression of β-catenin.e upregulation of β-catenin mediated by Wnt signaling induces the apoptosis in carcinoma cells (Bordonaro et al., 2007). Accordingly, the Wnt inhibition blocks the e ff ect of HDAC1 knockout, con fi rming a role for this pathway in the HDAC1-mediated modulation of cell death during the early postnatal days (Jacob et al., 2011).
GPR126 and GPR44
GPR126 is a receptor belonging to the GPCR family, which proved necessary for myelination in zebrafish and mouse (Monk et al., 2009, 2011). From a mechanistic point of view, GPR126 was shown to act mainly by modulating intracellular cAMP levels, being able to couple both G-inhibitory (Gi) and G-stimulatory (Gs) proteins (Mogha et al., 2013). However, the Gs-mediated action seems to be more important, since GPR126 activation increases intracellular cAMP levels. As a consequence, intracellular cAMP upregulation leads to PKA and Oct6 activation, which in turn initiates a transcriptional cascade; Oct6 and Sox10, alongside other factors, promote Krox20-mediated myelination (Monk et al., 2015).e GRP126 Gi coupling is likely involved in fi ne cAMP tuning during development and, probably, in the indirect modulation of Rac1 through a Gi βγ subunits mediated mechanism, also involving PI3K (Mogha et al., 2013).
Recent observations suggested that GPR126 may play a role also in the regeneration process following nerve injury (Mogha et al., 2016). In this context, GPR126 was proved to be necessary for the upregulation of several chemokines (e.g., tumor necrosis factor, TNF), and some of them downstream targets, such as Ccl2, Ccl3, and Cxcl10. The role of cAMP modulation on these effects still needs to be elucidated (Mogha et al., 2016).
GPR44 is another GPCR involved in myelin formation and maintenance (Trimarco et al., 2014). A recent study showed that NRG-1 type III upregulates prostaglandin D2 (PD2) synthase, which produces PD2 in turn activating GPR44 (Trimarco et al., 2014). Consequently, GPR44 activation leads to the de-phosphorylation of the nuclear factor of activated T-cells (NFAT) isoform c4 (NFATc4), that controls the expression of Krox20 and P0 (Kao et al., 2009). Importantly, also GPR44 is involved in the modulation of NRG-1 type III signaling in SCs (Trimarco et al., 2014).
PI3K/Akt/mTOR
NRG-1/ErbB signaling is the main determinant of SC development. NRG-1/ErbB mediated activation of the PI3K pathway leads to the phosphorylation of 3-phosphoinositide dependent protein kinase-1 (PDK1), which in turn converts phosphatidylinositol diphosphate (PIP2) to phosphatidylinositol triphosphate (PIP3), then activating Akt. Different studies supported the importance of this pathway in SC development (Maurel and Salzer, 2000; Ogata et al., 2006). However, NRG1 has a role also in myelination control (Taveggia, 2016).
Ablation of the phosphatase and tensin homolog (PTEN), a lipid phosphatase whose effects are opposite to PDK1, was reported to induce increased SC wrapping and axon hypermyelination (Goebbels et al., 2010).ese e ff ects were ameliorated by the mTOR inhibitor rapamycin, demonstrating they were due to Akt-mediated mTOR activation (Goebbels et al., 2012). Prenatal inactivation of mTOR in SCs impaired myelination, supporting the hypothesis of its role in the myelination process (Sherman et al., 2012). Interestingly, the mTOR-mediated control of peripheral myelination seems to be due to mTORC1. Indeed, only the selective ablation of Raptor, a speci fi c mTORC1 adaptor, led to impaired myelination and abnormal cell sorting, whereas the ablation of the mTORC2 adaptor Rictor had no effect (Norrmén et al., 2014). Another hypothesis dealing with the role of mTOR in myelination is based on the relevance of the Raptor-mediated control of the sterol regulatory element-binding protein 1 (SREBP-1) expression, which a ff ects lipid metabolism independently from ErbB signaling (Norrmén et al., 2014).
Overall, the fi ne mechanism carried out by mTOR during myelination is not completely clear, although mTOR is broadly considered a key regulator of protein translation in the PNS (Laplante and Sabatini, 2013).
Mitogen-Activated Protein Kinase (MAPK), Erk and p38
Both MAPK, Erk1/2 and p38, were shown to be fundamental in the control of SC di ff erentiation, although their action is partially contradictory. Indeed, they were proved to act either as positive or negative regulators of myelination.
Some evidence suggested that MAPK is an intracellular pathway downstream NRG-1/ErbB, mediating some effects on myelination. Erk1/2 ablation during development was shown to impaire SC differentiation and myelination (Newbern et al., 2011). In agreement, the activation of the Erk1/2 pathway led to increased myelin growth (Ishii et al., 2013; Sheean et al., 2014). In particular, the constitutive activation of the Erk pathways could replace NRG-1/ErbB signaling in myelination (Sheean et al., 2014), even when other pathways downstream NRG-1 control, such as phosphor-lipase C-γ (PLCγ) and PI3K-Akt-mTOR, were not activated. Accordingly, the phosphatase Shp2, which activates the Erk1/2 signaling pathway, was shown to be necessary for SC di ff erentiation and myelination, since its conditional knockout led to a phenotype overlapping the ErbB2 knockout mice (Grossmann et al., 2009). Deletion of the sca ff olding protein Gab1 reduced Erk activation, resulting in hypomyelination (Shin et al., 2014). In addition, Erk pathway may activate also the pro-myelinating transcriptional factor YY1 (He et al., 2010).
Also the p38 MAPK signaling pathway seems to play a pro-myelinating role at the early stages of myelination, since its inhibition at the time when myelination strats, was reported to block myelin formation (Fragoso et al., 2003).
In contrast, di ff erent studies suggested that the activation of MAPK pathways might lead to the negative regulation of myelination and to the induction of the SC repairing phenotype. Indeed, the inhibition of Erk1/2 and p38 MAPK pathways was shown to enhance cell myelination in culture, while p38 activation blocks the cAMP-mediated myelin gene expression and SC di ff erentiation (Syed et al., 2010; Yang et al., 2012).In vivo, Erk1/2 were rapidly activated in the distal stump aer nerve injury (Harrisingh et al., 2004; Guertin et al., 2005), and their inhibition blocked SC de-di ff erentiation from the myelinating toward the repairing SC phenotype (Harrisingh et al., 2004; Napoli et al., 2012). Importantly, Raf-1 mediated Erk1/2 activation led to SC de-di ff erentiation and de-myelination, even in the absence of nerve injury (Napoli et al., 2012).e signal initiating Erk1/2 activation aer injury is not clear, although some evidence suggested that it could be ErbB2.is hypothesis is based on the observation that its blockade produced e ff ects similar to Erk1/2 deletion, such as myelin breakdown and SC proliferation (Guertin et al., 2005; Napoli et al., 2012; Kim et al., 2013). In addition, p38 MAPK plays similar roles aer nerve injury. Its inhibition, indeed, was shown to lessenin vivode-myelination after nerve injury as well asin vitroSC de-di ff erentiation (Yang et al., 2012).
In conclusion, it is evident that MAPK activation in SCs may act under di ff erent circumstances, as positive or negative regulator of myelination. MAPK seems to play a pro-myelinating role during development and an anti-myelinating role after nerve injury. This dualistic role may depend on the lasting of activation. Indeed, transient activation during developmental stages may be linked to positive myelination modulation, while long-term activation following nerve injury may lead to its negative modulation (Glenn and Talbot, 2013; Kim et al., 2013).
PLCγ-NFAT
The PLCγ-NFAT pathway was suggested to be important for SCs, in particular for the modulation of gene expression during SC myelination and maturation. NRG-1 binding to ErbB2/ErbB3 receptor complex activates also PLCγ, leading to increased intracellular Ca2+levels and activation of the protein phosphatase calcineurin B. This phosphatase is responsible for cytosolic NFAT dephosphorylation, speci fi cally for what regards NFATc3 and c4 (Kao et al., 2009). ErbB inhibitors proved able to block such an e ff ect, whereas PI3K and MAPK inhibitors did not influence it, suggesting that these two pathways are not involved in the modulation of NFAT dephosphorylation.
NFAT proteins may accumulate in the nucleus, where NFATc4 synergistically co-activates, alongside Sox10, the myelin-speci fi c enhancer (MSE) region of Krox20, inducing its expression (Kao et al., 2009).is mechanism of control is likely dependent on increased intracellular cAMP levels, necessary for NFAT nuclear translocation (Kipanyula et al., 2013). Moreover, NFATc4 and Sox10 exert also a synergic control of P0 promoter, regulating myelination (Kao et al., 2009). Hereaer, given that P0 is also dependent by Krox20, these observations mean that NFAT modulate P0 expression directly and indirectly (Kao et al., 2009).
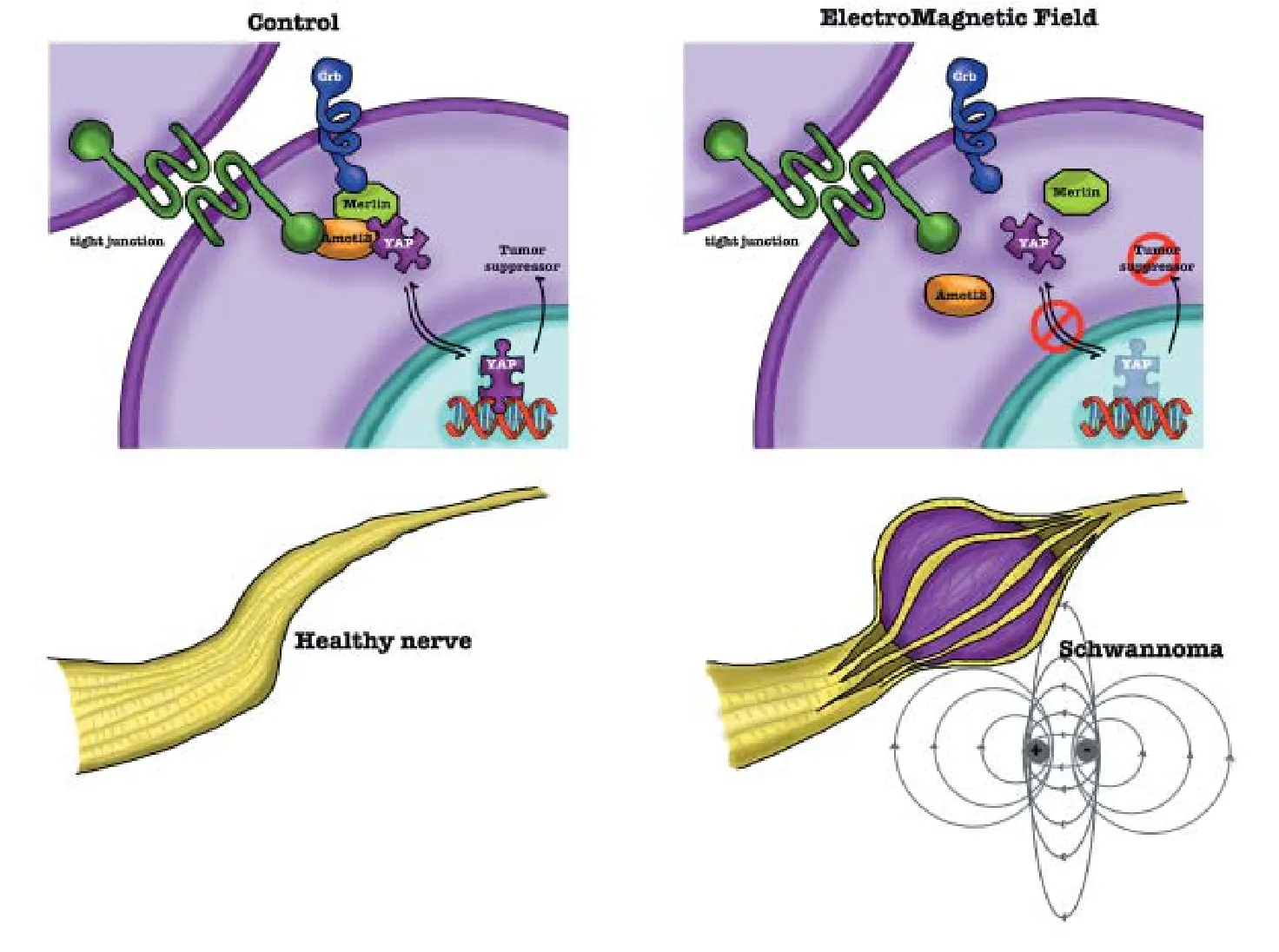
Figure 3 Scheme of Hippo/YAP involvement in SCs.
Neuroactive Steroids/GABA Receptors
Neuroactive steroids, including progestagens, are hormones active and synthesized in the nervous system. The progesterone metabolite 5α-pregnan-3α-ol-20-one, named tetrahydroprogesterone or ALLO, is the most important neuroactive steroid (Baulieu and Robel, 1990), targeting both neurons and glial cells in the central nervous system and in the PNS. In particular, the PNS is a target of neuroactive steroids, since it expresses the common progestagen receptors, such as the classic progesterone receptor (PR) and the non-classic GABA-A receptor (Faroni and Magnaghi, 2011). It is well established that ALLO may act through the GABA-A receptor, modulating Cl-ion flux and exerting non-genomic actions (Puia et al., 1990, 2015), albeit alternative mechanisms have been recently hypothesized (Cooke et al., 2013; Pang et al., 2013). SCs synthesize ALLO and simultaneously may be a target of its actions (Faroni and Magnaghi, 2011).
In the PNS, ALLO participates in the control of myelination, nerve regeneration and likely nociception (Magnaghi et al., 2006, 2010; Faroni and Magnaghi, 2011; Melfi et al., 2017).
It was mentioned (see above) that, in SCs, Src is directly activated by ALLO through a GABA-A dependent mechanism, involving the modulation of FAK (Mel fi et al., 2017).is determines remodeling and proliferation of SCs, associated to a promotion ofin vitromyelination and increase in the internodes distance (Figure 2C) (Mel fi et al., 2017).
Interestingly, several proofs collected in the last decade demonstrated that also the GABA-B receptor is relevant in the control of SC development, maturation and plasticity (Procacci et al., 2012; Faroni et al., 2014; Magnaghi et al., 2014; Corell et al., 2015). Hitherto, it was not established whether the GABA-B receptor in the PNS, in particular in SCs, might be a direct target of neuroactive steroids, such as ALLO. Interestingly, the cross-regulation between GABA-A and GABA-B receptors in SCs was partially characterized (Magnaghi et al., 2006), representing an alternative mechanism of SC regulation that needs further investigation.
c-Jun
Recently, the transcription factor c-Jun was shown to be the key modulator of SC plasticity towards the repairing phenotype.
As a matter of fact, c-Jun expression changes during SC development. Its expression is low or absent in SC precursors, but later it is upregulated in immature SCs,viaKrox20-mediated mechanism (Jessen and Mirsky, 2016). In mature SCs, c-Jun is strongly downregulated, although it is still detectable in non-myelinating and (to a lesser extent) in myelinating SCs (Jessen and Mirsky, 2016). Meanwhile c-Jun is a negative modulator of SC myelination, suppressing som myelin genes including Krox20, P0 and MBP (Parkinson et al., 2004, 2008).
Importantly, c-Jun is rapidly upregulated in injured nerves. In fact studies performed in SCs obtained from conditional knockout mice proved that c-Jun is not essential during development, but it is required for functional regeneration and post-injury axonal recovery (Arthur-Farraj et al., 2012). After nerve injury, c-Jun mediates the de-differentiation from the SC myelinating phenotype, as well as the subsequent activation of the repairing program in PNS. c-Jun was shown to be necessary for the upregulation of important factors involved in axonal growth and survival, such as GDNF, BDNF, p75-NTR and N-cadherin. In particular, GDNF and N-cadherin are directly targeted by c-Jun (Arthur-Farraj et al., 2012; Fontana et al., 2012).
Interestingly, c-Jun seems to play a role in the so called myelinophagy process, which is a specialized mTOR-independent autophagy process by which SCs start to removemyelin following nerve damage (Jessen and Mirsky, 2016). c-Jun also participates in the macrophage recruitment aer nerve injury (Arthur-Farraj et al., 2012).
Mechanical Cues
Mechanobiology is a field of science studying the effect of physical forces, such as stretching and compression, on living systems (Halder et al., 2012). By activating mechanotransduction systems, cells translate the physical stimuli into biochemical signals, controlling multiple aspects of cell behavior, including growth, di ff erentiation and tumor progression (Dupont et al., 2011). For instance, physical properties of the extracellular matrix and mechanical forces are integral to morphogenetic processes in embryonic development, defining tissue architecture (Mammoto and Ingber, 2010; Dupont et al., 2011). For a long time, the biology of dysmyelination and demyelination have been studied under myelin pathological conditions, although the study of mechanical stimuli that guide the development of myelinating glial cells was only recently addressed (Jagielska et al., 2012; Lourenço et al., 2016). SCs were shown to be mechanosensitive, albeit the nature of forces that affect their differentiationin vivoremains to be fully established, as well as the potential implication of these pathogenic alterations in tumorigenesis (Poitelon et al., 2017).
In this context, the Hippo pathway (see above) has been described as one of the most important e ff ector of SC changes following physical challenges. YAP and TAZ were proved to be activated by mechanical stimuli, in turn regulating SC proliferation and transcription of basal lamina receptor genes (Poitelon et al., 2016). Recently, the exposure to electromagnetic fields has been indicated as an environmental challenge affecting SCs development (Lacy-Hulbert et al., 1998; Colciago et al., 2015).e electromagnetic fi eld exposure induces YAP dysregulation, changing its intracellular localization; these e ff ects were associated with increased SC proliferation and decreased differentiation. In normal cells YAP is mainly localized in the nucleus, where it controls proliferation and apoptosis, while in SCs exposed to electromagnetic field YAP is mostly present in the cytoplasm (Colciago et al., 2015), likely losing its tumor suppressor capability (Figure 3).
Taken together, these fi ndings suggest mechanobiology as an emerging and promising fi eld of study in SC biology, even its relevance and importance in the pathophysiology of SCs needs further studies.
Conclusions
SCs are the main glial cells in the PNS, involved in several hereditary, metabolic and traumatic diseases. In the last thirty years, a huge amount of data in the scienti fi c literature pointed out the role of growth factors, neuregulins, integrins, hormones and neurotransmitters in SC regulation, under different pathophysiological conditions. In this review, we presented some of the more recent findings on novel signaling pathways that proved important in regulating the different stages of SCs, from development to maturation, myelination, plasticity and nerve repair.
Author contributions:LFC wrote most of the chapters; VB wrote chapter on Src and drew all fi gures; SM wrote the chapters on Nf2 and YAP; MB and DC wrote chapter on mechanical cues and references; VM supervised and proofread the entire review.
Con fl icts of interest:None declared.
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and signi fi cance of this paper.
Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR (2012) c-Jun reprograms schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 75:633-647.
Bare DJ, Lauder JM, Wilkie MB, Maness PF (1993) p59fyn in rat brain is localized in developing axonal tracts and subpopulations of adult neurons and glia. Oncogene 8:1429-1436.
Baulieu EE, Robel P (1990) Neurosteroids: a new brain function? J Steroid Biochem Mol Biol 37:395-403.
Benninger Y, Thurnherr T, Pereira JA, Krause S, Wu X, Chrostek-Grasho ff A, Herzog D, Nave KA, Franklin RJM, Meijer D, Brakebusch C, Suter U, Relvas JB (2007) Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development. J Cell Biol 177:1051-1061.
Bordonaro M, Lazarova DL, Sartorelli AC (2007)e activation of beta-catenin by Wnt signaling mediates the e ff ects of histone deacetylase inhibitors. Exp Cell Res 313:1652-1666.
Brodhun M, Stahn V, Harder A (2017) Pathogenesis and molecular pathology of vestibular schwannoma. HNO 65:362-372.
Calalb MB, Polte TR, Hanks SK (1995) Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol 15:954-963.
Cheng HL, Steinway ML, Russell JW, Feldman EL (2000) GTPases and phosphatidylinositol 3-kinase are critical for insulin-like growth factor-I-mediated Schwann cell motility. J Biol Chem 275:27197-27204.
Colciago A, Melfi S, Giannotti G, Bonalume V, Ballabio M, Caffino L, Fumagalli F, Magnaghi V (2015) Tumor suppressor Nf2/merlin drives Schwann cell changes following electromagnetic fi eld exposure through Hippo-dependent mechanisms. Cell Death Discov 1:15021.
Cooke PS, Nanjappa MK, Yang Z, Wang KK (2013)erapeutic e ff ects of progesterone and its metabolites in traumatic brain injury may involve non-classical signaling mechanisms. Front Neurosci 7:108.
Corell M, Wicher G, Radomska KJ, Dağlıkoca ED, Godskesen RE, Fredriksson R, Benedikz E, Magnaghi V, Fex Svenningsen A (2015) GABA and its B-receptor are present at the node of Ranvier in a small population of sensory fi bers, implicating a role in myelination. J Neurosci Res 93:285-295.
Dugas JC, Notterpek L (2011) MicroRNAs in oligodendrocyte and Schwann cell di ff erentiation. Dev Neurosci 33:14-20.
Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S (2011) Role of YAP/TAZ in mechanotransduction. Nature 474:179-183.
Faroni A, Castelnovo LF, Procacci P, Caffino L, Fumagalli F, Melfi S, Gambarotta G, Bettler B, Wrabetz L, Magnaghi V (2014) Deletion of GABA-B receptor in Schwann cells regulates remak bundles and small nociceptive C- fi bers. Glia 62:548-565.
Faroni A, Magnaghi V (2011)e neurosteroid allopregnanolone modulates specific functions in central and peripheral glial cells. Front Endocrinol 2:103.
Faroni A, Mobasseri SA, Kingham PJ, Reid AJ (2015) Peripheral nerve regeneration: experimental strategies and future perspectives. Adv Drug Deliv Rev 82-83:160-167.
Feltri ML, Poitelon Y, Previtali SC (2016) How Schwann cells sort axons: new concepts. Neuroscientist 22:252-265.
Fernandez-Valle C, Wood PM, Bunge MB (1998) Localization of focal adhesion kinase in di ff erentiating Schwann cell/neuron cultures. Microsc Res Tech 41:416-430.
Fontana X, Hristova M, Da Costa C, Patodia S,ei L, Makwana M, Spencer-Dene B, Latouche M, Mirsky R, Jessen KR, Klein R, Raivich G, Behrens A (2012) C-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. J Cell Biol 198:127-141.
Fragoso G, Robertson J, Athlan E, Tam E, Almazan G, Mushynski WE (2003) Inhibition of p38 mitogen-activated protein kinase interferes with cell shape changes and gene expression associated with Schwann cell myelination. Exp Neurol 183:34-46.
Fu SY, Gordon T (1997)e cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol 14:67-116.
Giovannini M, Bonne NX, Vitte J, Chareyre F, Tanaka K, Adams R, Fisher LM, Valeyrie-Allanore L, Wolkenstein P, Goutagny S, Kalamarides M (2014) mTORC1 inhibition delays growth of neuro fi bromatosis type 2 schwannoma. Neuro Oncol 16:493-504.
Glenn TD, Talbot WS (2013) Signals regulating myelination in peripheral nerves and the Schwann cell response to injury. Curr Opin Neurobiol 23:1041-1048.
Glozak MA, Sengupta N, Zhang X, Seto E (2005) Acetylation and deacetylation of non-histone proteins. Gene 363:15-23.
Goebbels S, Oltrogge JH, Kemper R, Heilmann I, Bormuth I, Wolfer S, Wichert SP, Möbius W, Liu X, Lappe-Siee C, Rossner MJ, Groszer M, Suter U, Frahm J, Boretius S, Nave KA (2010) Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J Neurosci 30:8953-8964.
Goebbels S, Oltrogge JH, Wolfer S, Wieser GL, Nientiedt T, Pieper A, Ruhwedel T, Groszer M, Sereda MW, Nave KA (2012) Genetic disruption of Pten in a novel mouse model of tomaculous neuropathy. EMBO Mol Med 4:486-499.
Grossmann KS, Wende H, Paul FE, Cheret C, Garratt AN, Zurborg S, Feinberg K, Besser D, Schulz H, Peles E, Selbach M, Birchmeier W, Birchmeier C (2009)e tyrosine phosphatase Shp2 (PTPN11) directs Neuregulin-1/ErbB signaling throughout Schwann cell development. Proc Natl Acad Sci U S A 106:16704-16709.
Grove M, Brophy PJ (2014) FAK Is required for schwann cell spreading on immature basal lamina to coordinate the radial sorting of peripheral axons with myelination. J Neurosci 34:13422-13434.
Grove M, Kim H, Santerre M, Krupka AJ, Han SB, Zhai J, Cho JY, Park R, Harris M, Kim S, Sawaya BE, Kang SH, Barbe MF, Cho SH, Lemay MA, Son YJ (2017) YAP/TAZ initiate and maintain Schwann cell myelination. Elife 6:e20982.
Grove M, Komiyama NH, Nave KA, Grant SG, Sherman DL, Brophy PJ (2007) FAK is required for axonal sorting by Schwann cells. J Cell Biol 176:277-282.
Guertin AD, Zhang DP, Mak KS, Alberta JA, Kim HA (2005) Microanatomy of axon/glial signaling during Wallerian degeneration. J Neurosci 25:3478-3487.
Guo L, Lee AA, Rizvi TA, Ratner N, Kirschner LS (2013a)e protein kinase A regulatory subunit R1A (Prkar1a) plays critical roles in peripheral nerve development. J Neurosci 33:17967-17975.
Guo L, Moon C, Niehaus K, Zheng YY, Ratner N (2012) Rac1 controls schwann cell myelination through cAMP and NF2/merlin. J Neurosci 32:17251-17261.
Guo L, Moon C, Zheng Y, Ratner N (2013b) Cdc42 regulates Schwann cell radial sorting and myelin sheath folding through NF2/merlin-dependent and independent signaling. Glia 61:1906-1921.
Had fi eld KD, Smith MJ, Urquhart JE, Wallace AJ, Bowers NL, King AT, Rutherford SA, Trump D, Newman WG, Evans DG (2010) Rates of loss of heterozygosity and mitotic recombination in NF2 schwannomas, sporadic vestibular schwannomas and schwannomatosis schwannomas. Oncogene 29:6216-6221.
Halder G, Dupont S, Piccolo S (2012) Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol 13:591-600.
Harrisingh MC, Perez-Nadales E, Parkinson DB, Malcolm DS, Mudge AW, Lloyd AC (2004) The Ras/Raf/ERK signaling pathway drives Schwann cell dedi ff erentiation. EMBO J 23:3061-3071.
He Y, Kim JY, Dupree J, Tewari A, Melendez-Vasquez C, Svaren J, Casaccia P (2010) Yy1 as a molecular link between neuregulin and transcriptional modulation of peripheral myelination. Nat Neurosci 13:1472-1480.
Hirokawa Y, Tikoo A, Huynh J, Utermark T, Hanemann CO, Giovannini M, Xiao GH, Testa JR, Wood J, Maruta H (2004) A clue to the therapy of neuro fi bromatosis type 2: NF2/merlin is a PAK1 inhibitor. Cancer J 10:20-26.
Ishii A, Furusho M, Bansal R (2013) Sustained activation of ERK1/2 MAPK in oligodendrocytes and schwann cells enhances myelin growth and stimulates oligodendrocyte progenitor expansion. J Neurosci 33:175-186.
Jacob C, Christen CN, Pereira JA, Somandin C, Baggiolini A, Lötscher P, Ozçelik M, Tricaud N, Meijer D, Yamaguchi T, Matthias P, Suter U (2011) HDAC1 and HDAC2 control the transcriptional program of myelination and the survival of Schwann cells. Nat Neurosci 14:429-436.
Jacob C, Lötscher P, Engler S, Baggiolini A, Varum Tavares S, Brügger V, John N, Büchmann-Møller S, Snider PL, Conway SJ, Yamaguchi T, Matthias P, Sommer L, Mantei N, Suter U (2014) HDAC1 and HDAC2 control the speci fi cation of neural crest cells into peripheral glia. J Neurosci 34:6112-6122.
Jagielska A, Norman AL, Whyte G, Vliet KJ Van, Guck J, Franklin RJ (2012) Mechanical environment modulates biological properties of oligodendrocyte progenitor cells. Stem Cells Dev 21:2905-2914.
Jessen KR, Mirsky R (2005)e origin and development of glial cells in peripheral nerves. Nat Rev Neurosci 6:671-682.
Jessen KR, Mirsky R (2016)e repair Schwann cell and its function in regenerating nerves. J Physiol 594:3521-3531.
Kao SC, Wu H, Xie J, Chang CP, Ranish JA, Graef IA, Crabtree GR (2009) Calcineurin/NFAT signaling is required for neuregulin-regulated Schwann cell di ff erentiation. Science 323:651-654.
Kim HA, Mindos T, Parkinson DB (2013) Plastic fantastic: Schwann cells and repair of the peripheral nervous system. Stem Cells Transl Med 2:553-557.
Kim M, Kim T, Johnson RL, Lim DS (2015) Transcriptional co-repressor function of the hippo pathway transducers YAP and TAZ. Cell Rep 11:270-282.
Kipanyula MJ, Woodhoo A, Rahman M, Payne D, Jessen KR, Mirsky R (2013) Calcineurin-nuclear factor of activated t cells regulation of Krox-20 expression in Schwann cells requires elevation of intracellular cyclic AMP. J Neurosci Res 91:105-115.
Kissil JL, Wilker EW, Johnson KC, Eckman MS, Ya ff e MB, Jacks T (2003) Merlin, the product of the Nf2 tumor suppressor gene, is an inhibitor of the p21-activated kinase, Pak1. Mol Cell 12:841-849.
Lacy-Hulbert A, Metcalfe JC, Hesketh R (1998) Biological responses to electromagnetic fi elds. FASEB J 12:395-420.
Laplante M, Sabatini DM (2013) Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci 126:1713-1719.
Li W, Cooper J, Zhou L, Yang C, Erdjument-Bromage H, Zagzag D, Snuderl M, Ladanyi M, Hanemann CO, Zhou P, Karajannis MA, Giancotti FG (2014) Merlin/NF2 loss-driven tumorigenesis linked to CRL4(DCAF1)-mediated inhibition of the hippo pathway kinases Lats1 and 2 in the nucleus. Cancer Cell 26:48-60.
Li W, Giancotti FG (2010) Merlin’s tumor suppression linked to inhibition of the E3 ubiquitin ligase CRL4 (DCAF1). Cell Cycle 9:4433-4436.
Lopez-Anido C, Poitelon Y, Gopinath C, Moran JJ, Ma KH, Law WD, Antonellis A, Feltri ML, Svaren J (2016) Tead1 regulates the expression of Peripheral Myelin Protein 22 during Schwann cell development. Hum Mol Genet 25:3055-3069.
Lopez-Anido C, Sun G, Koenning M, Srinivasan R, Hung HA, Emery B, Keles S, Svaren J (2015) Di ff erential Sox10 genomic occupancy in myelinating glia. Glia 63:1897-1914.
Lourenço T, Paes de Faria J, Bippes CA, Maia J, Lopes-da-Silva JA, Relvas JB, Grãos M (2016) Modulation of oligodendrocyte di ff erentiation and maturation by combined biochemical and mechanical cues. Sci Rep 6:21563.
Magnaghi V, Ballabio M, Consoli A, Lambert JJ, Roglio I, Melcangi RC (2006) GABA receptor-mediated effects in the peripheral nervous system: A cross-interaction with neuroactive steroids. J Mol Neurosci 28:89-102.
Magnaghi V, Castelnovo LF, Faroni A, Cavalli E, Caffino L, Colciago A, Procacci P, Pajardi G (2014) Nerve regenerative effects of GABA-B ligands in a model of neuropathic pain. Biomed Res Int 2014:368678.
Magnaghi V, Parducz A, Frasca A, Ballabio M, Procacci P, Racagni G, Bonanno G, Fumagalli F (2010) GABA synthesis in Schwann cells is induced by the neuroactive steroid allopregnanolone. J Neurochem 112:980-990.
Mammoto T, Ingber DE (2010) Mechanical control of tissue and organ development. Development 137:1407-1420.
Mathis S, Goizet C, Tazir M, Magdelaine C, Lia AS, Magy L, Vallat JM (2015) Charcot-Marie-Tooth diseases: an update and some new proposals for the classi fi cation. J Med Genet 52:681-690.
Maurel P, Salzer JL (2000) Axonal regulation of Schwann cell proliferation and survival and the initial events of myelination requires PI 3-kinase activity. J Neurosci 20:4635-4645.
McLean GW, Komiyama NH, Serrels B, Asano H, Reynolds L, Conti F, Hodivala-Dilke K, Metzger D, Chambon P, Grant SGN, Frame MC (2004) Speci fi c deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression. Genes Dev 18:2998-3003.
Mel fi S, Colciago A, Giannotti G, Bonalume V, Ca ffi no L, Fumagalli F, Magnaghi V (2015) Stressing out the Hippo/YAP signaling pathway: toward a new role in Schwann cells. Cell Death Dis 6:e1915.
Melfi S, Montt Guevara MM, Bonalume V, Ruscica M, Colciago A, Simoncini T, Magnaghi V (2017) Src and phospho-FAK kinases are activated by allopregnanolone promoting Schwann cell motility, morphology and myelination. J Neurochem 141:165-178.
Meng Z, Moroishi T, Guan KL (2016) Mechanisms of Hippo pathway regulation. Genes Dev 30:1-17.
Mindos T, Dun XP, North K, Doddrell RD, Schulz A, Edwards P, Russell J, Gray B, Roberts SL, Shivane A, Mortimer G, Pirie M, Zhang N, Pan D, Morrison H, Parkinson DB (2017) Merlin controls the repair capacity of Schwann cells aer injury by regulating Hippo/YAP activity. J Cell Biol 216:495-510.
Mogha A, Benesh AE, Patra C, Engel FB, Schoneberg T, Liebscher I, Monk KR (2013) Gpr126 functions in Schwann cells to control di ff erentiation and myelination via G-protein activation. J Neurosci 33:17976-17985.
Mogha A, Harty BL, Carlin D, Joseph J, Sanchez NE, Suter U, Piao X, Cavalli V, Monk KR (2016) Gpr126/Adgrg6 has schwann cell autonomous and nonautonomous functions in peripheral nerve injury and repair. J Neurosci 36:12351-12367.
Monk KR, Feltri ML, Taveggia C (2015) New insights on schwann cell development. Glia 63:1376-1393.
Monk KR, Naylor SG, Glenn TD, Mercurio S, Perlin JR, Dominguez C, Moens CB, Talbot WS (2009) A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science 325:1402-1405.
Monk KR, Oshima K, Jörs S, Heller S, Talbot WS (2011) Gpr126 is essential for peripheral nerve development and myelination in mammals. Development 138:2673-2680.
Moroishi T, Hansen CG, Guan KL (2015)e emerging roles of YAP and TAZ in cancer. Nat Rev Cancer 15:73-79.
Napoli I, Noon LA, Ribeiro S, Kerai AP, Parrinello S, Rosenberg LH, Collins MJ, Harrisingh MC, White IJ, Woodhoo A, Lloyd AC (2012) A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron 73:729-742.
Newbern JM, Li X, Shoemaker SE, Zhou J, Zhong J, Wu Y, Bonder D, Hollenback S, Coppola G, Geschwind DH, Landreth GE, Snider WD (2011) Speci fi c functions for ERK/MAPK signaling during PNS development. Neuron 69:91-105.
Norrmén C, Figlia G, Lebrun-Julien F, Pereira JA, Trötzmüller M, Köfeler HC, Rantanen V, Wessig C, van Deijk ALF, Smit AB, Verheijen MHG, Rüegg MA, Hall MN, Suter U (2014) mTORC1 controls PNS myelination along the mTORC1-RXRγ-SREBP-lipid biosynthesis axis in Schwann cells. Cell Rep 9:646-660.
Ogata T, Yamamoto S, Nakamura K, Tanaka S (2006) Signaling axis in schwann cell proliferation and di ff erentiation. Mol Neurobiol 33:51-62. Okada T, Lopez-Lago M, Giancotti FG (2005) Merlin/NF-2 mediates contact inhibition of growth by suppressing recruitment of Rac to the plasma membrane. J Cell Biol 171:361-371.
Parkinson DB, Bhaskaran A, Arthur-Farraj P, Noon LA, Woodhoo A, Lloyd AC, Feltri ML, Wrabetz L, Behrens A, Mirsky R, Jessen KR (2008) c-Jun is a negative regulator of myelination. J Cell Biol 181:625-637.
Parkinson DB, Bhaskaran A, Droggiti A, Dickinson S, D’Antonio M, Mirsky R, Jessen KR (2004) Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J Cell Biol 164:385-394.
Pereira JA, Benninger Y, Baumann R, Gonçalves AF, Ozçelik M,urnherr T, Tricaud N, Meijer D, Fässler R, Suter U, Relvas JB (2009) Integrin-linked kinase is required for radial sorting of axons and Schwann cell remyelination in the peripheral nervous system. J Cell Biol 185:147-161.
Piccolo S, Dupont S, Cordenonsi M (2014)e biology of YAP/TAZ: Hippo signaling and beyond. Physiol Rev 94:1287-1312.
Poitelon Y, Lopez-Anido C, Catignas K, Berti C, Palmisano M, Williamson C, Ameroso D, Abiko K, Hwang Y, Gregorie ff A, Wrana JL, Asmani M, Zhao R, Sim FJ, Wrabetz L, Svaren J, Feltri ML (2016) YAP and TAZ control peripheral myelination and the expression of laminin receptors in Schwann cells. Nat Neurosci 19:879-887.
Poitelon Y, Nunes GD, Feltri ML (2017) Myelinating cells can feel disturbances in the force. Oncotarget 8:5680-5681.
Procacci P, Ballabio M, Castelnovo LF, Mantovani C, Magnaghi V (2012) GABA-B receptors in the PNS have a role in Schwann cellsdi ff erentiation? Front Cell Neurosci 6:68.
Puia G, Ravazzini F, Castelnovo LF, Magnaghi V (2015) PKCε and allopregnanolone: functional cross-talk at the GABAA receptor level. Front Cell Neurosci 9:83.
Puia G, Santi MR, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E (1990) Neurosteroids act on recombinant human GABAA receptors. Neuron 4:759-765.
Quintes S, Brinkmann BG, Ebert M, Fröb F, Kungl T, Arlt FA, Tarabykin V, Huylebroeck D, Meijer D, Suter U, Wegner M, Sereda MW, Nave KA (2016) Zeb2 is essential for Schwann cell differentiation, myelination and nerve repair. Nat Neurosci 19:1050-1059.
Raphael AR, Talbot WS (2011) New insights into signaling during myelination in zebra fi sh. Curr Top Dev Biol 97:1-19.
Salzer JL (2015) Schwann cell myelination. Cold Spring Harb Perspect Biol 7:a020529.
Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT (1994) Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol 14:1680-1688.
Schwartz MA, Shattil SJ (2000) Signaling networks linking integrins and rho family GTPases. Trends Biochem Sci 25:388-391.
Shah NM, Marchionni MA, Isaacs I, Stroobant P, Anderson DJ (1994) Glial growth factor restricts mammalian neural crest stem cells to a glial fate. Cell 77:349-360.
Sheean ME, McShane E, Cheret C, Walcher J, Müller T, Wulf-Goldenberg A, Hoelper S, Garratt AN, Krüger M, Rajewsky K, Meijer D, Birchmeier W, Lewin GR, Selbach M, Birchmeier C (2014) Activation of MAPK overrides the termination of myelin growth and replaces Nrg1/ErbB3 signals during Schwann cell development and myelination. Genes Dev 28:290-303.
Sherman DL, Krols M, Wu LM, Grove M, Nave KA, Ganglo ff YG, Brophy PJ (2012) Arrest of myelination and reduced axon growth when Schwann cells lack mTOR. J Neurosci 32:1817-1825.
Shin YK, Jang SY, Park SY, Park JY, Kim JKP, Kim JKP, Suh DJ, Lee HJ, Park HT (2014) Grb2-associated binder-1 is required for neuregulin-1-induced peripheral nerve myelination. J Neurosci 34:7657-7662.
Stuhlmiller TJ, García-Castro MI (2012) Current perspectives of the signaling pathways directing neural crest induction. Cell Mol Life Sci 69:3715-3737.
Svaren J (2014) MicroRNA and transcriptional crosstalk in myelinating glia. Neurochem Int 77:50-57.
Syed N, Reddy K, Yang D, Taveggia C, Salzer JL, Maurel P, Kim HA (2010) Soluble neuregulin-1 has bi-functional, concentration-dependent e ff ects on Schwann cell myelination. J Neurosci 30:6122-6131.
Taveggia C (2016) Schwann cells–axon interaction in myelination. Curr Opin Neurobiol 39:24-29.
Terashima T, Yasuda H, Terada M, Kogawa S, Maeda K, Haneda M, Kashiwagi A, Kikkawa R (2001) Expression of Rho-family GTPases (Rac, cdc42, RhoA) and their association with p-21 activated kinase in adult rat peripheral nerve. J Neurochem 77:986-993.
Thaxton C, Lopera J, Bott M, Fernandez-Valle C (2008) Neuregulin and laminin stimulate phosphorylation of the NF2 tumor suppressor in Schwann cells by distinct protein kinase A and p21-activated kinase-dependent pathways. Oncogene 27:2705-2715.
Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi AB, Seitanidou T, Babinet C, Charnay P (1994) Krox-20 controls myelination in the peripheral nervous system. Nature 371:796-799.
Torres-Martin M, Lassaletta L, de Campos JM, Isla A, Gavilan J, Pinto GR, Burbano RR, Latif F, Melendez B, Castresana JS, Rey JA (2013) Global pro fi ling in vestibular schwannomas shows critical deregulation of MicroRNAs and upregulation in those included in chromosomal region 14q32 Galardy PJ, ed. PLoS One 8:e65868.
Trimarco A, Forese MG, Alfieri V, Lucente A, Brambilla P, Dina G, Pieragostino D, Sacchetta P, Urade Y, Boizet-Bonhoure B, Boneschi FM, Quattrini A, Taveggia C (2014) Prostaglandin D2 synthase/ GPR44: a signaling axis in PNS myelination. Nat Neurosci 17:1682-1692.
Woodhoo A, Alonso MBD, Droggiti A, Turmaine M, D’Antonio M, Parkinson DB, Wilton DK, Al-Shawi R, Simons P, Shen J, Guillemot F, Radtke F, Meijer D, Feltri ML, Wrabetz L, Mirsky R, Jessen KR (2009) Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat Neurosci 12:839-847.
Woodhoo A, Sommer L (2008) Development of the Schwann cell lineage: from the neural crest to the myelinated nerve. Glia 56:1481-1490.
Xing Z, Chen HC, Nowlen JK, Taylor SJ, Shalloway D, Guan JL (1994) Direct interaction of v-Src with the focal adhesion kinase mediated by the Src SH2 domain. Mol Biol Cell 5:413-421.
Yang DP, Kim J, Syed N, Tung YJ, Bhaskaran A, Mindos T, Mirsky R, Jessen KR, Maurel P, Parkinson DB, Kim HA (2012) p38 MAPK activation promotes denervated Schwann cell phenotype and functions as a negative regulator of Schwann cell di ff erentiation and myelination. J Neurosci 32:7158-7168.
Zhang H, Liu CY, Zha ZY, Zhao B, Yao J, Zhao S, Xiong Y, Lei QY, Guan KL (2009) TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition. J Biol Chem 284:13355-13362.
Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, Liu P, Anders RA, Pan D (2010)e Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell 19:27-38.
Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL (2008) TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 22:1962-1971.
Zhao YL, Takagawa K, Oya T, Yang HF, Gao ZY, Kawaguchi M, Ishii Y, Sasaoka T, Owada K, Furuta I, Sasahara M (2003) Active Src expression is induced aer rat peripheral nerve injury. Glia 42:184-193.
Zhou Y, Notterpek L (2016) Promoting peripheral myelin repair. Exp Neurol 283:573-580.
Valerio Magnaghi, Ph.D., valerio.magnaghi@unimi.it.
10.4103/1673-5374.211172
*< class="emphasis_italic">Correspondence to: Valerio Magnaghi, Ph.D., valerio.magnaghi@unimi.it.
orcid: 0000-0002-6903-7042 (Valerio Magnaghi)
- 中国神经再生研究(英文版)的其它文章
- Mitochondrial quality control in amyotrophic lateral sclerosis: towards a common pathway?
- Umbilical cord: an unlimited source of cells di ff erentiable towards dopaminergic neurons
- SoxC transcription factors in retinal development and regeneration
- Targeting 14-3-3 adaptor protein-protein interactions to stimulate central nervous system repair
- BDNF pro-peptide: a novel synaptic modulator generated as an N-terminal fragment from the BDNF precursor by proteolytic processing
- Impact of glucocorticoid on neurogenesis

