Littoral-cell angioma of the spleen: a case report
Dongming Liu, Zhaohui Chen, Tongtong Wang, Baichang Zhang, Hongyuan Zhou, Qiang Li
Department of Hepatobiliary Oncology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin 300060, China
Littoral-cell angioma of the spleen: a case report
Dongming Liu, Zhaohui Chen, Tongtong Wang, Baichang Zhang, Hongyuan Zhou, Qiang Li
Department of Hepatobiliary Oncology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin 300060, China
Littoral-cell angioma (LCA), a primary angioma which clinically belongs to splenic hemangioma, can be mostly found in normal spleen red sinus shore cells of reticuloendothelial cell system. The cells of LCA strongly express endothelial and tissue cell associated antigens that indicate a dual differentiation characteristic; whereas only endothelial cell markers are positive in normal spleen red sinus shore cells. Diagnosis of LCA relies on histopathology. Regular follow-up is needed to monitor recurrence and metastasis.
Splenic hemangioma; case report; spleen red sinus shore cells
Introduction
Littoral-cell angioma (LCA) is a type of primary splenic hemangioma clinically and is mostly found in the normal spleen red sinus shore cells1-4. Furthermore, LCA belongs to the reticuloendothelial cell system. A typical feature of LCA is that the associated antigens of endothelial cells and tissue cells can be expressed strongly at the same time, thereby representing the characteristic of double differentiation; by contrast, normal spleen red sinus shore cells are only positive for endothelial cell markers5,6. LCA was reported and named by Falk first in 19917-9; such cases are extremely rare, and the most documented are single case reports. At present, internal reports are less than 170 cases10. The Department of Hepatobiliary Oncology at Tianjin Medical University Cancer Institute and Hospital received a case of LCA of the spleen in March 2016, which is reported as follows.
Case report
A male patient of 46 years old was admited after discovering abdominal mass in general examination for 1 week, with no abnormalities. Laboratory and equipment examination: hemoglobin 151 g/L, red blood cells 4.97 × 1012/L, white blood cells 10.04 × 109/L, platelets 180 × 1012/L, also with no abnormalities. MRI examination shows the following: anterior spleen tumor, considerable for hemangioma; liver multiple small cysts. Diagnosis considers high possibility of spleen hemangioma (Figure 1). Elective resection of retroperitoneal mass under general anesthesia was performed. Surgical exploration: pelvic, colon, small intestine, liver, stomach, abdominal aorta around the omentum (–). The spleen mass was 5 cm in diameter and cystic solid. Intraoperative diagnosis is splenic space occupying, and hamartoma may not be excluded; we then carried out excision of splenic mass. Postoperative pathology showed LCA, with extramedullary hematopoiesis, proposing blood and bone marrow examination to exclude lymphoid hematopoietic system diseases and follow-up. Immunohistochemistry: CD68 (+), lysozyme (+), S-100 (partial +), CD34 (+), FV III factor (partial +), CD117 (extramedullary hematopoietic cell +), CD8(–), and Ki-67 (extramedullary hematopoietic cells +, Figure 2A). As for the disclosure of all the above information, the patient has signed informed consent.
Discussion
LCA is different from primary splenic neoplasms,hemangioma, and lymphangioma; LCA is more than simply a splenic carcinoid with vascular luminal structures1,3. LCA usually presents recurrent multiple lesions in the spleen, but a minority of single lesions is also present2, as seen in this case report. LCA has a wide range of incidence rates among the population; with an age distribution from 1 to 77 years old, of which the majority are middle-aged patient. The incidence rate for both men and women has no significant difference2,3. The clinical symptoms of this case are atypical; the majority of patients’ visits are due to unexplained splenomegaly and hypersplenism, following the characteristics of LCA phagocytic cells2. Preoperative diagnosis is mainly based on imaging examination (MRI, enhanced CT, and so on). Given that LCA is easily misdiagnosed with splenic neoplasms and lymphangioma, among others, postoperative pathology is used for clear diagnoses. The related features are as follows: tumor has composition of anastomotic vascular cavity, similar to the splenic sinus. These vascular cavities have reciprocal migration with the surrounding normal splenic sinus, and more vascular cavities are irregular and sponge-like. At the same time, taking this patient as an example, the immunohistochemistry showed double differentiation of tumor cells, endothelial cell markers (such as CD34 and CD31, as shown in Figure 2B) mostly indicated strong positive reactions, and tissue cell markers (such as CD68, as shown in Figure 2C) showed varying degrees of positive reaction, which sometimes can also be negative. The principle of LCA treatment is surgical excision, i.e. laparotomy or laparoscopic surgery. However, studies have shown that LCA has an important relationship with malignant tumors. Regular follow-up in patients with LCA is extremely important. At more than three months after surgery, no tumor recurrence or metastasis was found in the patient. However, given that the patient showed extramedullary hematopoiesis, blood and bone marrow should be promptly checked to exclude lymphatic hematopoietic system diseases, and regular follow-up is needed.
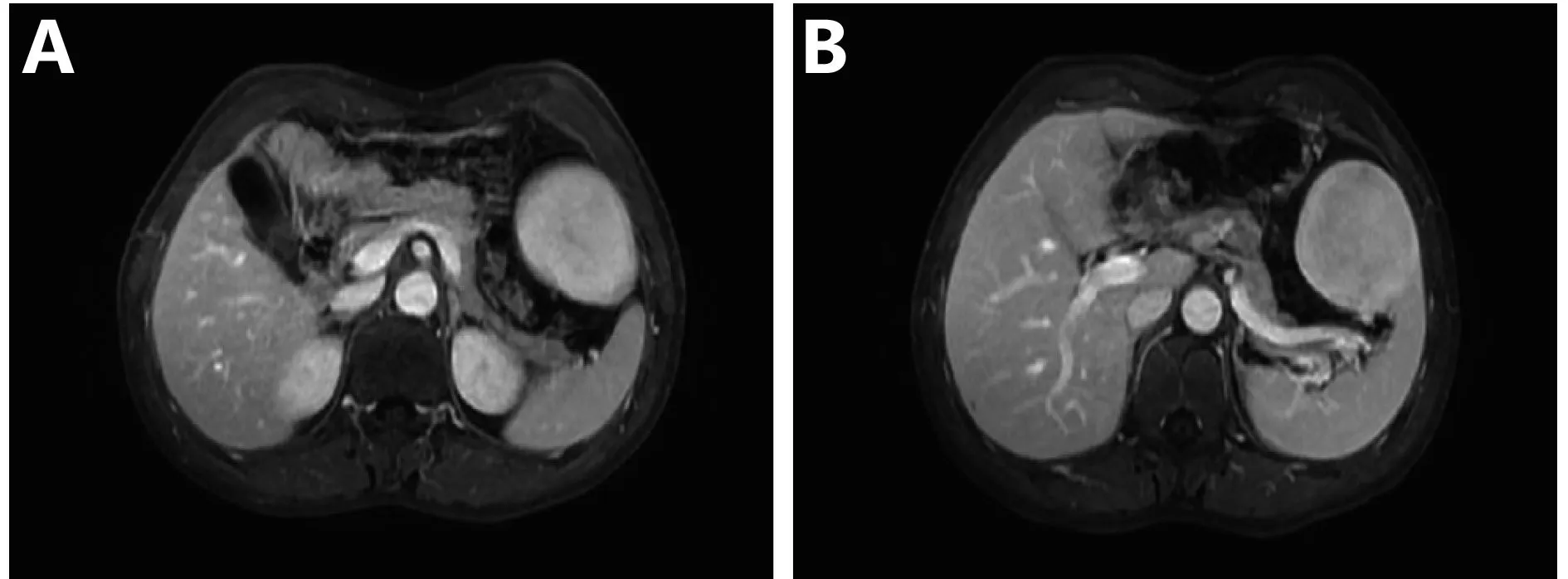
Figure 1 MRI of littoral cell angioma. (A) MRI performance with a clear boundary between lesion and spleen. (B) MRI performance of littoral cell angioma during the venous period.

Figure 2 Histopathology and immunohistochemistry features of LCA. (A) Histopathology of LCA (H&E staining, 10×). (B) CD34 (+) staining (IHC staining, 200×). (C) CD68 (+) staining (IHC staining, 400×).
Acknowlegements
This article was published originally in Chinese Journal of Clinical Oncology 2016; 43(19): 877 (in Chinese).
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.Bailey A, Vos J, Cardinal J. Littoral cell angioma: a case report. World J Clin Cases. 2015; 3: 894-9.
2.Yuan CH, Xiu DR, Zhang TL. A case report of littoral cell angioma. Chin J Hepatobiliary Surg. 2013; 19: 300, 320
3.Falk S, Stutte HJ, Frizzera G. Littoral cell angioma. A novel splenic vascular lesion demonstrating histiocytic differentiation. Am J Surg Pathol. 1991; 15: 1023-33.
4.Cui XW, Ignee A, De Molo C, Schreiber-Dietrich D, Woenckhaus M, Dietrich CF. Littoral cell angioma of the spleen. Z Gastroenterol. 2013; 51: 209-12.
5.Ursuleac I, Iosif C, Bîrlă R, Dobrea C, Găman AM, Arsene D, et al. Littoral cell angioma of the spleen-a surprising cause of anemia. Rom J Morphol Embryol. 2013; 54: 885-8.
6.Gao C, Li YC, Xiao XM, Qi F, Liu T. Littoral cell angioma in the spleen. Br J Hosp Med (Lond). 2015; 76: 55
7.Lin XY, Li JM, Wang QX, Feng JZ, Zhao MQ, Zhong WX, et al. Littoral cell angioma of the spleen: report of three cases and a review of the literature. Chin Med J (Engl). 2011; 124: 3423-6.
8.Cosme A, Tejada A, Bujanda L, Vaquero M, Elorza JL, Ojeda E, et al. Littoral-cell angioma of the spleen: a case report. World J Gastroenterol. 2007; 13: 6603-4.
9.Wang YJ, Li F, Cao F, Sun JB, Liu JF, Wang YH. Littoral cell angioma of the spleen. Asian J Surg. 2009; 32: 167-71.
10.Pilz JB, Sperschneider T, Lutz T, Loosli B, Maurer CA. Littoral cell angioma in main and accessory intrapancreatic spleen presenting as splenic rupture. Am J Surg. 2011; 201: e15-7.
11.Bhatt S, Huang JT, Dogra V. Littoral cell angioma of the spleen. Am J Roentgenol. 2007; 188: 1365-6.
Cite this article as: Liu D, Chen Z, Wang T, Zhang B, Zhou H, Li Q, et al. Littoral-cell angioma of the spleen: a case report. Cancer Biol Med. 2017; 14: 194-5. doi: 10.20892/j.issn.2095-3941.2016.0094
Qiang Li
E-mail: liqiang4016@yahoo.com
November 12, 2016; accepted February 13, 2017.
Available at www.cancerbiomed.org
Copyright © 2017 by Cancer Biology & Medicine
 Cancer Biology & Medicine2017年2期
Cancer Biology & Medicine2017年2期
- Cancer Biology & Medicine的其它文章
- Supplementary materials
- Erratum to Genetic and molecular changes in ovarian cancer
- Prognosis of gestational choriocarcinoma diagnosed incidentally during laparoscopy for a presumed cornual pregnancy: a report of five cases
- Aggressive primary hepatic epithelioid hemangioendothelioma: a case report and literature review
- Rapid response of brain metastasis to crizotinib in a patient with KLC1-ALK fusion and MET gene amplification positive non-small cell lung cancer: a case report
- Concomitant-chemoradiotherapy-associated oral lesions in patients with oral squamous-cell carcinoma
