Exploring pre-degenerative alterations in humans using induced pluripotent stem cell-derived dopaminergic neurons
Exploring pre-degenerative alterations in humans using induced pluripotent stem cell-derived dopaminergic neurons
Understanding the cellular and molecular mechanisms underlying human neurological disorders is hindered by both the complexity of the disorders and the lack of suitable experimental models recapitulating key pathological features of the disease.is is a crucial issue since a limited understanding of pathogenic mechanisms precludes the development of drugs counteracting the progression of the disease. Among neurological disorders, Parkinson’s disease (PD) is likely caused by a variable combination of genetic and environmental factors leading to the progressive degeneration of dopamine (DA) neurons in the substantia nigra pars compacta (SNp) (Kalia and Lang, 2015). Animal models and post-mortem studies have provided indications that multiple cellular processes may be altered in both familiar and sporadic PD pathogenesis including protein folding and aggregation, protein membrane trafficking and mitochondrial function (Kalia and Lang, 2015). However, the various genetic models of PD do not fully recapitulate the characteristics of human disease, while postmortem tissues from patients oen represent the end stage of disease and may exhibit neurochemical alterations due to chronic pharmacological treatments.e possibility to reprogram human somatic cells into cells with pluripotent potential (human induced pluripotent stem cell, hiPSC) (Takahashi et al., 2007) and to di ff erentiate hiPSC into any cell type, including neurons, thus provides an innovative approach to model neurological diseases. On this line, more information about the pathogenesis of PD has been recently provided by iPSC technology. In particular, important disease-related pathologic events such as aberrant autophagy and mitochondrial and cytoskeletal abnormalities have been described (Zhang et al., 2017), thus pointing to these models as a valuable tool for mimicking PD phenotypein vitro. More intriguingly, at present, hiPSC likely represents a unique experimental model for recognizing pre-degenerative neuronal dysfunctions that prelude the development of full-blown PD.e prerequisite for investigating pre-degenerative defects is a comprehensive elucidation of the physiological properties of hiPSC-derived DA neurons, especially focusing on the expression and function of “core” molecules playing a critical role in neuronal physiology.e iPSC technology overcomes the inability to directly examine living human neurons, thus providing a platform for investigating the properties of any proteins in their naturally occurring neuronal population. On this line, using hiPSC-derived neurons form healthy subjects, a pharmacological characterization of various ligand-and voltage-gate ion channels has been recently reported (Dage et al., 2014; Chatzidaki et al., 2015). Moreover, a physiological characterization of human midbrain DA neurons differentiated from hiPSC has been provided, showing the ability of neurons to synthetize and release DA and the capability to reuptake DA into the cells. In the same model, the electrophysiological properties of DA neurons and the neuronal mitochondrial homeostasis have been also examined (Hart fi eld et al., 2014).
Human iPSC as a model for investigating the dopamine D2 and D3 receptors properties in DA neurons: We recently used the hiPSC technology to perform an extensive characterization of human DA D2 and D3 receptors (D2R/D3R) expression and function. D2R/D3R are G protein-coupled receptors that belong to the D2-like family of DA receptors. From a functional point of view, D2R/D3R signal through the recruitment of the G alpha i/o protein to inhibit cAMP production thus decreasing protein kinase activity (PKA). In addition, stimulation of these receptors is linked to the activation of other intracellular pathways such as the extracellular signal regulated kinase 1/2 (Erk1/2) and Akt cascade (Fiorentini et al., 2015). Among the various area of distribution, in the central nervous system D2R/D3R are co-localized in DA neurons of the mesencephalic nuclei of the SNp and ventral tegmental area. In these brain areas, D2R/D3R are classically considered autoreceptors inhibiting DA release. However, more recent evidence from studies in both cultured DA neurons and animal models have demonstrated the D2R/ D3R involvement in a variety of processes critical for midbra in DA neuron function. In particular, D2R/D3R have been found to contribute to the proliferation and di ff erentiation of neuronal progenitors, both during brain development (Belinsky et al., 2013) and in the adult brain (O’Kee ff e et al., 2009). Moreover, a role of D2R/D3R in supporting DA neuron neurotrophic changes and in preventing pathological alterations that may subsequently lead to neurodegeneration have been also proposed (Fiorentini et al., 2015).
On this line we extensively analyzed D2R/D3R expression and function all throughout the differentiation process of hiPSC into DA neurons. By using a modified version of the dual-SMAD inhibition protocol (Kriks et al., 2011), human cultures enriched in TH/DAT positive neu rons (~40%) expressing genes and proteins typical of authentic, terminally di ff erentiated midbrain DA neurons (mDA) (Bono et al., 2017), were obtained at day 50 of di ff erentiation.
We investigated the involvement of D2R/D3R in mDA neuron di ff erentiation showing that the mRNA encoding for both D2R and D3R was already detected in the hiPSC stage. Interestingly, D2R/D3R stimulation at the very onset stage of di ff erentiation signi fi cantly increased the number of neurons expressing both the pan-neuronal marker MAP2 and the DA marker TH, an e ff ect speci fi cally counteracted by the D2R/D3R antagonist sulpiride (Figure 1, panel a–c). Similarly, sulpiride, when given alone, significantly impaired the production of MAP2-and TH-positive neurons (Bono et al., 2017), thus proving evidence that during development, in the human brain, D2R/D3R are critically involved in the amplification of the multipotent/ neuronal progenitor cell population before the acquisition of a fi nally di ff erentiated DA phenotype.
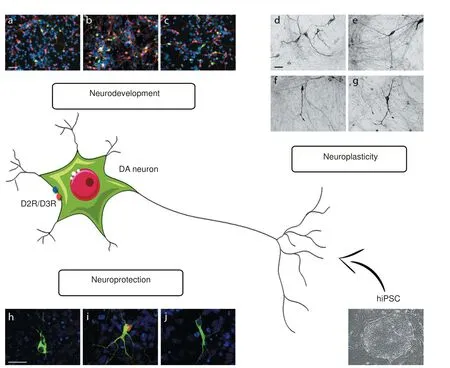
Figure 1 Role of D2R/D3R receptors expressed in human iPSC-derived dopaminergic neurons.
hiPSC-derived mDA neurons also allowed us to point out the ability of D2R/D3R in protecting DA neurons from toxic damage. At day 50 of di ff erentiation, confocal analyses revealed a broad distribution of the synaptic protein alpha synuclein (alpha-syn) in both cell body and dendrites of TH-positive mDA neurons (Figure 1, panel h). Energy starvation, induced by glucose deprivation, resulted in alpha–syn aggregation into inclusions that are reminiscent of pathological alpha-syn aggregates (Figure 1, panel i), the histopathological hallmark of PD.ese observations thus indicate that hiPSC-derived mDA neurons represent a usefulin vitrotool for modeling neurodegenerative events typical of PD, such as alpha-syn aggregation. Interestingly, in TH-positive mDA neurons, alpha-syn aggregation was counteract by D2R/D3R agonists (Figure 1, panel j). Morphological analysis of TH-positive mDA neurons also showed an association between alpha-syn aggregates and morphological alterations, such as defective neuronal dendritic arborization, that occurs later than alpha-syn aggregation (Bono et al., 2017). As shown for alpha-syn inclusions, the structural alterations observed in TH-positive mDA neurons were also counteracted by D2R/D3R stimulation (Bono et al., 2017), suggesting a D2R/ D3R crucial role in triggering key intracellular events with neuroprotective potential.
Together, a very comprehensive characterization of D2R/D3R expression and function in human mDA has been provided using the iPSC technology. It is important to note that the individual role of D2R and D3R in mediating the various e ff ects on mDA neurons has not been de fi ned due to the lack of selective ligands. However, based on mRNA analysis, hiPSC-derived mDA neurons at day 50 of di ff erentiation express both D2R and D3R, with the D2R expressed at higher levels than the D3R. Thus, in line with previous evidence (Fiorentini et al., 2015), it is likely that, even if expressed at low levels, the D3R should provide a crucial contribution to D2-like agonist-induced effects described in mDA neurons.
Human iPSC as a model for investigating pre-degenerative defects in DA neurons: Our data suggest that abnormalities in D2R/D3R expression and function could represent an early event that may underlie the special vulnerability of DAneurons of the SNp, at least in some PD subtypes. On this line, there is evidence that various genes associated with the genetic forms of PD, such as VP35, DNAJC13, LRRK2, GAK, RABXX and RAB398 may be implicated in the trafficking of proteins between intracellular compartments and plasma membrane, which may include receptors for neurotransmitters and their functional partners (Kalia and Lang, 2015). Interestingly, impairment in the expression and function of D2R, leading to neuronal synaptic dysfunctions, has been observed in transgenic mice carrying LRRK2 mutation (Wallings et al., 2015).
On this line, given that D2R/D3R are crucially involved in the homeostasis of DA neurons by regulating their development, morphological plasticity and neuroprotection (Bono et al., 2017), the possibility that abnormalities in the D2R/D3R pathway could increase the susceptibility of mDA neurons to neurotoxic damages, in at least some PD patients, is to be taken into account. Defective D2R/D3R expression, membrane trafficking and function during neuronal development could in fact decrease the number of mature TH-positive neurons. On the other hand, alterations in the D2R/D3R signaling may be responsible for the inability of DA neurons to respond to di ff erent trophic stimuli both in physiological and pathological conditions as well as to counteract toxic stimuli.erefore, in such PD patients, where abnormalities in the D2R/D3R function represent a prodromal molecular event, other signaling pathways than D2R/D3R have to be targeted for sustaining and protecting DA neurons against disease. More generally, it is well accepted that various and heterogeneous fundamental cellular processes are implicated in pathogenesis of PD, leading to DA neuron degeneration. On this line, the iPSC technology may represent the most useful strategy to model PD using patient-derived cells in order to disclose, in each patient, a hierarchically relevant pre-degenerative event that precedes DA neuron death, thus opening the possibility to consider or eventually to exclude pharmacological strategies able to slow or to stop disease progression.
Conclusions: To date, the possibility to reprogram human somatic cells into iPSC (Takahashi et al., 2007) and to di ff erentiate them into neurons is an innovative approach to model human neurons both in physiological and pathological conditions. On one hand, reprogramming iPSC from healthy human subjects to allow neurons to exhibit the advantage of directly investigating the properties of neuronal proteins in their naturally occurring environment. On the other hand, iPSC-derived neurons from patients, by recapitulating all stages of a disease, represent an invaluable tool to reveal the disease onset.is is of utmost importance in view of the fact that uncovering early pathological features provides the possibility for evaluating potential neuroprotective strategies to be considered as disease modifying therapies.
Federica Bono*, Chiara Fiorentini
Division of Pharmacology, Department of, Molecular and Translational Medicine, University of Brescia, Brescia, Italy
*Correspondence to: Federica Bono, Ph.D., federica.bono@unibs.it.
orcid: 0000-0001-9716-5327 (Federica Bono)
Accepted:2017-06-19
How to cite this article:Bono F, Fiorentini C (2017) Exploring pre-degenerative alterations in humans using induced pluripotent stem cell-derived dopaminergic neurons. Neural Regen Res 12(7):1068-1070.
Open access statement:is is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Contributor agreement: A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check: This paper has been checked twice with duplication-checking soware ienticate.
Peer review: A double-blind and stringent peer review process has been performed to ensure the integrity, quality and signi fi cance of this paper.
Belinsky GS, Sirois CL, Rich MT, Short SM, Moore AR, Gilbert SE, Antic SD (2013) Dopamine receptors in human embryonic stem cell neurodifferentiation. Stem Cells Dev 22:1522-1540.
Bono F, Savoia P, Guglielmi A, Gennarelli M, Piovani G, Sigala S, Leo D, Espinoza S, Gainetdinov RR, Devoto P, Spano P, Missale C, Fiorentini C (2017) Role of dopamine D2/D3 receptors in development, plasticity, and neuroprotection in human iPSC-derived midbrain dopaminergic neurons. Mol Neurobiol doi:10.1007/s12035-016-0376-3.
Chatzidaki A, Fouillet A, Li J, Dage J, Millar NS, Sher E, Ursu D (2015) Pharmacological characterisation of nicotinic acetylcholine receptors expressed in human iPSC-derived neurons. PLoS One 10:e0125116.
Dage JL, Colvin EM, Fouillet A, Langron E, Roell WC, Li J, Mathur SX, Mogg AJ, Schmitt MG, Felder CC, Merchant KM, Isaac J, Broad LM, Sher E, Ursu D (2014) Pharmacological characterization of ligand- and voltage-gated ion channels expressed in human iPSC-derived forebrain neurons. Psychopharmacology 231:1105-1124.
Fiorentini C, Savoia P, Bono F, Tallarico P, Missale C (2015)e D3 dopamine receptor: From structural interactions to function. Eur Neuropsychopharmacol 25:1462-1469.
Hart fi eld EM, Yamasaki-Mann M, Ribeiro Fernandes HJ, Vowles J, James WS, Cowley SA, Wade-Martins R (2014) Physiological characterisation of human iPS-derived dopaminergic neurons. PLoS One 9:e87388.
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386:896-912.
Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L (2011) Dopamine neurons derived from human ES cells e ffi ciently engrain animal models of Parkinson’s disease. Nature 48:547-551.
O’Keeffe GC, Tyers P, Aarsland D, Dalley JW, Barker RA, Caldwell MA (2009) Dopamine-induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. Proc Natl Acad Sci U S A 106:8754-8759.
Zhang Q, Chen W, Tan S, Lin T (2017) Stem cells for modeling and therapy of Parkinson’s disease. Hum Geneer 28:85-98.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fi broblasts by de fi ned factors. Cell 131:861-872.
Wallings R, Manzoni C, Bandopadhyay R (2015) Cellular processes associated with LRRK2 function and dysfunction. FEBS J 282:2806-2826.
10.4103/1673-5374.211184
- 中国神经再生研究(英文版)的其它文章
- SoxC transcription factors in retinal development and regeneration
- Umbilical cord: an unlimited source of cells di ff erentiable towards dopaminergic neurons
- Targeting 14-3-3 adaptor protein-protein interactions to stimulate central nervous system repair
- RACK1 regulates neural development
- Schwann cell development, maturation and regeneration: a focus on classic and emerging intracellular signaling pathways
- BDNF pro-peptide: a novel synaptic modulator generated as an N-terminal fragment from the BDNF precursor by proteolytic processing

