Concomitant-chemoradiotherapy-associated oral lesions in patients with oral squamous-cell carcinoma
Sadia Minhas, Muhammad Kashif, Wasif Altaf, Nadeem Afzal, Abdul Hanan NagiOral Pathology Department, Akhtar Saeed Medical and Dental College, Lahore 5000, Pakistan;Immunology Department, University of Health Sciences, Punjab 5600, Pakistan;Dentistry Department, Fauji Foundation Hospital, Lahore 5000, Pakistan;Morbid Anatomy and Histopathology Department, University of Health Sciences, Punjab 5600, Pakistan
Concomitant-chemoradiotherapy-associated oral lesions in patients with oral squamous-cell carcinoma
Sadia Minhas1*, Muhammad Kashif2*, Wasif Altaf3, Nadeem Afzal2, Abdul Hanan Nagi41Oral Pathology Department, Akhtar Saeed Medical and Dental College, Lahore 54000, Pakistan;2Immunology Department, University of Health Sciences, Punjab 54600, Pakistan;3Dentistry Department, Fauji Foundation Hospital, Lahore 54000, Pakistan;4Morbid Anatomy and Histopathology Department, University of Health Sciences, Punjab 54600, Pakistan
Objective: Oral squamous-cell carcinoma (OSCC) accounts for >90% of oral cancers affecting adults mostly between the fourth to seventh decades of life. The most common OSCC treatment is concomitant chemoradiotherapy (CCRT) having both locoregional and distant control, but CCRT has acute and chronic toxic effects on adjacent normal tissue. This study aimed to determine the side effects of CCRT on the oral mucosa and to characterize the clinicopathology of oral lesions in patients with OSCC.
Methods: This descriptive, cross-sectional study was certified by the Ethical Review Committee (UHS/Education/126-12/2728) of the University of Health Sciences, Lahore, Pakistan. OSSC patients (n=81) with various histological subtypes, grades, and stages were recruited, and findings on their oral examination were recorded. These patients
Results: The most common presentation of OSCC was a nonhealing ulcer (63%) involving tongue (55.6%). Clinical findings included mucositis (92.6%) and xerostomia of mild, moderate, and severe degrees in 11.1%, 46.9%, and 35.8% cases, respectively. Ulcers (87.7%), palpable lymph nodes (64.2%), limited mouth opening (64.2%) and fistula (40.7%) were also observed. In females, the association of radiotherapy dosage with limited mouth opening, xerostomia, and histological grading was statistically significant (P<0.05). The association of chemotherapy drugs with xerostomia (P=0.003) was also statistically significant.
Conclusions: CCRT induced mucositis, xerostomia, and trismus in patients with OSCC.
OSCC; CCRT; oral lesions; xerostomia; trismus
Introduction
Oral cancers are the eighth most common cancer worldwide, with the highest incidence in males1. According to the American Cancer Society, the incidence and prevalence of oral cancer is increasing in developed countries, and the mortality rate due to oral cancer is also rising in developing countries. In Southeast Asia, oral cancer is the second most common cancer and the second most common cause of death among males. In the same region, approximately onethird of worldwide cases and half of the deaths from oral cancer have been reported2.
More than 90% of oral cancers are squamous-cell carcinoma (SCC), which is frequently linked to heavy alcohol consumption and tobacco smoking1. Oral squamous-cell carcinoma (OSCC) is the most common tumor in Sri Lanka, Bhutan, India, Iran, Afghanistan, Maldives, Nepal, and Pakistan3.
The Pakistan Medical Research Council has reported that oral cancer is the most common tumor in males, the second most common tumor in females after breast carcinoma, and the second most common tumor in both the genders4.
Radiation and chemotherapy are the most widely used cancer treatments. However, they are expensive and associated with many adverse reactions that increase patient morbidity and mortality5.
Acute reactions often occur in tissues with brisk cellular turnover rates, e.g., mucous membranes, which is responsible for the acute morbidity of the treatment. The damage may not become obvious in tissues with slow cellular turnover rates for months or years after therapy6,7. The effects of radiation therapy on normal tissues are divided into acute and chronic effects8. Oral complications include mucositis(stomatitis), xerostomia (dry mouth), loss of taste, infections (bacterial, fungal, or viral), dental caries, and osteoradionecrosis9. Oral mucositis significantly complicates cancer treatment due to weight loss, high risk of infection, pain, depression, dysphagia, and decreased quality of life14.
After the first week of conventional doses of radiation, erythema of oral mucosa obviously progresses in the next few weeks to mucositis, which ranges from small patches to ulcerated areas. Mucositis represents dead epithelial cells, inflammatory cells, and fibrin; it is sometimes superimposed by bacteria or yeast, and then patients complain of oral/throat pain during treatment8. Mucositis usually persists throughout treatment, peaks at the end of the irradiation period, and continues 1–3 weeks even after treatment cessation10. The frequency of mucositis with 5-florouracil chemotherapy agents is as high as 40%–70%. Frequencies of mucositis are considerably higher in patients receiving CCRT compared with those receiving radiation therapy alone11. Mucositis develops and resolves through four phases: initial inflammatory/vascular phase, epithelial phase, ulcerative/ bacteriological phase, and healing phase12. With increased intensity of radiation and chemotherapy, adverse effects particularly oral mucositis also increase13. Oral mucositis significantly complicates cancer treatment due to weight loss, high risk of infection, pain, depression, dysphagia, and decreased quality of life14.
Loss of taste is a frequent complication of cancer treatment that begins early and progresses rapidly during the second week of treatment. Patients may complain of diminished acuity, strange sensation, or absolute lack of taste. Xerostomia is also a frequent problem that exacerbates taste loss. The improvement of taste is a slow process, and volume, composition, and production of saliva are also affected by radiation. Saliva production decreases to almost 50% after a week of treatment. Patients frequently complain ropy, tenacious, and thickened saliva, which may cause difficulty in speech, swallowing, and taste loss8.
Chronic complications of radiotherapy consist of mandible osteoradionecrosis, which is vert destructive. Softtissue fibrosis and ischemia are long-term effects of radiation therapy and may not be resolved at all. The main mechanism of osseous involvement is damage to the periosteal tissue and small vasculatures, the haversian canals. Fortunately, osteoradionecrosis is a rare complication, and the occurrence of osteoradionecrosis of maxilla is much less15-17. Trismus may result because of the fibrosis of masticatory muscles. Delayed wound healing can be a result of high-dose preoperative radiation. Without careful dental care during and after radiation therapy, patients are liable to accelerated caries and decay8.
Chemotherapy drugs damage tissues of rapidly dividing cells, i.e., skin, hair and nails. They also affect the mouth, bone marrow, and the lining of the digestive system. Side effects include mouth ulcers, change in taste, high risk of developing infections, tiredness, fatigue, diarrhea, constipation, easy bruising, and hair fall18. Similarly, toxic effects of CCRT are sore mouth, anemia, strange taste, increased risk of bleeding, nausea and vomiting, hair loss, lethargy, tingling and numbness in the fingers or toes, upset bowel (diarrhea/constipation), and tinnitus and highfrequency hearing loss19.
In the present study, OSCC patients during or at the end of CCRT subjected to primary surgical treatment were selected to determine the incidence of mucositis, xerostomia, and trismus.
Patients and methods
This descriptive study involved 81 histopathologically diagnosed OSCC patients from INMOL (Institute of Nuclear Medicine and Oncology Lahore) Hospital, Lahore, Pakistan who were on concomitant chemo-radiotherapy. After obtaining written informed consent, details of each patient (age, gender, addictive habits, site of tumor, clinical stage, and histologic grade) were recorded.
These patients underwent CCRT, with five fractions in five days per week to total radiotherapy dosages of 70, 90, and 119 Gy accordingly. The chemotherapy drugs cisplatin and 5-fluorouracil were given in 4, 6, and 8 cycles depending on the stage of tumor and nutritional status of patient. Adverse effects of CCRT were noted. Oral mucositis was categorized according to EORTC/RTOG criteria (Table 1)20.
For the severity of xerostomia, the CTCAE grading scale (version 3.0) was used21,22. Xerostomia was classified as grade mild, moderate, and severe (Table 2).
A mean RTOG/EORTC score was calculated from theobservations of two experts and then compared with the patients’ reported scores. Each patient was asked to complete a patient-reported xerostomia questionnaire comprising eight items (Table 3) that rated symptoms on an 11-point ordinal Likert scale from 0 to 10, with higher scores representing discomfort due to greater dryness. Each point score was added, and the total was changed linearly to generate the final score varying from 0 to 100, with higher scores representing greater levels of xerostomia (Table 1)23.

Table 1 Oral mucositis scale according to EORTC/ RTOG
Trismus was measured as follows:
● In dentulous patients, distance was calculated between incisal edges of mandibular and maxillary central incisors.
● In edentulous patients, distance was calculated between mandibular and maxillary alveolar ridges24.
All data were entered and analyzed using SPSS 20.0. Chisquared test was used to determine statistical association among the study variables. APvalue ≤0.05 was considered as statistically significant. Regarding the ethics of medical research in humans, authors observed the declaration of World Medical Association in Helsinki (2008). This study was also approved by the Ethical Review Committee of the University of Health Sciences (UHS), Lahore, Pakistan.

Table 2 Clinical grading of xerostomia

Table 3 Xerostomia questionnaire
Results
The age of OSCC patients was between 46–55 years, with a mean SD of 53.5±14.1 years; the male-to-female ratio was 1.4:1 (64.2% males and 35.8% females) (Figure 1). The most common OSCC presentation was a nonhealing ulcer (63%); the tumor sites were tongue (55.6%), buccal mucosa (27.2%), floor of mouth (9.9%), retromolar area (4.9%), and lip and palate (1.2%) (Figure 2). The history of smoking and pan chewing was 45.7% and 12.3%, respectively, whereas 29.6% of patients were without any history of addiction (Figure 3).

Figure 1 Incidence of OSCC in different age groups.
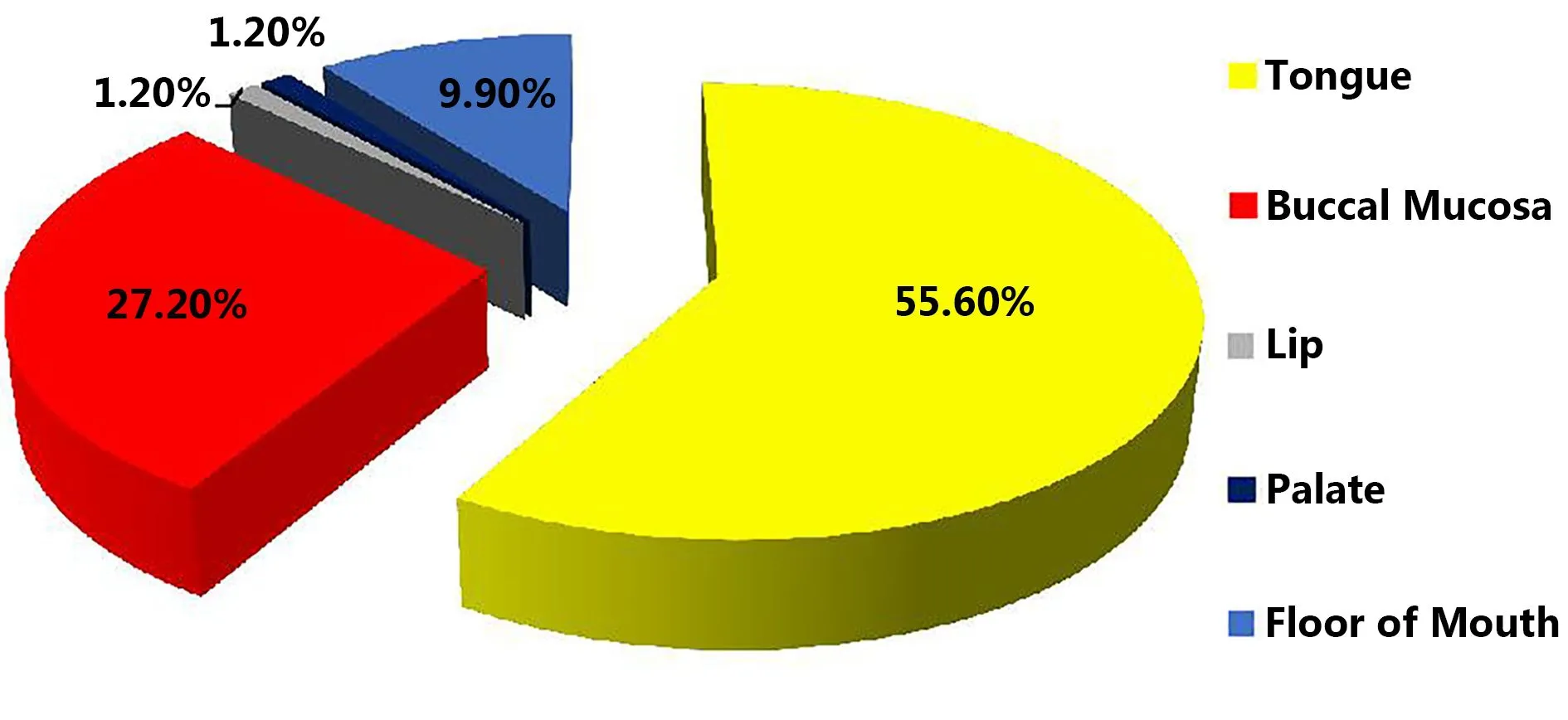
Figure 2 The site distribution of OSCC.

Figure 3 Addictive habits in OSCC patients.
Well, moderately, and poorly differentiated tumors were observed in 29.6%, 51.96%, and 14.8%, respectively. Regarding clinical staging of tumor, T4 stage was observed in the majority (57%), followed by T3 (28.4%), T2 (12.3%), and T1 (2.5%). Significant association was observed between the site of tumor and gender (P=0.036), habits of patients (P=0.018), gross appearance of tumor (P=0.001), and histological grading of tumors (P=0.024). A significant association was observed between age and clinical stage of tumors (P=0.035).
Associated complications included mucositis (92.6%) (Figure 4), xerostomia (mild, 11.1%; moderate, 46.9%; and severe, 35.8%), and fungal infection (60.5%). Limited mouth opening, palpable lymph nodes and fistula (Figure 5) were present in 64.2%, 64.2%, and 40.7%, respectively. A significant association was observed between dosage of radiotherapy and limited mouth opening, xerostomia (Figure 6), and histological grading. Similarly, a significant association was observed between chemotherapy drugs and xerostomia (P=0.003).

Figure 4 A 45-year-old female with no history of addiction, presented with OSCC of tongue. At the end of CCRT grade 4 mucositis was observed with sever xerostomia and poor oral hygiene as well as decayed and carious teeth.

Figure 5 A patient presented with OSCC of tongue and at the end of CCRT, a fistula was noted on the right lower border of the mandible.

Figure 6 A patient presented with OSCC of the posterior lateral border of tongue. At the end of CCRT, the patient complained about the thick, ropy saliva with grade 3 mucositis and loss of taste sensation as there was loss of papilla from the tongue surface.
Discussion
Most of the clinical features observed in patients with OSCC in this study were in accordance with earlier studies. Oral cancer mostly occurs in elderly people. The frequency of oral cancers increases with increased age25. The majority of studies reported OSCC in males, consistent with the findings of the current study, i.e., high incidence in the fifth to seventh decades of life26-28. The incidence of oral cancers has been recently noted at a younger age, and the same has been observed in the present study28,29. Approximately 17% of patients were under 40 years of age30or somewhere in the fourth decade of life31.
Tongue was the most commonly affected intraoral site in the present study, consistent with previous reports also suggesting the predilection of tongue for OSCC development32,33. However, diverse results have been reported, such as the high proportion of OSCC on the floor of mouth, proceeded by the tongue34,35. The intraoral spread of OSCC may be associated with the cultural and geographic differences in addictive habits of individuals, e.g., smokeless tobacco chewing in the subcontinent. The most common site of OSCC in these areas is buccal mucosa36.
OSCC treatment includes surgery, radiation therapy, chemotherapy, and CCRT depending on a patient’s risk factors, such as stage of cancer, age, immune status, and presence of comorbidities37. Chemotherapy is the choice foradvanced tumor stages, tumors with no option of resection, and patients unsuitable for surgery, etc.33In the current study, most patients presented with advanced tumor stage, i.e., T4. The chief goal of chemotherapy is to eliminate systemic micrometastasis, and the goal of administrating chemotherapy plus radiation concurrently is to develop regional and systemic control of tumor38. Currently, CCRT is the most excellent therapy for patients with regionally advanced solid tumors because it improves their survival rates and loco-regional control with organ protection39.
Radiation therapy and chemotherapy have severe side effects that decrease a patient’s quality of life40. Given that both radiotherapy and chemotherapy are used to treat locally advanced oral cavity tumors, the incidence of toxicities also increases.
In the present study, mucositis was observed in most patients during CCRT. International studies on mucositis and CCRT toxicity are in accordance with the present study. An increased incidence of mucositis has been reported with increased intensity of chemotherapy and radiotherapy, and in some cases, they appear as a “wave-like” pattern throughout the irradiation period41. Trotti et al.42observed grade ≥3 mucositis in 20%–30% of patients treated with radiation therapy alone versus 40%–90% of patients treated with radiation plus concurrent chemotherapy. Sonis et al.43reported that 64% of radiotherapy patients and 78% of chemotherapy patients had clinically visible mucositis. Apparently, the incidence of mucositis with radiotherapy is lower, but when chemotherapy is added to radiotherapy, the incidence and severity of mucositis increases. To prevent this complication of CCRT, patients are advised to maintain proper oral hygiene and dental care. Current clinical management of oral mucositis is mainly focused on palliative measures, such as nutritional support, maintenance of good oral hygiene, and pain management.
A severe complication of radiation therapy for head and neck tumors is radiation-induced xerostomia, which frequently occurs at the initial stage of treatment and strongly influences a patient’s everyday living44. The injury advances and becomes irreversible with increased radiation contact dose to the salivary glands45-47. The association between dosage of CCRT and xerostomia is significant45,48-50, consistent with the present study.
Patients receiving radiation alone or on radio-chemotherapy have a considerably higher risk for trismus51,52. The present study observed a significant association between dosage of radiotherapy and trismus. With increased dose of radiotherapy, the frequency of trismus also increases. This finding agreed with that of Teguh et al.53, who reported that radiation-induced trismus varied with radiation dose, fraction of radiation, treatment technique, and overall time of treatment.
Conclusions
Frequent problems of patients with oral cancer on CCRT included mucositis, xerostomia, trismus, and difficulty in drinking and eating. All these toxicities were significantly associated with CCRT dose. Pretreatment measures should be taken to lessen these complications so that the patient’s nutritional status would not be compromised and the patient’s quality of life would not be affected.
Acknowledgements
All authors acknowledge the support extended by the staff of the Morbid Anatomy and Histopathology, UHS, Lahore and INMOL Hospital, Lahore.
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.Scully C, Bagan JV. Oral squamous cell carcinoma: overview of current understanding of aetiopathogenesis and clinical implications. Oral Dis. 2009; 15: 388-99.
2.Petti S, Masood M, Scully C. The magnitude of tobacco smokingbetel quid chewing-alcohol drinking interaction effect on oral cancer in South-East Asia. A meta-analysis of observational studies. PLoS One. 2013; 8: e78999
3.International Agency for Research on Cancer. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012[Internet]. Geneva: World Health Organization. Available from: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx.
4.Mahmood Z, Khurshid A, Jafarey NA. Incidence of the cancers of oral cavity and pharynx in Pakistan. Pak J Med Res. 1986; 25: 173-81.
5.Dose AM. The symptom experience of mucositis, stomatitis, and xerostomia. Semin Oncol Nurs. 1995; 11: 248-55.
6.Joiner MC, van der Kogel AJ, Steel GG. Introduction: the significance of radiobiology and radiotherapy for cancer treatment. In: van der Kogel AJ, editor. Basic Clinical Radiobiology. 4th ed. London: Hodder Arnold Publication; 2009. p.1-10.
7.Hall EJ, Giaccia AJ. Radiobiology for the Radiobiologist. 7th ed. Philadelphia: Lippincott, Williams and Wilkins; 2011.
8.Schreiber GJ, Meyers AD, Calhoun KH, Talavera F. General principles of radiation therapy[Internet]. [Accessed 2016 Sept 18].Available from: http://emedicine.medscape.com/article/846797-overview#aw2aab6b6.
9.Zlotolow IM. General consideration in prevention and treatment of oral manifestation of cancer therapies. In: Berget AP, Weissman DE, editors. Principles and Practice of Supportive Oncology. Philadelphia: Lippincott Raven; 1998. p. 237.
10.Scully C, Epstein JB. Oral health care for the cancer patient. Eur J Cancer B Oral Oncol. 1996; 32: 281-92.
11.Murphy BA. Clinical and economic consequences of mucositis induced by chemotherapy and/or radiation therapy. J Support Oncol. 2007; 5: 13-21.
12.Sonis ST, Eilers JP, Epstein JB, LeVeque FG, Liggett Jr WH, Mulagha MT, et al. Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Cancer. 1999; 85: 2103-13.
13.Volpato LER, Silva TC, Oliveira TM, Sakai VT, Machado MAAM. Radiation therapy and chemotherapy-induced oral mucositis. Braz J Otorhinolaryngol. 2007; 73: 562-8.
14.Silverman Jr S. Diagnosis and management of oral mucositis. J Support Oncol. 2007; 5: 13-21.
15.Curi MM, Lauria L. Osteoradionecrosis of the jaws: a retrospective study of the background factors and treatment in 104 cases. J Oral Maxillofac Surg. 1997; 55: 540-4.
16.Tong ACK, Leung ACF, Cheng JCF, Sham J. Incidence of complicated healing and osteoradionecrosis following tooth extraction in patients receiving readiotherapy for treatment of nasopharyngeal carcinoma. Austr Dent J. 1999; 44: 187-94.
17.Thorn JJ, Hansen HS, Specht L, Bastholt L. Osteoradionecrosis of the jaws: clinical characteristics and relation to the field of irradiation. J Oral Maxillofac Surg. 2000; 58: 1088-93.
18.Cancer Research UK. Chemotherapy side effects[Internet]. Cancer Research UK. [Accessed 2016 Sept 23]. Available from: http://www.cancerresearchuk.org/cancerhelp/aboutcancer/treatme nt/chemotherapy/chemotherapy-side-effects.
19.Cancer Research UK. Chemoradiation for mouth and oropharyngeal cancer[Internet]. Cancer Research UK. [Accessed 2016 Sept 23]. Available from: http://www.cancerresearchuk.org/cancer-help/type/mouthcancer/treatment/chemoradiation.
20.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995; 31: 1341-6.
21.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Rad Oncol. 2003; 13: 176-81.
22.Wada A, Uchida N, Yokokawa M, Yoshizako T, Kitagaki H. Radiation-induced xerostomia: objective evaluation of salivary gland injury using MR sialography. Am J Neuroradiol. 2009; 30: 53-8.
23.Eisbruch A, Kim HM, Terrell JE, Marsh LH, Dawson LA, Ship JA. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001; 50: 695-704.
24.Dhanrajani PJ, Jonaidel O. Trismus: aetiology, differential diagnosis and treatment. Dent Update. 2002; 29: 88-94.
25.Ariyoshi Y, Shimahara M, Omura K, Yamamoto E, Mizuki H, Chiba H, et al. Epidemiological study of malignant tumors in the oral and maxillofacial region: survey of member institutions of the Japanese Society of Oral and Maxillofacial Surgeons, 2002. Int J Clin Oncol. 2008; 13: 220-8.
26.Ling W, Mijiti A, Moming A. Survival pattern and prognostic factors of patients with squamous cell carcinoma of the tongue: a retrospective analysis of 210 cases. J Oral Maxillofac Surg. 2013; 71: 775-85.
27.Seoane-Romero JM, Vázquez-Mahía I, Seoane J, Varela-Centelles P, Tomás I, López-Cedrún JL. Factors related to late stage diagnosis of oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal. 2012; 17: e35-40.
28.Bhurgri Y, Bhurgri A, Usman A, Pervez S, Kayani N, Bashir I, et al. Epidemiological review of head and neck cancers in Karachi. Asian Pac J Cancer Prev. 2006; 7: 195-200.
29.Iamaroon A, Pattanaporn K, Pongsiriwet S, Wanachantararak S, Prapayasatok S, Jittidecharaks S, et al. Analysis of 587 cases of oral squamous cell carcinoma in northern Thailand with a focus on young people. Int J Oral Maxillofac Surg. 2004; 33: 84-8.
30.Halboub ES, Al-Anazi YM, Al-Mohaya MA. Characterization of Yemeni patients treated for oral and pharyngeal cancers in Saudi Arabia. Saudi Med J. 2011; 32: 1177-82.
31.Sherin N, Simi T, Shameena PM, Sudha S. Changing trends in oral cancer. Indian J Cancer. 2008; 45: 93-6.
32.De Stefani E, Boffetta P, Deneo-Pellegrini H, Ronco AL, Acosta G, Ferro G, et al. The effect of smoking and drinking in oral and pharyngeal cancers: a case-control study in Uruguay. Cancer Lett. 2007; 246: 282-9.
33.Kaminagakura E, Werneck da Cunha I, Soares FA, Nishimoto IN, Kowalski LP. CCND1 amplification and protein overexpression in oral squamous cell carcinoma of young patients. Head Neck. 2011; 33: 1413-9.
34.Udeabor SE, Rana M, Wegener G, Gellrich NC, Eckardt AM. Squamous cell carcinoma of the oral cavity and the oropharynx in patients less than 40 years of age: a 20-year analysis. Head Neck Oncol. 2012; 4: 28
35.Ribeiro ACP, Silva ARS, Simonato LE, Salzedas LMP, Sundefeld MLMM, Soubhia AMP. Clinical and histopathological analysis of oral squamous cell carcinoma in young people: a descriptive study in Brazilians. Br J Oral Maxillofac Surg. 2009; 47: 95-8.
36.Ghoshal S, Mallick I, Panda N, Sharma SC. Carcinoma of the buccal mucosa: analysis of clinical presentation, outcome and prognostic factors. Oral Oncol. 2006; 42: 533-9.
37.ul Haq ME, Abid H, Hanif MK, Warraich RA, Mahmood HS, Saddique K. Frequency and pattern of oral and maxillo-facial carcinomas. Ann King Edw Med Univ. 2009; 15: 171-5.
38.Seiwert TY, Salama JK, Vokes EE. The chemoradiation paradigm in head and neck cancer. Nat Clin Pract Oncol. 2007; 4: 156-71.
39.Vokes EE, Kies MS, Haraf DJ, Stenson K, List M, Humerickhouse R, et al. Concomitant chemoradiotherapy as primary therapy for locoregionally advanced head and neck cancer. J Clin Oncol. 2000; 18: 1652-61.
40.Patel SC, Carpenter WR, Tyree S, Couch ME, Weissler M, Hackman T, et al. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol. 2011; 29: 1488-94.
41.Wygoda A, Maciejewski B, Skladowski K, Hutnik M, Pilecki B, Golen M, et al. Pattern analysis of acute mucosal reactions in patients with head and neck cancer treated with conventional and accelerated irradiation. Int J Radiat Oncol Biol Phys. 2009; 73: 384-90.
42.Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003; 66: 253-62.
43.Sonis ST, Eilers JP, Epstein JB, LeVeque FG, Liggett Jr WH, Mulagha MT, et al. Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Cancer. 1999; 85: 2103-13. (12,)
44.Wescott WB, Mira JG, Starcke EN, Shannon IL, Thornby JI. Alterations in whole saliva flow rate induced by fractionated radiotherapy. Am J Roentgenol. 1978; 130: 145-9.
45.Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999; 45: 577-87.
46.Roesink JM, Moerland MA, Battermann JJ, Hordijk GJ, Terhaard CH. Qantitative dose-volume response analysis of changes in parotid gland function after radiotheraphy in the head-and-neck region. Int J Radiat Oncol Biol Phys. 2001; 51: 938-46.
47.Radfar L, Sirois DA. Structural and functional injury in minipig salivary glands following fractionated exposure to 70 Gy of ionizing radiation: an animal model for human radiation-induced salivary gland injury. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003; 96: 267-74.
48.Berk LB, Shivnani AT, Small Jr W. Pathophysiology and management of radiation-induced xerostomia. J Support Oncol. 2005; 3: 191-200.
49.Jellema AP, Doornaert P, Slotman BJ, Leemans CR, Langendijk JA. Does radiation dose to the salivary glands and oral cavity predict patient-rated xerostomia and sticky saliva in head and neck cancer patients treated with curative radiotherapy? Radiother Oncol. 2005; 77: 164-71.
50.Sullivan CA, Haddad RI, Tishler RB, Mahadevan A, Krane JF. Chemoradiationinduced cell loss in human submandibular glands. Laryngoscope. 2005; 115: 958-64.
51.Weber C, Dommerich S, Pau HW, Kramp B. Limited mouth opening after primary therapy of head and neck cancer. Oral Maxillofac Surg. 2010; 14: 169-73.
52.Khojastepour L, Bronoosh P, Zeinalzade M. Mandibular bone changes induced by head and neck radiotherapy. Indian J Dent Res. 2012; 23: 774-7.
53.Teguh DN, Levendag PC, Voet P, van der Est H, Noever I, de Kruijf W, et al. Trismus in patients with oropharyngeal cancer: relationship with dose in structures of mastication apparatus. Head Neck. 2008; 30: 622-30.
Cite this article as: Minhas S, Kashif M, Altaf W, Afzal N, Nagi AH. Concomitant-chemoradiotherapy-associated oral lesions in patients with oral squamous-cell carcinoma. Cancer Biol Med. 2017; 14: 176-82. doi: 10.20892/j.issn.2095-3941.2016.0096
*These authors have contributed equally to this work
Muhammad Kashif
E-mail: drkashifazam@gmail.com
November 28, 2016; accepted December 23, 2016.
Available at www.cancerbiomed.org
Copyright © 2017 by Cancer Biology & Medicine
70, 90, and 119 Gy of radiotherapy dosages in combination with the chemotherapy drugs cisplatin and 5-fluorouracil. Data were analyzed using SPSS 20.0.
 Cancer Biology & Medicine2017年2期
Cancer Biology & Medicine2017年2期
- Cancer Biology & Medicine的其它文章
- Developmental pathways associated with cancer metastasis: Notch, Wnt, and Hedgehog
- Cellular immunity augmentation in mainstream oncologic therapy
- Mesenchymal stromal cells’ role in tumor microenvironment: involvement of signaling pathways
- Simplified microsatellite instability detection protocol provides equivalent sensitivity to robust detection strategies in Lynch syndrome patients
- Significance of stromal-1 and stromal-2 signatures and biologic prognostic model in diffuse large B-cell lymphoma
- Immunohistochemical evaluation of vitamin D receptor(VDR) expression in cutaneous melanoma tissues and four VDR gene polymorphisms
