Targeting 14-3-3 adaptor protein-protein interactions to stimulate central nervous system repair
Andrew Kaplan, Alyson E. Fournier
Department of Neurology and Neurosurgery, Montréal Neurological Institute, McGill University, Montréal, Québec, Canada
Targeting 14-3-3 adaptor protein-protein interactions to stimulate central nervous system repair
Andrew Kaplan*, Alyson E. Fournier
Department of Neurology and Neurosurgery, Montréal Neurological Institute, McGill University, Montréal, Québec, Canada
How to cite this article:Kaplan A, Fournier AE (2017) Targeting 14-3-3 adaptor protein-protein interactions to stimulate central nervous system repair. Neural Regen Res 12(7):1040-1043.
Funding: Research in the AEF lab was funded by the Canadian Institutes for Health Research and the Multiple Sclerosis Society of Canada.
axon regeneration; 14-3-3; gcnl, fusicoccin; optic nerve; spinal cord injury
Accepted: 2017-06-19
Introduction
Central nervous system (CNS) axons have a poor capacity for re-growth aer injury.is leads to permanent disruption of neuronal communication and directly underlies paralysis and sensory loss in spinal cord injuries.erapies that induce regrowth of damaged axons or sprouting from intact axons are needed to restore functional connectivity. While genetic manipulations such as phosphatase and tensin homolog (PTEN) knockout have demonstrated that it is possible to elicit substantial axon regeneration and sprouting (Sun et al., 2011), there is now a need for drugs that can be used to repair CNS injuries. Recently, several small molecule drugs including taxol, epothilones, gabapentinoids and statins are raising significant interest in the field for their ability to stimulate axon growth and regeneration (Hellal et al., 2011; Ruschel et al., 2015; Li et al., 2016; Tedeschi et al., 2016). These drugs have well defined safety profiles and could eventually be re-purposed for treatment of CNS injury. The continued discovery and development of drugs with axon growth activity will enhance the potential for the eventual success of a new therapy for spinal cord injury. We recently identified a new pharmacological strategy for enhancing axon growth and regeneration. We discovered that a family of adaptor proteins called 14-3-3s can be targeted with a small molecule called fusicoccin-A to enhance axon regeneration. Here we outline the functions of 14-3-3 proteins and discuss the potential use of fusicoccin-A and related compounds as therapeutic agents to target 14-3-3s to induce CNS repair.
14-3-3 Adaptor Proteins
14-3-3s are a family of regulatory adaptor proteins with highly conserved structure and function from yeast to humans.ey are soluble cytoplasmic proteins that are distinguished by a groove which serves as the canonical binding site for ‘client proteins.’ere are hundreds of 14-3-3 client proteins with diverse functions. 14-3-3s typically bind in a stereotyped fashion at phosphorylated serine/threonine motifs. Binding can have a wide array of consequences including alteration of stability, subcellular localization, activity, and protein-protein-interactions. In humans, there are seven isoforms encoded by unique genes.e residues lining the groove that bind the phospho-peptide of the client protein are identical between isoforms (Kaplan et al., 2017a).e strict conservation of the groove between isoforms means that there is major overlap in client proteins. However, variations in binding affinities for a particular client and differing expression levels of each isoform can result in certain functions that are primarily carried out by speci fi c isoforms.
14-3-3 proteins are ubiquitous, but are most highly expressed in the CNS. 14-3-3s are critical mediators of nervous system development and axon guidance (Kent et al., 2010; Yam et al., 2012).e roles of 14-3-3s in the adult nervous system are not as well characterized. Interestingly, 14-3-3s are transported into the central axonal process of dorsal root ganglion neurons aer conditioning lesions to the peripheral process (Mar et al., 2014). This suggests that 14-3-3s may be involved in promoting central axon regeneration aer a peripheral lesion. Supporting this idea, inhibition of 14-3-3s with a peptide that blocks interactions with client proteins impairs axon growth in multiple neuronal types (Mar et al., 2014; Lavalley et al., 2016; Kaplan et al., 2017b). Moreover, we found that 14-3-3 knockdown impairs, whereas over-expression enhances, axon regrowth aerin vitroinjury of cortical neurons in a scratch assay. Based on these studies, we aimed to develop a pharmacological approach to harness the pro-growth activity of 14-3-3s in neurons.is led to the discovery that stabilization of 14-3-3 adaptor protein-protein interactions with a natural small molecule called fusicoccin-A promotes axon growth and regeneration (Kaplan et al., 2017b).
Fusicoccin-A: Stabilizer of 14-3-3 Protein-Protein Interactions (PPIs)
Fusicoccin-A (FC-A) is a small molecule produced by Phomopsis amygdali fungi that is known to stabilize 14-3-3 interactions with client proteins. FC-A binds to a solvent-exposed hydrophobic pocket created by the docking of a client motif within the 14-3-3 binding groove, simultaneously binding both proteins (Figure 1). Structural and biochemical analyses indicate that FC-A can stabilize a growing repertoire of 14-3-3: client interactions. Many of these include disease-relevant molecules. For instance, FC-A stabilizes 14-3-3 binding to cystic fibrosis transmembrane conductance regulator (CFTR) and promotes its tra ffi cking to the plasma membrane, a key therapeutic strategy for the treatment of cystic fi brosis (Stevers et al., 2016). FC-A derivatives are also known to potentiate the e ff ects of multiple anti-tumor drugs in cancer models (Miyake et al., 2015). Stabilization of 14-3-3 PPIs with FC-A and derivatives is an innovative approach for the development of drugs that may have applications in many indications.
Fusicoccin-A Stimulates Axon Growth and Regeneration
Inhibition of 14-3-3 PPIs impairs neurite outgrowth in cultured neurons (Mar et al., 2014; Lavalley et al., 2016; Kaplan et al., 2017b). We therefore reasoned that stabilization of 14-3-3 PPIs with FC-A might enhance axon growth. We found that FC-A markedly stimulates neurite outgrowth from rodent cortical neurons at the time of plating as well as aer scratch injury in mature cultures. FC-A also stimulated outgrowth from primary human fetal neurons, indicating that this activity is conserved from rodent to human. Suggesting that 14-3-3s are required for this e ff ect, knockdown of 14-3-3s or inhibition of 14-3-3 PPIs with a small molecule inhibitor attenuates FC-A-induced growth (Kaplan et al., 2017b). To test whether FC-A could enhance axon regeneration aerin vivoinjury we used the optic nerve crush model and examined the e ff ect of local intravitreal injections of FC-A on retinal ganglion cell axon regeneration. We found that two injections of FC-A, one immediately after injury and one 7 days later, stimulated axon regeneration up to 0.5-1 mm past the lesion. Optic nerve crush results in massive retinal ganglion cell death in the retina and it is possible that FC-A could influence cell survival. However, we found no difference in retinal ganglion cell density between control and FC-A-treated mice.is suggests that FC-A promotes axon regeneration without improving cell survival. It is interesting to speculate that the e ff ects of FC-A on regeneration in this model could be greatly enhanced by combining the treatment with a pharmacological or genetic blockade of apoptosis.
We also examined whether FC-A can promote axonal repair in the spinal cord using the dorsal hemisection model and assessing the corticospinal axon tract. Immediately aer injury, we locally applied FC-A in a rapidly-polymerizing fi brin adhesive onto the injury site. C3 transferase, a protein inhibitor of RhoA in clinical trials under the trade-name Cethrin, has been applied in the hemisection model using a similar method of administration, resulting in axon regeneration distal to the lesion (Dergham et al., 2002). We found that FC-A reduced axonal die-back, but not regeneration past the lesion. Because FC-A is a small molecule, it is likely that it readily di ff used from the fi brin gel and that the duration of exposure directly at the injury site was quite shortlived. Future studies could utilize osmotic mini-pumps to continuously deliver the compound intrathecally or it could be administered by repeated injectionsviaa systemic route. In xenogratumor models in mice, FC-A derivatives have been administered systemically at doses that were e ff ective in reducing tumor burden without adverse events or renal or hepatic toxicity (Kawakami et al., 2012).is suggests that systemic treatment with FC-A might be a safe and e ff ective approach to repair axonal damage. However, it is currentlyunknown whether these compounds can cross the bloodbrain barrier. Further analysis of the pharmacokinetics of these compounds will be required to answer these questions. Because these compounds target 14-3-3, a fundamental regulator of axonal outgrowth, it is possible that they could be e ff ective in stimulating repair in the context of other injuries and diseases of the CNS, including stroke and neurodegeneration.
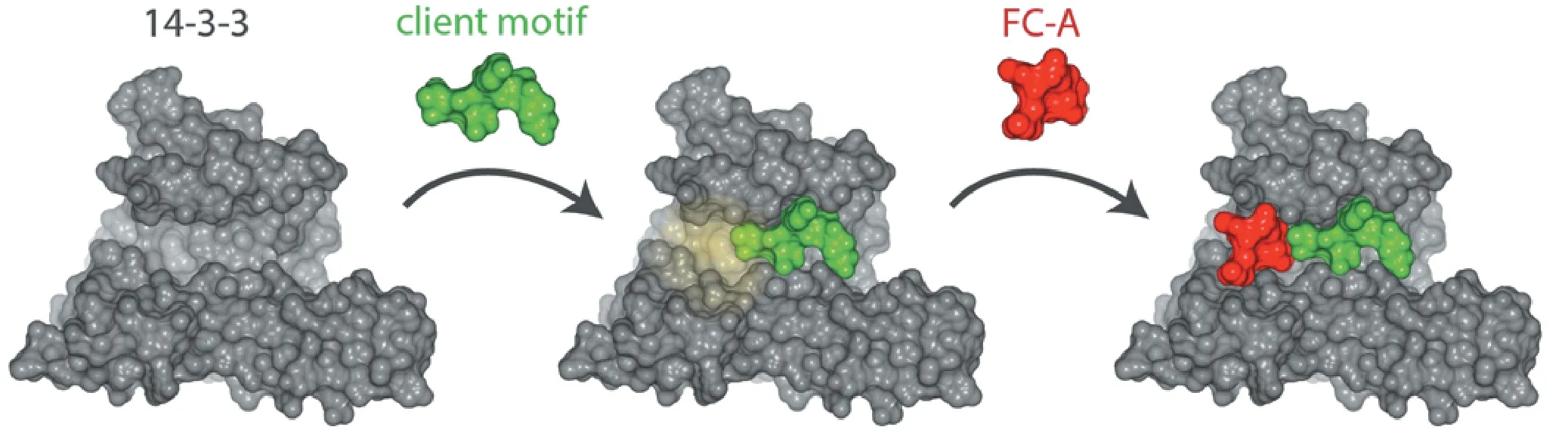
Figure 1 Fusicoccin-A (FC-A) binds to interface of 14-3-3 and a client motif docked within groove.
An important question that remains is how FC-A might affect other cell types involved in response to CNS injury. For instance, FC-A has been shown to induce cytoskeletal reorganization in an astrocytoma cell line (Bury et al., 2013). Cotylenin-A, a closely related compound, has been shown to induce differentiation in myeloid leukemia cell lines (Asahi et al., 1997).is suggests the possibility that FC-A could affect the astrocytic and inflammatory responses to injury. Our results with isolated cortical neurons indicate that the e ff ect on axon outgrowth is cell autonomous, however it is also possible that the compound could influence axon regeneration by a ff ecting other cell types in the lesion environment.e e ff ect of FC-A on secondary events aer injury including astrocytic scar formation, inflammation and re-myelination remains to be examined. It is interesting to speculate that targeting 14-3-3, a master regulator of cell signaling, could yield beneficial effects through actions on multiple cell types.
FC-A Stimulates Axon Growth through a Mechanism Involving GCN1 Downregulation
The mechanisms underlying the effects of FC-A on axon growth remain unclear, as the extent and identity of 14-3-3 PPIs that are acted on by FC-A are unknown. In an e ff ort to identify 14-3-3:client complexes that might be targeted by FC-A to induce axon growth, we identi fi ed a regulator of translation called GCN1. In yeast, GCN1 has a well-characterized role in shutting down global translation in response to multiple sources of stress by inducing eukaryotic initiation factor 2 (eIF2) phosphorylationviaactivation of its kinase GCN2. Although a similar mechanism is thought to occur in mammalian cells, the role of GCN1 is less clear (Castilho et al., 2014). We found that GCN1 binding to 14-3-3 is stabilized by FC-A and that this induces GCN1 turnover in neurons. Suggesting a role in FC-A-induced neurite outgrowth, loss of GCN1 enhanced basal growth and occluded a further increase by a low dose of FC-A. However, a higher dose of FC-A persisted in stimulating growth from GCN1-depleted neurons, suggesting the involvement of additional mechanisms. Intriguingly, previous work has shown that a protein called IMPACT which competes with GCN2 to bind GCN1, promotes neurite outgrowth (Ro ff e et al., 2013). Together this supports the idea that antagonism of GCN1 may trigger axon regeneration. Previously it has been shown that axonal injury in the CNS induces a global downregulation of pro-growth signaling molecules. It has been suggested that this accounts for failed regeneration in the CNS (Belin et al., 2015). Stress response pathways such as GCN1-GCN2 could be manipulated with drugs to counteract this downregulation of growth molecules. Further characterization of the molecular players in these stress pathways and their e ff ects on axon regeneration will be required to validate this approach.
14-3-3 PPIs as Druggable Targets
There is no indication that systemic targeting of 14-3-3s with FC-A-related compounds has any toxicity in rodents at efficacious doses in tumor models (Kawakami et al., 2012). However, because 14-3-3 proteins are ubiquitously expressed, it is desirable to identify which 14-3-3 PPIs are specifically involved in axon regeneration and develop compounds that can selectively target these PPIs. Individual 14-3-3:client complexes like 14-3-3:GCN1 may play more tissue-restricted roles, reducing the potential for side e ff ects. Extensive structural and biochemical analyses with FC-A have provided strong proof-of-concept for the druggability of the pocket formed by 14-3-3:client complexes and compounds can be tailored to target specific clients. FC-A is a starting point for such therapeutics. For instance, a semi-synthetic derivative of FC-A has been developed that is speci fi c for a pocket created when 14-3-3 binds to client protein only at C-terminal motifs (Anders et al., 2013). Because a total synthesis of FC-A has not been completed, efforts have also been made to identify synthetic 14-3-3:client stabilizers. A high-throughput screen to identify compounds that could stabilize a 14-3-3:peptide interaction resulted in the identi fi cation of two structurally distinct molecules, epibestatin and pyrrolidone 1. Crystal structures of these compounds bound to the pocket created by 14-3-3 and the client peptide showed unique modes of interaction that underlie their ability to enhance the stability of the complex (Rose et al., 2010).ese studies provided proof-of-concept that 14-3-3:client interfaces are druggable with synthetic small molecules. Further optimization of FC-A or the development of client-specific small molecules could provide new leads for the development of drugs to repair damaged CNS axons.
Conclusion
14-3-3 adaptor proteins play critical roles in axon outgrowth.ese multi-functional proteins are key regulators of many client proteins. FC-A stabilizes multiple 14-3-3 complexes and stimulates axon regeneration. The stereotyped fashion in which 14-3-3s bind to their client proteins in a conserved groove offers the opportunity to develop drugs that can target specific 14-3-3:client complexes. We have identified GCN1 as one client protein that is important for the axon outgrowth activity of FC-A, suggesting that this could serve as a more specific target. Further study into the mechanisms underlying the e ff ect of FC-A on axon growth could yield additional molecular players involved in this process. Subsequent chemical modification of FC-A could result in client-speci fi c drugs. Such drugs could be used to target 14-3-3 PPIs with speci fi c roles in axon growth and could be the basis of a new approach to treat CNS injury.
Author contributions:AK and AEF wrote the manuscript.
Con fl icts of interest:None declared.
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and signi fi cance of this paper.
Open peer review reports:
Reviewer 1:Teresa Caprile, Universidad de Concepcion, Chile.
Comments to author:e authors of this manuscript have discussed the role of fusicoccin-A on stabilization of 14-3-3 adaptor protein-protein interactions.e paper is interesting although I would suggest to give more detail about the e ff ect of this molecule during axon regeneration (Kaplan et al., 2017).
Reviewer 2: Denis S. Barry, University of Dublin Trinity College, Ireland. Comments to author:e article is to the point in parts, and contains new information that will be of interest to readers of the fi eld. See additional fi le for more details.
Additional fi le: Open peer review report 2.
Anders C, Higuchi Y, Koschinsky K, Bartel M, Schumacher B,iel P, Nitta H, Preisig-Muller R, Schlichthorl G, Renigunta V, Ohkanda J, Daut J, Kato N, Ottmann C (2013) A semisynthetic fusicoccane stabilizes a protein-protein interaction and enhances the expression of K+ channels at the cell surface. Chem Biol 20:583-593.
Asahi K, Honma Y, Hazeki K, Sassa T, Kubohara Y, Sakurai A, Takahashi N (1997) Cotylenin A, a plant-growth regulator, induces the differentiation in murine and human myeloid leukemia cells. Biochem Biophys Res Commun 238:758-763.
Belin S, Nawabi H, Wang C, Tang S, Latremoliere A, Warren P, Schorle H, Uncu C, Woolf CJ, He Z, Steen JA (2015) Injury-induced decline of intrinsic regenerative ability revealed by quantitative proteomics. Neuron 86:1000-1014.
Bury M, Andol fi A, Rogister B, Cimmino A, Megalizzi V, Mathieu V, Feron O, Evidente A, Kiss R (2013) Fusicoccin a, a phytotoxic carbotricyclic diterpene glucoside of fungal origin, reduces proliferation and invasion of glioblastoma cells by targeting multiple tyrosine kinases. Transl Oncol 6:112-123.
Castilho BA, Shanmugam R, Silva RC, Ramesh R, Himme BM, Sattlegger E (2014) Keeping the eukaryotic initiation factor 2 (eIF2) alpha kinase Gcn2 in check. Biochim Biophys Acta 1843:1948-1968.
Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L (2002) Rho signaling pathway targeted to promote spinal cord repair. J Neurosci 22:6570-6577.
Hellal F, Hurtado A, Ruschel J, Flynn KC, Laskowski CJ, Umlauf M, Kapitein LC, Strikis D, Lemmon V, Bixby J, Hoogenraad CC, Bradke F (2011) Microtubule stabilization reduces scarring and causes axon regeneration aer spinal cord injury. Science 331:928-931.
Kaplan A, Bueno M, Fournier AE (2017a) Extracellular functions of 14-3-3 adaptor proteins. Cell Signal 31:26-30.
Kaplan A, Morquette B, Kroner A, Leong S, Madwar C, Sanz R, Banerjee SL, Antel J, Bisson N, David S, Fournier AE (2017b) Small-molecule stabilization of 14-3-3 protein-protein interactions stimulates axon regeneration. Neuron 93:1082-1093 e1085.
Kawakami K, Hattori M, Inoue T, Maruyama Y, Ohkanda J, Kato N, Tongu M, Yamada T, Akimoto M, Takenaga K, Sassa T, Suzumiy J, Honma Y (2012) A novel fusicoccin derivative preferentially targets hypoxic tumor cells and inhibits tumor growth in xenogras. Anticancer Agents Med Chem 12:791-800.
Kent CB, Shimada T, Ferraro GB, Ritter B, Yam PT, McPherson PS, Charron F, Kennedy TE, Fournier AE (2010) 14-3-3 proteins regulate protein kinase a activity to modulate growth cone turning responses. J Neurosci 30:14059-14067.
Lavalley NJ, Slone SR, Ding H, West AB, Yacoubian TA (2016) 14-3-3 Proteins regulate mutant LRRK2 kinase activity and neurite shortening. Hum Mol Genet 25:109-122.
Li H, Kuwajima T, Oakley D, Nikulina E, Hou J, Yang WS, Lowry ER, Lamas NJ, Amoroso MW, CroGF, Hosur R, Wichterle H, Sebti S, Filbin MT, Stockwell B, Henderson CE (2016) Protein prenylation constitutes an endogenous brake on axonal growth. Cell Rep 16:545-558.
Mar FM, Simoes AR, Leite S, Morgado MM, Santos TE, Rodrigo IS, Teixeira CA, Misgeld T, Sousa MM (2014) CNS axons globally increase axonal transport after peripheral conditioning. J Neurosci 34:5965-5970.
Miyake T, Honma Y, Urano T, Kato N, Suzumiya J (2015) Combined treatment with tamoxifen and a fusicoccin derivative (ISIR-042) to overcome resistance to therapy and to enhance the antitumor activity of 5- fl uorouracil and gemcitabine in pancreatic cancer cells. Int J Oncol 47:315-324.
Ro ff e M, Hajj GN, Azevedo HF, Alves VS, Castilho BA (2013) IMPACT is a developmentally regulated protein in neurons that opposes the eukaryotic initiation factor 2alpha kinase GCN2 in the modulation of neurite outgrowth. J Biol Chem 288:10860-10869.
Rose R, Erdmann S, Bovens S, Wolf A, Rose M, Hennig S, Waldmann H, Ottmann C (2010) Identi fi cation and structure of small-molecule stabilizers of 14-3-3 protein-protein interactions. Angew Chem Int Ed Engl 49:4129-4132.
Ruschel J, Hellal F, Flynn KC, Dupraz S, Elliott DA, Tedeschi A, Bates M, Sliwinski C, Brook G, Dobrindt K, Peitz M, Brustle O, Norenberg MD, Blesch A, Weidner N, Bunge MB, Bixby JL, Bradke F (2015) Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration aer spinal cord injury. Science 348:347-352.
Stevers LM, Lam CV, Leysen SF, Meijer FA, van Scheppingen DS, de Vries RM, Carlile GW, Milroy LG, Thomas DY, Brunsveld L, Ottmann C (2016) Characterization and small-molecule stabilization of the multisite tandem binding between 14-3-3 and the R domain of CFTR. Proc Natl Acad Sci U S A 113:E1152-1161.
Sun F, Park KK, Belin S, Wang D, Lu T, Chen G, Zhang K, Yeung C, Feng G, Yankner BA, He Z (2011) Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature 480:372-375.
Tedeschi A, Dupraz S, Laskowski CJ, Xue J, Ulas T, Beyer M, Schultze JL, Bradke F (2016)e calcium channel subunit alpha2delta2 suppresses axon regeneration in the adult CNS. Neuron 92:419-434.
Yam PT, Kent CB, Morin S, Farmer WT, Alchini R, Lepelletier L, Colman DR, Tessier-Lavigne M, Fournier AE, Charron F (2012) 14-3-3 proteins regulate a cell-intrinsic switch from sonic hedgehog-mediated commissural axon attraction to repulsion aer midline crossing. Neuron 76:735-749.
Andrew Kaplan, Ph.D., Andrew.kaplan@mail.mcgill.ca.
10.4103/1673-5374.211176
*< class="emphasis_italic">Correspondence to: Andrew Kaplan, Ph.D., Andrew.kaplan@mail.mcgill.ca.
orcid: 0000-0002-9336-4369 (Andrew Kaplan)
- 中国神经再生研究(英文版)的其它文章
- Mitochondrial quality control in amyotrophic lateral sclerosis: towards a common pathway?
- Umbilical cord: an unlimited source of cells di ff erentiable towards dopaminergic neurons
- SoxC transcription factors in retinal development and regeneration
- RACK1 regulates neural development
- Schwann cell development, maturation and regeneration: a focus on classic and emerging intracellular signaling pathways
- BDNF pro-peptide: a novel synaptic modulator generated as an N-terminal fragment from the BDNF precursor by proteolytic processing

