Di ff usion-weighted magnetic resonance imaging re fl ects activation of signal transducer and activator of transcription 3 during focal cerebral ischemia/ reperfusion
Wen-juan Wu, Chun-juan Jiang Zhui-yang Zhang Kai Xu, Wei Li
1 Department of Radiology, Nanjing Medical Unversity A ffi liated Wuxi Second People’s Hospital, Wuxi, Jiangsu Province, China
2 Department of Radiology, A ffi liated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu Province, China
Di ff usion-weighted magnetic resonance imaging re fl ects activation of signal transducer and activator of transcription 3 during focal cerebral ischemia/ reperfusion
Wen-juan Wu1,2, Chun-juan Jiang1, Zhui-yang Zhang1, Kai Xu2, Wei Li1,*
1 Department of Radiology, Nanjing Medical Unversity A ffi liated Wuxi Second People’s Hospital, Wuxi, Jiangsu Province, China
2 Department of Radiology, A ffi liated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu Province, China
How to cite this article:Wu WJ, Jiang CJ, Zhang ZY, Xu K, Li W (2017) Di ff usion-weighted3 magnetic resonance imaging re fl ects activation of signal transducer and activator of transcription 3 during focal cerebral ischemia/reperfusion. Neural Regen Res 12(7):1124-1130.
Graphical Abstract
orcid: 0000-0002-3808-4399 (Wei Li)
Signal transducer and activator of transcription (STAT) is a unique protein family that binds to DNA, coupled with tyrosine phosphorylation signaling pathways, acting as a transcriptional regulator to mediate a variety of biological e ff ects. Cerebral ischemia and reperfusion can activate STATs signaling pathway, but no studies have con fi rmed whether STAT activation can be veri fi ed by di ff usion-weighted magnetic resonance imaging (DWI) in rats aer cerebral ischemia/reperfusion. Here, we established a rat model of focal cerebral ischemia injury using the modi fi ed Longa method. DWI revealed hyperintensity in parts of the lehemisphere before reperfusion and a low apparent di ff usion coe ffi cient. STAT3 protein expression showed no signi fi cant change aer reperfusion, but phosphorylated STAT3 expression began to increase after 30 minutes of reperfusion and peaked at 24 hours. Pearson correlation analysis showed that STAT3 activation was correlated positively with the relative apparent di ff usion coe ffi cient and negatively with the DWI abnormal signal area.ese results indicate that DWI is a reliable representation of the infarct area and re fl ects STAT phosphorylation in rat brain following focal cerebral ischemia/reperfusion.
nerve regeneration; cerebral ischemia/reperfusion; magnetic resonance imaging; di ff usion weighted imaging; signal transducer and activator of transcription 3; phosphorylated signal transducer and activator of transcription 3; apparent di ff usion coe ffi cient; relative apparent di ff usion coe ffi cient; immunohistochemistry; western blot assay; neural regeneration
Introduction
Cerebral ischemia/reperfusion injury is an important pathophysiological process that underlies cerebrovascular disease. Magnetic resonance imaging (MRI) can reveal ischemic brain tissue. In hyperacute cerebral infarction (< 6 hours), the infarcted area can be seen in diffusion-weighted MRI (DWI), and magnetic resonance perfusion imaging can show the location and extent of the ischemic zone at around 10 minutes (Beck et al., 2014).
Cerebral ischemia/reperfusion can activate signal transducers and activators of transcription (STATs) (Li et al., 2015b).e Janus kinase (JAK)-STAT pathway is activated after cerebral ischemia. Membrane receptor signaling by various ligands induces activation of JAK kinases, which then leads to tyrosine phosphorylation of various STAT transcription factors (Kim et al., 2017). STAT1 and STAT3 are members of the STAT family, and phosphorylated (p-) STAT3 is the activated form of STAT3 (Jia et al., 2017). These proteins play an important role in neuronal survival and antiapoptosis. p-STAT3 is a mediator of growth factors, hormones and cytokines, and exerts its protective and regenerative effects in cerebral ischemia/reperfusion partly through transcriptional upregulation of neuroprotective and neurotrophic genes (Jiang et al., 2012). In the present study, we analyzed the changes in DWI, STAT3 and p-STAT3 in the ischemic injury zone in a rat model of focal cerebral ischemia/reperfusion injury.
Materials and Methods
Animals
A total of 110 healthy male Sprague-Dawley rats, 45–60 days old and weighing 290–330 g, were provided by the Animal Center of Xuzhou Medical University, Jiangsu Province, China. The rats were randomized into three groups: sham (n= 10), 2-hour ischemia (n= 50), and 6-hour ischemia (n= 50). Rats in the ischemia groups underwent 2- or 6-hour ischemia followed by reperfusion for 0, 0.5, 2, 6, or 24 hours (n= 10 rats per time point). All rats were housed under diurnal lighting and had free access to food and water before the experiments. The protocols were approval by the Committee on Animal Experimental Guidelines of the A ffi liated Hospital of Xuzhou Medical University (XZMU-A201204-057R).
Focal cerebral ischemia injury modeling
A rat model of unilateral middle cerebral artery occlusion was established using the modi fi ed Longa method (Longa et al., 1989). Rats were anesthetized intraperitoneally with 10% chloral hydrate (3 mL/kg).e lecommon, external, and internal carotid arteries were exposedviaan incision in the neck and separated under a surgical microscope. The external carotid artery was ligated 0.8–1.0 cm from the common carotid artery, and the internal carotid artery was occluded. A 3-0 surgical mono fi lament nylon suture, blunted at the end, was gently inserted into the internal carotid artery through a small incision at the bifurcation. When the thread was extended 17–19 mm from the bifurcation and a slight resistance was felt, this indicated that the thread had been inserted into the origin of the middle cerebral artery at the circle of Willis, blocking blood fl ow in the middle cerebral artery trunk.e thread was ligated with a slipknot in the internal carotid artery, and the incision was sutured.e rats were returned to their cages with food and water and allowed to recover. Body temperature was maintained near 37°C using a heat pad. Aer 2 or 6 hours, the thread was withdrawn by approximately 10 mm to begin reperfusion. For the sham group, the procedure was identical except the thread was only inserted to a depth of 5 mm.
MRI scan
3.0 T MRI examination (Signa HD 3.0, GE Healthcare, Chicago, IL, USA) was performed at various time points aer ischemia/perfusion. A rat coil (Chenguang Medical Technology Co., Ltd., Shanghai, China) was used as the receiver coil.e rats were anesthetized by an intraperitoneal injection of 10% chloral hydrate.eir heads were then placed in the center of the coil in the prone position. Echo planar imaging was used with the following parameters: repetition time, 6,800 ms; echo time, 93 ms; field of view, 8 cm × 6 cm; matrix, 64 × 64; number of excitations, 2; thickness, 2.4 mm; slice gap, 0.2 mm. Aer the scan, DWI data were transmitted to the workstation for postprocessing to obtain apparent diffusion coefficient (ADC) profiles. The relative ADC (rADC) for the abnormal signal area on a slice with marked ischemia in the region of interest (ROI) was calculated as follows: rADC = ADCROI/ ADCcontralateral× 100%.e ratio of the DWI abnormal signal area (rS-DWI) on the selected slice (with marked ischemia) to that in the wholebrain slice area was calculated. Brains were removed aer scanning.
Immunohistochemistry
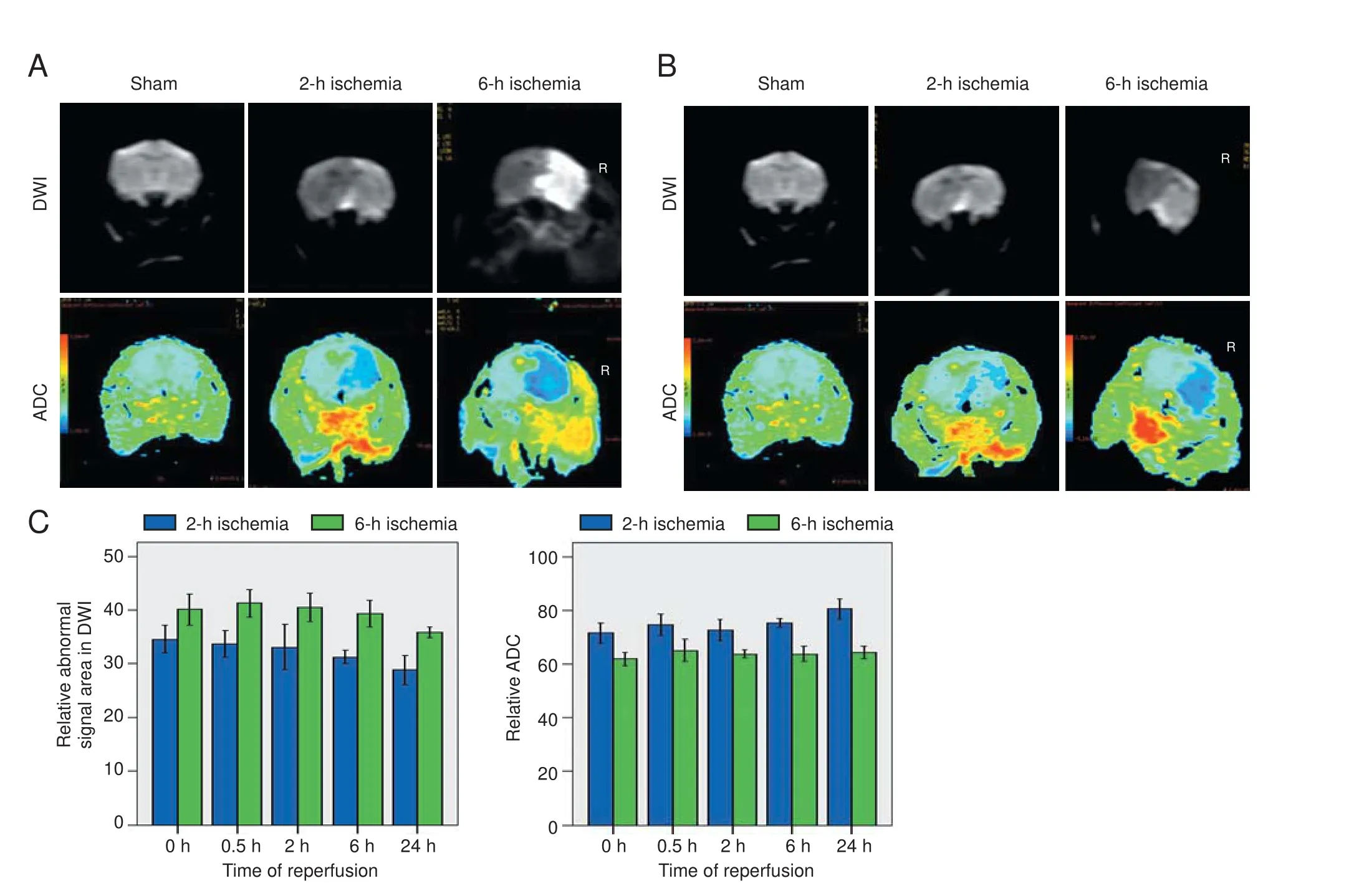
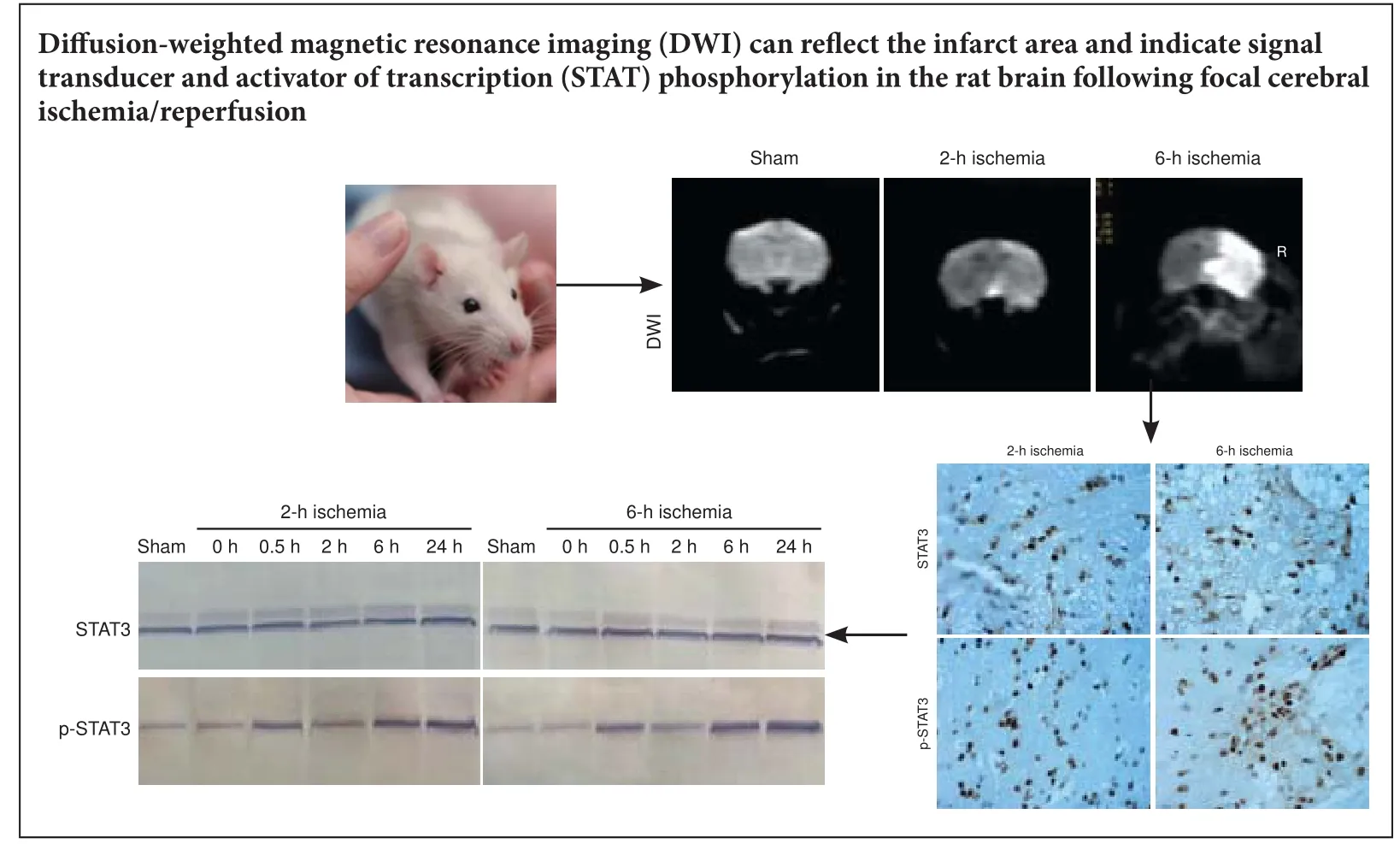
Figure 1 Brain di ff usion-weighted magnetic resonance imaging (DWI) and apparent di ff usion coeffi cient (ADC) in rat models of focal cerebral ischemia/reperfusion injury.

Figure 2 Immunohistochemical staining of STAT3 and p-STAT3 in the brain 24 h aer reperfusion (× 400).
Western blot assay
Statistical analysis
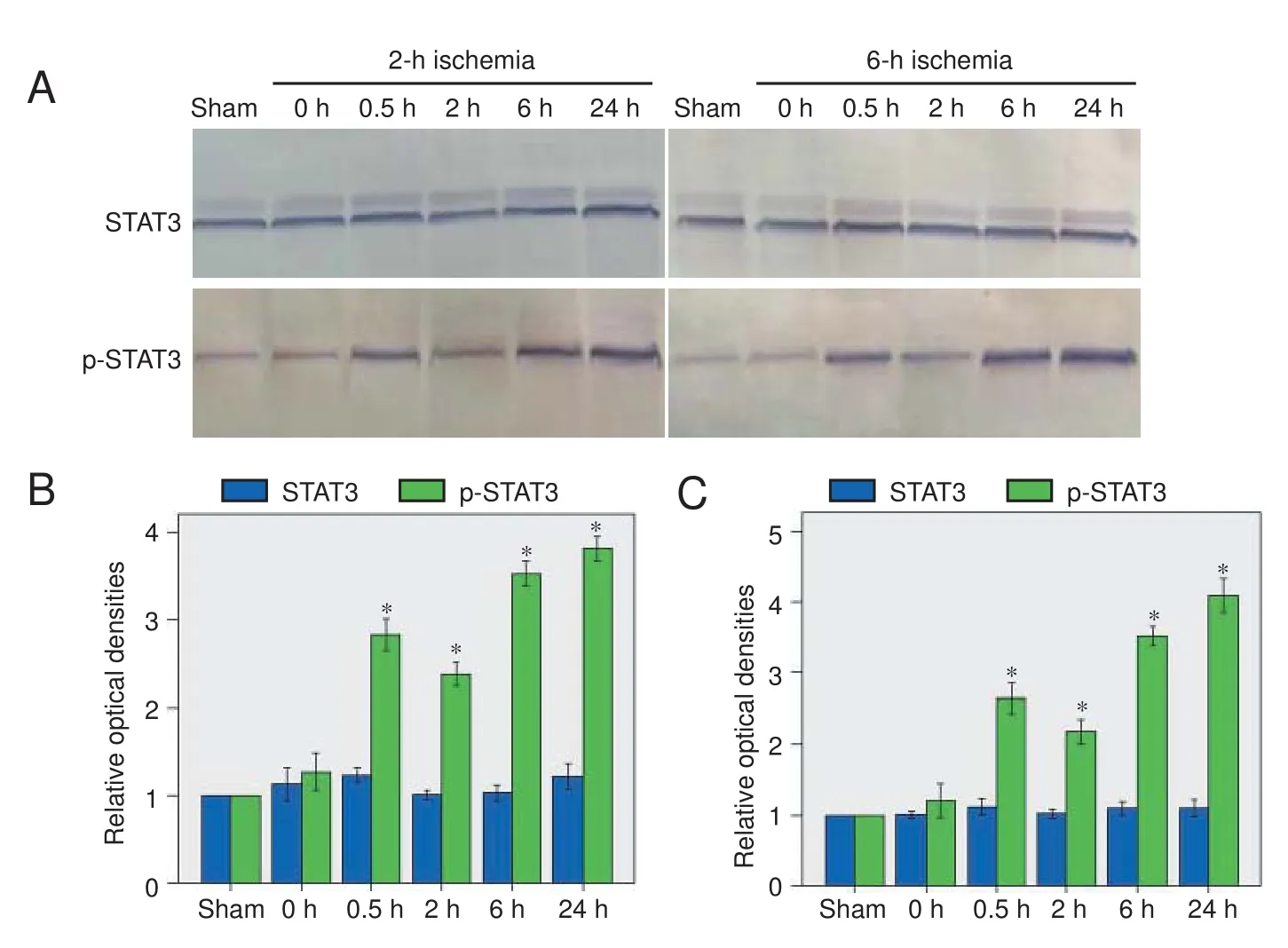
Figure 3 Changes in STAT3 expression and activation in the brain aer acute cerebral ischemia/reperfusion.
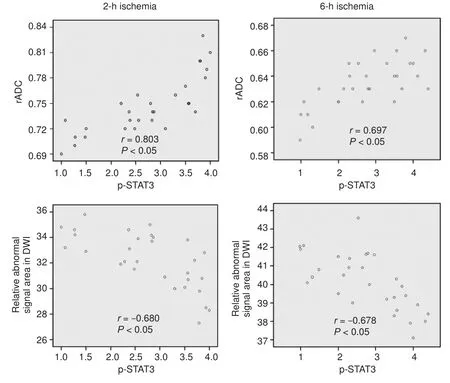
Figure 4 Correlation of phosphorylated signal transducer and activator of transcription 3 (p-STAT3) with relative apparent di ff usion coeffi cient (rADC) and relative abnormal signal area in di ff usion-weighted magnetic resonance imaging at di ff erent reperfusion time points.
SPSS 16.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Experimental data are expressed as the mean ± SD. One-way analysis of variance was used to compare DWI and ADC before and aer cerebral ischemia/ reperfusion, and STAT3 and p-STAT3 expression.e relationship between p-STAT3 expression and rADC or rS-DWI was analyzed by Pearson correlation analysis.P< 0.05 was considered statistically signi fi cant.
Results
DWI in rat brain before and aer acute cerebral ischemia/ reperfusion
In the sham group, DWI fi ndings were as expected, and ADC values were similar across both cerebral hemispheres. In both ischemia groups, before reperfusion, DWI showed patchy hyperintensity in the right corpus striatum and frontoparietal cortex, and ADC pseudocolor images showed an abnormal blue signal with blurred edges.e 6-hour ischemia group had larger rS-DWI and smaller ADC values than the 2-hour ischemia group (Figure 1). In the 2-hour ischemia group, aer 24 hours of reperfusion, the rS-DWI was signi fi cantly smaller than before reperfusion, and the ADC was partially restored. Aer 24 hours of reperfusion in the 6-hour group, the rS-DWI was slightly lower than before reperfusion, and the ADC value was slightly greater (Figure 1).
Changes in STAT3 expression and activation aer acute cerebral ischemia/reperfusion
Immunohistochemistry showed that p-STAT3-positive cells were rarely expressed in brain tissue from sham-operated rats, but in rats with ischemia, expression in the ischemic area increased with reperfusion time.e positive cells were circular or oval, and mainly astrocytes, followed in number by neurons. Aer 24 hours of reperfusion, there was neural nuclear condensation, cell body shrinkage and deformation, and larger astrocytes with abundant cytoplasm (Figure 2).
In the western blot assay, a low level of p-STAT3 was detected in brain tissue from sham-operated rats. In rats with ischemia, expression increased significantly after 0.5 hours of reperfusion, decreased slightly aer 2 hours, and peaked at 24 hours (Figure 3).
Correlation between p-STAT3 expression and DWI
Data from different time points after reperfusion showed that p-STAT3 expression was positively correlated with rADC (2-hour ischemia group:r= 0.803,P< 0.05; 6-hour ischemia group:r= 0.697,P< 0.05) and negatively correlated with the rS-DWI (2-hour ischemia group:r= −0.680,P<0.05; 6-hour ischemia group:r= −0.678,P< 0.05) (Figure 4).
Discussion
Semi-quantitative western blot assay revealed that STAT3 protein expression did not change signi fi cantly with di ff erent reperfusion periods following ischemia for 2 or 6 hours. By contrast, p-STAT3 expression increased gradually, whereas it was barely detectable in the sham group.e increase in p-STAT3 levels aer 0.5 hours of reperfusion may be related to the involvement of STAT3, c-Fos, and c-Jun in the transcriptional regulation of immediate early genes in neurons (Amantea et al., 2011).e signi fi cant increase in p-STAT3 levels after 24 hours of reperfusion may be related to ATP depletion during ischemia and significantly increased ATP levels after reperfusion; conversely, a marked proliferation of reactive glial cells and microglia was induced by ischemic brain damage, and increased cytokines and growth factors were released as reperfusion continued.
The basic pathology of the rS-DWI is that Na+/K+–ATP enzyme pump function is reduced due to ischemia and hypoxia, which leads to sodium retention and consequent cytotoxic edema, resulting in slowed molecular diffusion; this is demonstrated by the low ADC and DWI hyperintensity (Kim et al., 2006; Cereda et al., 2015; Lago et al., 2015; Song et al., 2015; Aoki et al., 2016; Freitag et al., 2016; Grams et al., 2016; Jiang et al., 2016; Kaseka et al., 2016; Kohno et al., 2016; Kvistad et al., 2016; Onofrj et al., 2016; Tamura et al., 2016; Xin and Han, 2016; Zhang et al., 2016; Zhou et al., 2016; Abdelgawad et al., 2017; Bekiesinska-Figatowska et al., 2017; Heiss and Zaro Weber, 2017). The decrease in ADC was highly consistent with the ATP-labeled defect area and decreased tissue pH area; and the decrease in ADC in ischemic brain damage was consistent with the level of cytotoxic edema caused by cell energy metabolism disorders (Anticoli et al., 2015; Baron et al., 2015; Brown et al., 2015; Eom et al., 2015; Gory et al., 2015; Kate et al., 2015; Landais, 2015; Li et al., 2015a; Makin et al., 2015; Mawet et al., 2015; Michałowska et al., 2015; Odland et al., 2015; Ostwaldt et al., 2015; Sasai et al., 2015; Yaghi et al., 2015). As infarction time increases (> 24 hours), there are corresponding increases in vasogenic edema, extracellular space water, di ff usion speed, and ADC values (Maruyama et al., 2015).
As the JAK-STAT signaling pathway is activated during cerebral ischemia, JAK1 expression in cortical pyramidal neurons and striatal cells increases, and STAT3 nuclear translocation also increases, in rats with focal cerebral ischemia/reperfusion injury; this results in extensive proliferation of reactive microglia and macrophages (Li and Zhang, 2003; Zechariah et al., 2010; Jiang et al., 2013; Feng et al., 2015; Jung et al., 2015; Deng et al., 2016). Previous studies have demonstrated that the gp130-STAT signaling pathway could be activated by the nuclear translocation of STAT3, and that this activation in astrocytes correlates closely with gp130 expression (Jang et al., 2014; Song et al., 2014; Xu et al., 2015; Zhang et al., 2015; Guo et al., 2016). Selection of the samples used in the present experiment was based on MRI fi ndings. STAT3 activation levels before and aer ischemia/reperfusion correlated with the rS-DWI in the ischemic region and rADC. This finding indicated that with aggravation of cytotoxic and vasogenic edema, thestatus of some membrane channels changed, which a ff ected certain cell signaling pathways and STAT3 activation.is correlation helps to identify biochemical changes of JAKSTAT signaling in brain tissue aer ischemia/reperfusion. Further research is needed to determine whether blocking STAT3 phosphorylation could prevent neuronal necrosis or apoptosis due to ischemia to achieve neuroprotective e ff ects and minimize ischemia/reperfusion injury, and whether it could be re fl ected in MRI.is correlation also provides a theoretical and experimental basis for the clinical treatment of cerebral ischemia.
Author contributions:WJW provided and analyzed data and wrote the paper. CJJ and KX participated in study conception and design, data analysis, statistical analysis, and provided technical or material support. ZZY guided the revision. WL was in charge of paper authorization and served as a principle investigator. All authors performed the experiments and approved the fi nal version of the paper.
Con fl icts of interest:
Research ethics:
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and signi fi cance of this paper.
Abdelgawad EA, Higazi MM, Abdelbaky AO, Abdelghany HS (2017) Diagnostic performance of CT cerebral blood volume colour maps for evaluation of acute infarcts; comparison with di ff usion-weighted MRI within 12hours of major stroke onset. J Neuroradiol 44:10-16.
Amantea D, Tassorelli C, Russo R, Petrelli F, Morrone LA, Bagetta G, Corasaniti MT (2011) Neuroprotection by leptin in a rat model of permanent cerebral ischemia: e ff ects on STAT3 phosphorylation in discrete cells of the brain. Cell Death Dis 2:e238.
Anticoli S, Pezzella FR, Pozzessere C, Gallelli L, Bravi MC, Caso V, Siniscalchi A (2015) Transient ischemic attack fast-track and longterm stroke risk: role of di ff usion-weighted magneticresonance imaging. J Stroke Cerebrovasc Dis 24:2110-2116.
Aoki J, Sakamoto Y, Kimura K (2016) Intravenous thrombolysis increases the rate of dramatic recovery in patients with acute stroke with an unknown onset time and negative FLAIR MRI. J Neuroimaging 26:414-419.
Baron CA, Kate M, Gioia L, Butcher K, Emery D, Budde M, Beaulieu C (2015) Reduction of di ff usion-weighted imaging contrast of acute ischemic stroke at short di ff usion times. Stroke 46:2136-2141.
Beck C, Kruetzelmann A, Forkert ND, Juettler E, Singer OC, Köhrmann M, Kersten JF, Sobesky J, Gerlo ff C, Fiehler J, Schellinger PD, Röther J, Thomalla G (2014) A simple brain atrophy measure improves the prediction of malignant middle cerebral artery infarction by acute DWI lesion volume. J Neurol 261:1097-1103.
Bekiesinska-Figatowska M, Duczkowska A, Szkudlinska-Pawlak S, Duczkowski M, Madzik J, Cabaj A, Krupa K, Peczkowski P, Bragoszewska H (2017) Diffusion restriction in the corticospinal tracts and the corpus callosum in neonates aer cerebral insult. Brain Dev 39:203-210.
Brown TA, Luby M, Shah J, Giannakidis D, Latour LL (2015) Magnetic resonance imaging in acute ischemic stroke patients with mild symptoms: an opportunity tostandardize intravenous thrombolysis. J Stroke Cerebrovasc Dis 24:1832-1840.
Cereda CW, Christensen S, Campbell BC, Mishra NK, Mlynash M, Levi C, Straka M, Wintermark M, Bammer R, Albers GW, Parsons MW, Lansberg MG (2015) A benchmarking tool to evaluate computer tomography perfusion infarct core predictions against a DWI standard. J Cereb Blood Flow Metab 36:1780-1789.
Deng R, Zhao FY, Zhang L, Li DY, Mu DZ (2016) Role of STAT3 signaling pathway in hypoxic-ischemic brain damage of neonatal rats. Zhongguo Dang Dai Er Ke Za Zhi 18:78-84.
Eom KS, Kim DW, Kang S (2015) Emergency microsurgical embolectomy in acute ischemic stroke with di ff usion-negative MRI. Neurol Neurochir Pol 49:432-435.
Feng Q, Wang YI, Yang Y (2015) Neuroprotective e ff ect of interleukin-6 in a rat model of cerebral ischemia. Exper Med 9:1695-1701.
Freitag MT, Bickelhaupt S, Ziener C, Meier-Hein K, Radtke JP, Mosebach J, Kuder TA, Schlemmer HP, Laun FB (2016) Selected clinically established and scienti fi c techniques of di ff usion-weighted MRI. In the context of imaging in oncology. Radiologe 56:137-147.
Gory B, Sivan-Hoffmann R, Riva R, Labeyrie PE, Eldesouky I, Sadeh-Gonike U, Signorelli F, Turjman F (2015) DWI lesions reversal in posterior circulation stroke aer reperfusion: Two illustrative cases and review of the literature. J Neuroradiol 42:184-187.
Grams RW, Kidwell CS, Doshi AH, Drake K, Becker J, Coull BM, Nael K (2016) Tissue-negative transient ischemic attack: is there a role for perfusion MRI? Am J Roentgenol 207:157-162.
Guo H, Zhou H, Lu J, Qu Y, Yu D, Tong Y (2016) Vascular endothelial growth factor: an attractive target in the treatment of hypoxic/ischemic brain injury. Neural Regen Res 11:174-179.
Heiss WD, Zaro Weber O (2017) Validation of MRI determination of the penumbra by PET measurements in ischemic stroke. J Nucl Med 58:187-193.
Hoffmann CJ, Harms U, Rex A, Szulzewsky F, Wolf SA, Grittner U, Lättig-Tünnemann G, Sendtner M, Kettenmann H, Dirnagl U, Endres M, Harms C (2015) Vascular signal transducer and activator of transcription-3 promotes angiogenesis and neuroplasticity long-term aer stroke. Circulation 131:1772-1782.
Jang SS, Choi JH, Im DS, Park S, Park JS, Park SM, Joe EH, Jou I, Suh YH (2014)e phosphorylation of STAT6 during ischemic reperfusion in rat cerebral cortex. Neuroreport 25:18-22.
Jia L, Wang F, Gu X, Weng Y, Sheng M, Wang G, Li S, Du H, Yu W (2017) Propofol postconditioning attenuates hippocampus ischemia-reperfusion injury via modulating JAK2/STAT3 pathway in rats after autogenous orthotropic liver transplantation. Brain Res 1657:202-207.
Jiang C, Xu Q, Xu K, Dai H, Zhang Z, Wu W, Ni J (2012) The effect of erythropoietin on focal cerebral ischemia reperfusion in rats by observing STAT1, STAT3 protein and MRI expression. Linchuang Fangshe Xue Zazhi 31:1036-1040.
Jiang C, Xu Q, Xu K, Dai H, Zhang Z, Wu W, Ni J (2013) Effects of erythropoietin on STAT1 and STAT3 levels following cerebral ischemia-reperfusion in rats. Int J Neurosci 123:684-690.
Jiang CJ, Wang ZJ, Zhao YJ, Zhang ZY, Tao JJ, Ma JY (2016) Erythropoietin reduces apoptosis of brain tissue cells in rats after cerebral ischemia/reperfusion injury: a characteristic analysis using magnetic resonance imaging. Neural Regen Res 11:1450-1455.
Jung JE, Karatas H, Liu Y, Yalcin A, Montaner J, Lo EH, van Leyen K (2015) STAT-dependent upregulation of 12/15-lipoxygenase contributes to neuronal injury aer stroke. J Cereb Blood Flow Metab 35:2043-2051.
Kaseka ML, Moharir M, deVeber G, MacGregor D, Askalan R, Dlamini N (2016) Prognostication value of descending corticospinal tract DWI signal in neonatal cerebral sinovenous thrombosis. Pediatr Neurol 59:90-94.
Kate MP, Riaz P, Gioia L, Sivakumar L, Jeerakathil T, Buck B, Beaulieu C, Butcher K (2015) Dynamic evolution of di ff usion-weighted imaging lesions in patients with minor ischemic stroke. Stroke 46:2318-2321.
Kim EY, Ryoo JW, Roh HG, Lee KH, Kim SS, Song IC, Chang KH, Na DG (2006) Reversed discrepancy between CT and di ff usion-weighted MR imaging in acute ischemic stroke. Am J Neuroradiol 27:1990-1995.
Kim HC, Kim E, Bae JI, Lee KH, Jeon YT, Hwang JW, Lim YJ, Min SW, Park HP (2017) Sevo fl urane postconditioning reduces apoptosis by activating the JAK-STAT pathway aer transient global cerebral ischemia in rats. J Neurosurg Anesthesiol 29:37-45.
Kohno N, Okada K, Yamagata S, Takayoshi H, Yamaguchi S (2016) Distinctive patterns of three-dimensional arterial spin-labeled perfusion magnetic resonance imaging in subtypes of acute ischemic stroke. J Stroke Cerebrovasc Dis 25:1807-1812.
Kvistad CE, Oygarden H, Logallo N,omassen L, Waje-Andreassen U, Moen G, Naess H (2016) A stress-related explanation to the increased blood pressure and its course following ischemic stroke. Vasc Health Risk Manag 12:435-442.
Lago A, Tembl JI, López-Cuevas R, Vallés J, Santos MT, Moscardó A, Parkhutik V (2015) Characterisation of DWI-MRI con fi rmed cerebral infarcts in patients with subarachnoid haemorrhage and their association with MMP-9 levels. Neurol Res 37:688-692.
Landais A (2015) Reversible splenium di ff usion weighted MRI changes associated with hypoglycemia. J Diabetes Complications 29:607-610.
Li HC, Zhang GY (2003) Activation of STAT3 induced by cerebral ischemia in rat hippocampus and its possible mechanisms. Sheng Li Xue Bao 55:311-316.
Li JL, Li CS, Fu JH, Zhang K, Xu R, Xu WJ (2015a) Evaluation of cranial and cervical arteries and brain tissue in transient ischemic attack patients with magnetic resonance angiography and di ff usion-weighted imaging. Med Sci Monit 21:1726-1731.
Li L, Li H, Li M (2015b) Curcumin protects against cerebral ischemia-reperfusion injury by activating JAK2/STAT3 signaling pathway in rats. Int J Clin Exp Med 8:14985-14991.
Liu X, Zhang X, Zhang J, Kang N, Zhang N, Wang H, Xue J, Yu J, Yang Y, Cui H, Cui L, Wang L, Wang X (2014) Diosmin protects against cerebral ischemia/reperfusion injury through activating JAK2/STAT3 signal pathway in mice. Neuroscience 268:318-327.
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84-91.
Makin SD, Doubal FN, Dennis MS, Wardlaw JM (2015) Clinically confirmed stroke with negative diffusion-weighted imaging magnetic resonance imaging:longitudinal study of clinical outcomes, stroke recurrence, and systematic review. Stroke 46:3142-3148.
Maruyama D, Fukuda K, Kataoka H, Morita Y, Nishimura K, Kawamura Y, Iihara K (2015) Evaluation of carotid artery outward remodeling by T1-weighted magnetic resonance imaging in carotid endarterectomy and stenting. J Vasc Surg 61:1464-1471.e1.
Mawet J, Eikermann-Haerter K, Park KY, Helenius J, Daneshmand A, Pearlman L, Avery R, Negro A, Velioglu M, Arsava EM, Ay H, Ayata C (2015) Sensitivity to acute cerebral ischemic injury in migraineurs: A retrospective case-control study. Neurology 85:1945-1949.
Michałowska I, Furmanek MI, Smaga E, Juraszyński Z, Zieliński T, Chełstowska S, Kuśmierczyk M, Szpakowski E, Mierzyńska A, Walecki JM (2015) Evaluation of brain lesions in patients aer coronary artery bypass graing using MRI with the emphasis on susceptibility-weighted imaging. Kardiochir Torakochirurgia Pol 12:1-7.
Odland A, Særvoll P, Advani R, Kurz MW, Kurz KD (2015) Are the current MRI criteria using the DWI-FLAIR mismatch concept for selection of patients with wake-up stroke to thrombolysis excluding too many patients? Scand J Trauma Resusc Emerg Med 23:22.
Onofrj V, Delli Pizzi S, Franciotti R, Taylor JP, Perfetti B, Caulo M, Onofrj M, Bonanni L (2016) Medio-dorsal thalamus and confabulations: Evidence from a clinical case and combined MRI/DTI study. Neuroimage Clin 12:776-784.
Ostwaldt AC, Usnich T, Nolte CH, Villringer K, Fiebach JB (2015) Case report of a young stroke patient showing interim normalization of the MRI diffusion-weighted imaging lesion. BMC Med Imaging 15:33.
Sasai H, Shimozawa N, Asano T, Kawamoto N, Yamamoto T, Kimura T, Kawamoto M, Matsui E, Fukao T (2015) Successive MRI fi ndings of reversible cerebral white matter lesions in a patient with cystathionine β-synthase de fi ciency. Tohoku J Exp Med 237:323-327.
Song CG, Yang X, Min LQ, Liu CX, Zhao CS (2014)e e ff ect of procyanidin on expression of STAT1 in type 2 diabetes mellitus SD rats with focal cerebral ischemia. Neuro Endocrinol Lett 35:68-72.
Song RR, Yu XF, Yerfan J, Sun JZ, Mao YY, Guo Y, Chen ZC, Zhang MM (2015) Cerebral lesions of DWI hyperintensity in patients with subacute stroke assessed by intravoxel incoherent motion technique. Zhejiang Da Xue Xue Bao Yi Xue Ban 44:632-637, 644.
Tamura G, Ihara S, Morota N (2016) Reversible di ff usion weighted imaging hyperintensities during the acute phase of ischemic stroke in pediatric moyamoya disease: a case report. Childs Nerv Syst 32:1531-1535.
Xin Y, Han FG (2016) Diagnostic accuracy of computed tomography perfusion in patients with acute stroke: A meta-analysis. J Neurol Sci 360:125-130.
Xu Q, Jiang C, Rong Y, Yang C, Liu Y, Xu K (2015)e e ff ects of fl udarabine on rat cerebral ischemia. J Mol Neurosci 55:289-296.
Yaghi S, Herber C, Willey JZ, Andrews HF, Boehme AK, Marshall RS, Lazar RM, Boden-Albala B (2015) Itemized NIHSS subsets predict positive MRI strokes in patients with mild deficits. J Neurol Sci 358:221-225.
Yang YL, Zhu WX, chen YH, Chen MN (2010) Protection of erythropoietin on cerebral ischemia/reperfusion injury. Zhongguo Yingyong Shenglixue Zazhi 26:152-153.
Zechariah A, ElAli A, Hermann DM (2010) Combination of tissue-plasminogen activator with erythropoietin induces blood-brain barrier permeability, extracellular matrix disaggregation, and DNA fragmentation aer focal cerebral ischemia in mice. Stroke 41:1008-1012.
Zhang S, Yao Y, Shi J, Tang X, Zhao L, Zhu W (2016) The temporal evolution of di ff usional kurtosis imaging in an experimental middle cerebral artery occlusion (MCAO) model. Magn Reson Imaging 34:889-895.
Zhang Y, Zheng J, Zhou Z, Zhou H, Wang Y, Gong Z, Zhu J (2015) Fractalkine promotes chemotaxis of bone marrow-derived mesenchymal stem cells towards ischemic brain lesions through Jak2 signaling and cytoskeletal reorganization. FEBS J 282:891-903.
Zhou IY, Guo Y, Igarashi T, Wang Y, Mandeville E, Chan ST, Wen L, Vangel M, Lo EH, Ji X, Sun PZ (2016) Fast di ff usion kurtosis imaging (DKI) with Inherent COrrelation-based Normalization (ICON) enhances automatic segmentation of heterogeneous diffusion MRI lesion in acute stroke. Nmr Biomed 29:1670-1677.
Copyedited by Slone-Murphy J, de Souza M, Yu J, Li CH, Qiu Y, Song LP, Zhao M
10.4103/1673-5374.211192
Accepted: 2017-04-14
*Correspondence to: Wei Li, 409631600@qq.com.
- 中国神经再生研究(英文版)的其它文章
- SoxC transcription factors in retinal development and regeneration
- Umbilical cord: an unlimited source of cells di ff erentiable towards dopaminergic neurons
- Targeting 14-3-3 adaptor protein-protein interactions to stimulate central nervous system repair
- RACK1 regulates neural development
- Schwann cell development, maturation and regeneration: a focus on classic and emerging intracellular signaling pathways
- BDNF pro-peptide: a novel synaptic modulator generated as an N-terminal fragment from the BDNF precursor by proteolytic processing

