Simplified microsatellite instability detection protocol provides equivalent sensitivity to robust detection strategies in Lynch syndrome patients
Hadi Babaei, Mehrdad Zeinalian, Mohammad Hassan Emami, Mortaza Hashemzadeh, Najmeh Farahani, Rasoul Salehi
1Department of Genetics and Molecular Biology, School of Medicine, Isfahan University of Medical Sciences, Isfahan 81744-176, Iran;2Clinic of Gastrointestinal Diseases, Poursina Hakim Research Center, Isfahan University of Medical Sciences, Isfahan 81744-176, Iran;3Cellular and Molecular Research Center, Shahrekord University of Medical Sciences, Shahrekord 43723, Iran
Simplified microsatellite instability detection protocol provides equivalent sensitivity to robust detection strategies in Lynch syndrome patients
Hadi Babaei1, Mehrdad Zeinalian1, Mohammad Hassan Emami2, Mortaza Hashemzadeh3, Najmeh Farahani1, Rasoul Salehi1
1Department of Genetics and Molecular Biology, School of Medicine, Isfahan University of Medical Sciences, Isfahan 81744-176, Iran;2Clinic of Gastrointestinal Diseases, Poursina Hakim Research Center, Isfahan University of Medical Sciences, Isfahan 81744-176, Iran;3Cellular and Molecular Research Center, Shahrekord University of Medical Sciences, Shahrekord 43723, Iran
Objective: Germline mutations in mismatch repair (MMR) genes cause Lynch syndrome (LS). LS is an inherited disease, and an important consequence of MMR deficiency is microsatellite instability (MSI) phenotype. MSI phenotype influences the efficacy of 5 fluorouracil (5-FU) chemotherapy. Reproducible, cost effective, and easy to perform laboratory tests are required to include MSI detection in routine laboratory practice. Evaluation of CAT25 as monomorphic short tandem repeat sequence enables CAT25 to be an efficient screening tool among hereditary nonpolyposis colorectal cancer (HNPCC) patients compared with other methods used currently.
Methods: Based on Amsterdam II criteria, 31 patients in 31 families were shortlisted from a total number of 1,659 colorectal cancer patients. MSI status was examined in these patients using CAT25 and a commercially available Promega MSI five-markerbased detection system as well as immunohistochemical (IHC) staining of four important MMR proteins. Patients were scored as high microsatellite instable (MSI-H), low (MSI-L), or stable (MSS). MSI status determined by CAT25 single mononucleotide marker was compared with that of five mononucleotide markers, Promega commercial kit, and IHC method.
Results: MMR protein deficiency was observed on 7/31 probands using IHC methodology and 6/31 categorized as MSI-H using commercial kit or CAT25 single marker. The sensitivity and specificity of the CAT25 single marker were the same as those detected by five-marker Promega commercial kit in our patients.
Conclusions: Based on our results, the performance of the CAT25 single mononucleotide marker for MSI status determination in our HNPCC patients is the same as that of the five-marker-based commercial kit.
Lynch syndrome; HNPCC; DNA mismatch repair; IHC; microsatellite instability
Introduction
Colorectal cancer (CRC) is one of the most common types of cancer worldwide1. Sporadic CRC is the main form of the disease, comprising greater than 90% of all CRC cases. Familial adenomatous polyposis (FAP) and Lynch syndrome (LS) or hereditary nonpolyposis colorectal cancer (HNPCC) are the most prevalent hereditary forms of CRC2. These hereditary forms are inherited in an autosomal dominant manner with 50% risk of transmission to the offspring3. Differentiation of sporadic CRCs from the hereditary forms is usually accomplished based on the available criterion, such as Amsterdam or Bethesda. However, categorization based on the abovementioned criteria must be confirmed through an appropriate genetic testing4. Germline mutations within the mismatch repair (MMR) genes can lead to LS (OMIM 120435) or HNPCC5. MMR gene mutations result in impaired base MMR error during DNA replication, allowing accumulation of mutations in the DNA. Risk of other cancers (except CRCs), such as ovary, gastrointestinal, and breast, is also rising considerably in HNPCC patients who have mutations in MMR genes6. Microsatellite instability (MSI) as a phenomenon is frequently observed in HNPCC patients, and approximately 10% is affected by sporadic CRC because of the inherent pitfall of DNA polymerase in replicating short tandem repeat (STR) DNA sequences, especially mononucleotide repeats7. Under these circumstances, newmononucleotide STR alleles can be produced because of unrepaired expansion or contraction of the existing STRs, which is called MSI phenotype. This phenomenon is currently used for screening and confirmation of LS and differentiates them from those affected by sporadic CRC8. Immunohistochemical (IHC) detection of MLH1, MSH2, MSH6, or PMS2 MMR protein deficiency9,10or by polymerase chain reaction (PCR)-based methods are used for MSI testing11. MSI-H tumors are predominantly located in the right or proximal colon and are characterized by poor differentiation, mucinous histology, and lymphocytic infiltration. Based on the available evidence, this pathologically unique subset of CRCs exhibited significant differences in clinical behavior. Meanwhile, the prognosis of MSI-H tumors is discreetly better than that of microsatellite stable (MSS) or MSI-low (MSI-L) tumors12. However, they seem to obtain no benefit from adjuvant 5-fluorouracil (5-FU) chemotherapy. This 5-FU resistance is proposed to stem from the incorporation of 5-FU metabolites into DNA instead of inhibiting its effective target, which is thymidylate synthase13. Therefore, MSI typing is likely to become a routine diagnostic procedure in all CRC patients; however, at present, it is usually applied to patients suspected for hereditary CRC prescreened by Amsterdam or Bethesda criteria14. To date, the standard testing procedure for the PCR-based detection of MSI status recommended by the National Cancer Institute/International Collaborative Group/HNPCC (NCI/ICG-HNPCC) is five mononucleotide markers for tumor and nontumor adjacent normal tissues. At present, the most frequently used PCR-based commercial MSI testing kit is a five mononucleotide marker kit developed by Promega, Madison, WI4. Five quasimonomorphic mononucleotide markers, including NR-21, BAT-25, MONO-27, NR-24, BAT-26, and 2 pentanucleotide repeats, involving penta C and penta D are used in this kit15. However, this technique is expensive, laborious, and difficult to optimize16. Much research has been conducted to evaluate the few number of STR markers in MSI testing without compromising the sensitivity and specificity of the test17-20.
Here, we aimed to evaluate the sensitivity and specificity of a new MSI marker, the T25 mononucleotide repeat of the caspase 2 gene (CAT25). Thirty-one families sorted according to the Amsterdam II criteria were included in this work. DNA was extracted from the tumor as well as normal adjacent formalin-fixed paraffin embedded tissues using QIAamp DNA FFPE Tissue Kit (Qiagen, Germany). All DNA samples were subjected to MSI detection using the Promega MSI testing system (MSI Analysis System, Version 1.2) and CAT25 in the house-developed protocol. In addition, IHC was performed on the tissue sections cut out from the same samples used for DNA extraction.
Materials and methods
Subjects
The study was approved by the Ethical Committee of Isfahan University of Medical Sciences. All patients provided informed consent. All patients diagnosed with CRC between 2000 and 2013 and registered at Poursina Hakim Research Center and Clinic, Isfahan, Iran were included in this study. The total number of patients screened in this study was 1, 659. Finally, 31 families were selected based on Amsterdam II criteria for HNPCC. Each of the following criteria must be fulfilled: three or more relatives with an associated cancer, two or more successive generations affected, one or more relatives diagnosed before the age of 50 years, and one should be a first-degree relative of the other two. Paraffin-embedded formalin-fixed tissue blocks were obtained from each proband to evaluate MSI status by either IHC or PCR-based techniques using multiplex commercially available MSI detection kit (Promega, USA) as well as our in-house developed monoplex CAT25 marker. FAP cases were excluded.
MSI status by immunohistochemistry
All tissue blocks were sectioned and subjected to IHC study to determine the MMR status as previously described9,10using standard techniques21.
MSI status by PCR-based methods
A commercial kit from Promega (MSI Analysis System, Version 1.2) was used for MSI typing. Five mononucleotide markers and two pentanucleotide markers (penta C and penta D) are included for probable cross contamination or samples’ mix up detection. Three categories of MSI status are observed by using Promega kit, MSI-H, MSI-L, and MSS showing more or less than 30% instabilities of the markers. Primer-BLAST software was used to design primers for CAT25 locus. Forward primer sequence is 5′-CCTAG AAACCTTTATCCCTGCTT-3′ and reverse primer sequence is 5′-GAGCTTGCAGTGAGCTGAGA-3′. PCR primers were labeled at the 5′ end with Cy5 for the subsequent analysis using ALFexpress DNA sequencer (Amersham Pharmasia Biotech). Up to 30 ng of DNA, 0.2 mmol of deoxynucleotide triphosphates, 10 pmol of each primer, 1× PCR buffer, 1.5mmol MgCl2, and 0.5 units Taq DNA polymerase were used for PCR reaction. The total volume reached 25 μL. The PCR stages are as follows: initial denaturation in 5 min at 94°C and then 32 cycles of denaturation at 94°C, annealing at 59°C, extension at 72°C for 30 s for each condition, and a final extension at 72°C for 5 min. For fragment analysis, 3 μL of appropriately diluted PCR products was mixed with 1.5 μL of formamide and 1.5 μL of DNA loading dye (Fermentas). After completion of the run, DNA fragments were analyzed using AlleleLink software provided by the manufacturer. Statistical analyses of the obtained data were performed by SPSS 16 software package (SPSS Inc., Chicago, IL, USA). Comparison of diagnostic sensitivity/specificity of the marker CAT25 ROC analysis was also conducted.
Results
Among the 1,659 CRC-affected patients based on Amsterdam II criteria, 31 probands representative of 31 families were selected in this study. The medical records of all 31 families included in this study were examined carefully and asked to complete a comprehensive questionnaire. Based on the data regarding cancer prevalence extracted from the patients’records and questionnaires, a total of 186 individuals from these 31 families were determined to be affected by various cancers. Eighty-six of them showed CRC (46.2%). The remaining 100 patients (53.8%) exhibited extracolonic cancers.
Immunohistochemistry results
Overall, loss of MMR expression was observed in 7/31probands (22.6%). An example of MSH2 expression in a patient with MMR is shown inFigure 1. Both MSH6 and MSH2 were negative on 4/7 (57.1%). MLH1 and PMS2 were negative on 2/7 (28.6%) and in one case. MSH6 was defective (14.3%). As we expected, only MSH6-deficient tumor was MSS (Figure 2). Data from all patients are summarized inTables 1and2.
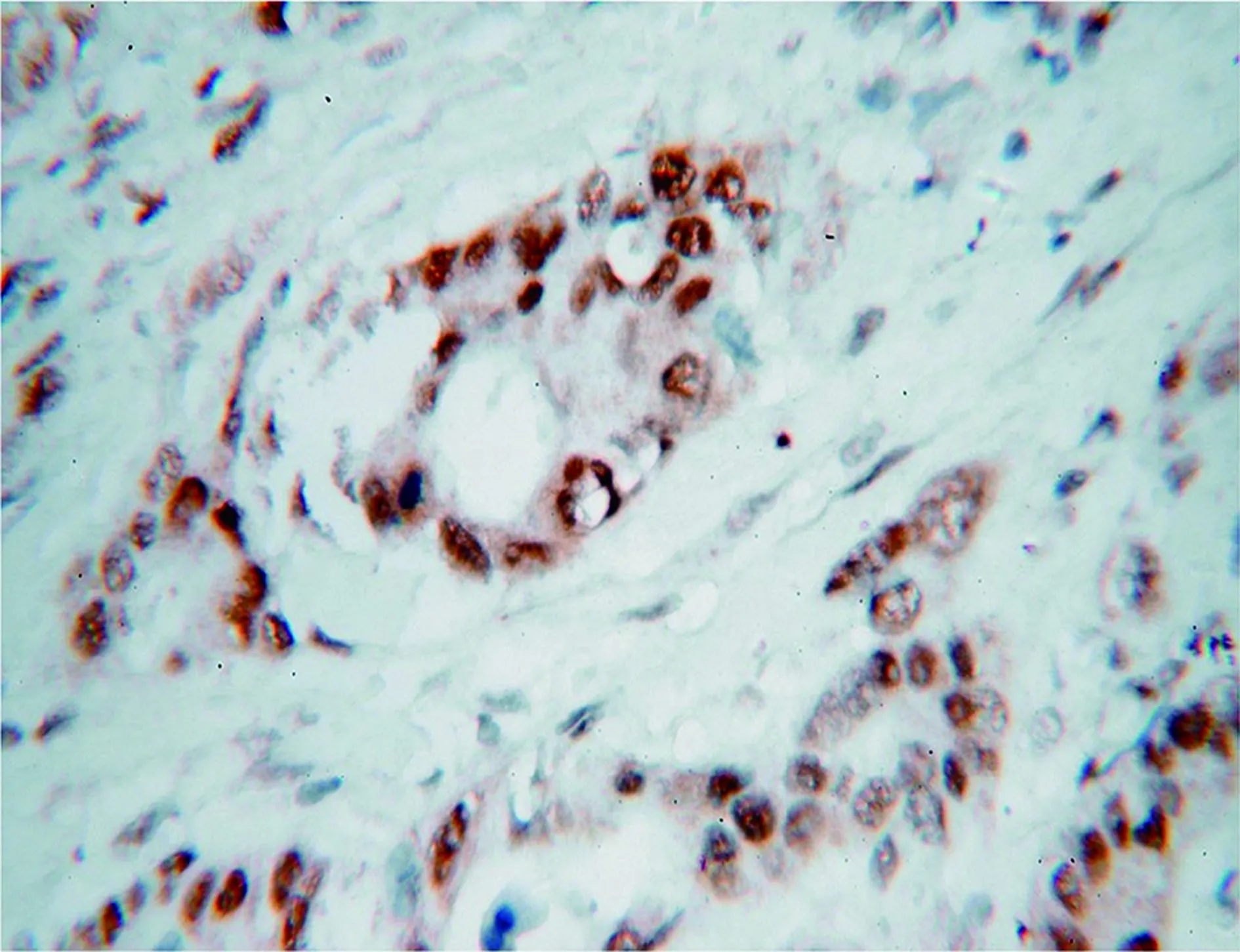
Figure 1 MSH2 expression in MMR-proficient sample (IHC staining, 40×).

Figure 2 Promega system results and IHC for MSH6-deficient patient. Stability in tumor tissue (A) compared with normal tissue (B). IHC staining demonstrating loss of MSH6 expression in the patient (C) (10 ×).

Table 1 Primary tumor site in patients according to their MMR status detected by IHC
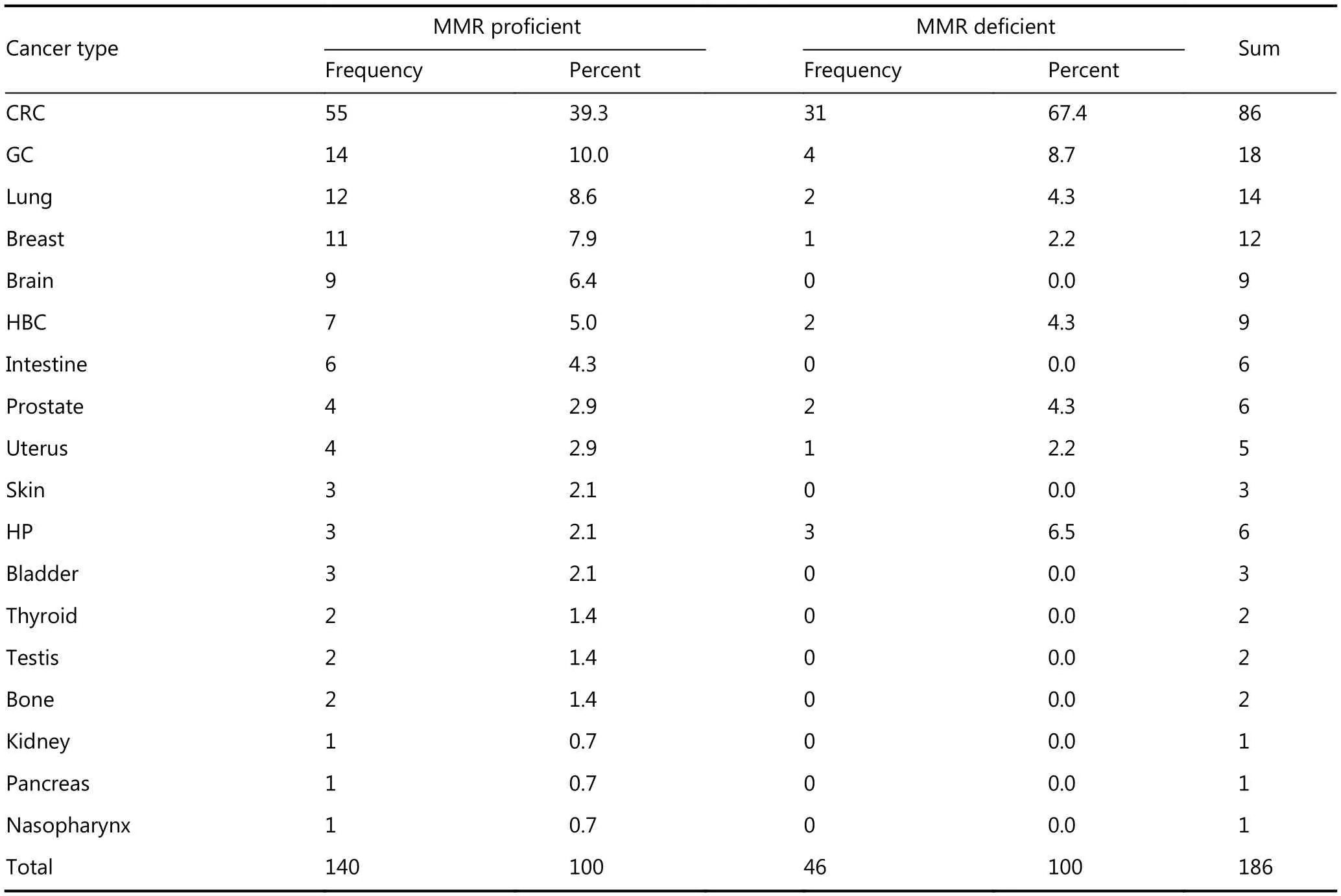
Table 2 Cancer type frequency according to their MMR status detected by IHC
MSI analysis using promega MSI detection system
Nine out of 31 patients (29%) showed MSI in their tumor tissues [six patients (19.4%) with MSI-H]. The first marker showed that the highest instability was BAT-26, followed by 7/31 MSI tumors (22.6%). Then, both BAT-25 and NR-24 markers showed instability on 6/31 (19.3%). Two markers were found unstable in 5/31 (MONO-27 and NR-21) (16.1%) tumors. The 4/6 (66.6%) CRC patients showed instability in all markers of the kit. Another showed stability only in one marker (MONO-27). The other exhibited two unstable markers (BAT-26 and NR-24) (Figure 3).Figure 4shows the example of fragment analysis results of the PCRproduct of the samples. The average age was 44.7, 51.7, and 36.0 years at the time of diagnosis of MSS, MSI-L, and MSIH probands, respectively (P=0.123).
The most frequent tumor sites were rectosigmoid (72.8%), rectum (66.7%), and right colon (50.0%) in CRC probands with MSS, MSI-L, and MSI-H phenotype, respectively. As we expected, 70% of the CRC tumors were located in the right side of the splenic flexure for MSI-H tumors. However, it was 18.6% for MSS tumors. The survival period of the probands was 6.1, 2.0, and 5.8 years for MSS, MSI-L, and MSI-H groups of the probands, respectively (P=0.341).
The average number of patients diagnosed with cancer among MSS, MSI-L, and MSI-H groups from the HNPCCfamilies was 5.4, 7.7, and 6.0 patients per family, respectively (P=0.12). In addition, on average, 2.2, 3.3, and 4.7 patients per family existed in CRC-affected members among our HNPCC families in MSS, MSI-L, and MSI-H groups, respectively (P=0.014). Meanwhile, the average number of affected members by extracolonic cancers in these three groups was 3.2, 4.3, and 1.3 patients per family (P=0.045). Among the MSS, MSI-L, and MSI-H groups of these families, the proportion of CRC patients to all cancer patients was 40.3%, 41.7%, and 65.1%, respectively. The stomach (18.3%), lung (15.5%), and breast (11.3%) were the most commonly involved organs in the MSS group of the studied families in extracolonic cancers. The lung (28.9%) and stomach (23.7%) in males and the breast (24.2%) and brain (15.2%) among the females were the most prevalently involved organs in extracolonic cancers. The most common involved organs were the breast (21.4%) and brain and hepatobiliary tract (14.3%) in MSI-L families and the stomach (26.7%) and hematopoietic system (20.0%) in MSIH families. The most prevalently involved organs in MSI-H families (except CRCs) among the males were the stomach (25.0%) and hematopoietic system (33.3%). In females, the majority of cases were CRC (84.2%) and the breast, uterus, and hepatobiliary tract (33.3% for all three cancers). The 8, 10, and 18 different organs were affected within MSI-H, MSIL, and MSS families, respectively. The most common pathological phenotype in MSS (31.8%) and MSI-L CRC tumors (66.7%) was “well differentiated adenocarcinoma”, whereas “moderately differentiated adenocarcinoma” was in MSI-H CRC tumors (50.0%). Moreover, more than half of the MSS, MSI-L, and MSI-H CRC tumors of the probands have been diagnosed in late pathological TNM stages (stage III or IV) (63.6%, 100%, and 83.3%, respectively). The proportion of the deceased probands among the MSS, MSI-L, and MSI-H groups was 31.8%, 100%, and 33.3%, respectively.

Figure 3 Number of tumors that were unstable or stable for each of 6 markers (5 markers in Promega kit plus a single CAT25 marker). For CAT25, 25 patients are grouped in MSS (orange) and 6 in MSI-H (blue).

Figure 4 Capillary electrophoresis results from the Promega system. A panel consisting of five mononucleotide marker was used for MSI determination via multiplex PCR. X axis is the size in bases. Y axis is the fluorescence intensity. Green peaks are amplification products from microsatellite loci, including three of five markers shown here (NR21, BAT25, and MONO27). Note the shift in the size (bases) of the amplification products in the tumor specimen compared with normal ones. An example of one shifted locus is demonstrated in the tumor sample (B) compared with normal one (A).
Sensitivity and specificity for CAT25 marker
CAT25 was unstable in all six patients with MSI-H (100%) but showed stability in MSS and MSI-L patients. Sensitivity and specificity of CAT25 marker compared with Promega were 100% (Figure 5). All MSI-H tumors were correctly identified as MSI-H by CAT25 marker. No MSS tumors were incorrectly identified as MSI-H by the marker compared with Promega system.
Discussion

Figure 5 MSI assay by single marker (CAT25) showing instability in tumor tissue (bottom) compared with normal tissue (top). The amplified products were separated by ALF express fragment analysis. Comparison of peak patterns with a shift in PCR product size of the tumor with normal ones represents instability. The arrows represent shifts in base pairs compared with normal tissue. The 22 and 23 lines are normal and tumoral tissues in one patient. The 24 and 25 lines are in another one. One example of MSS status detected by CAT25 marker is shown (right).
In this study, MSI, IHC, and different clinical criteria were analyzed for predicting and detecting loss of expression of MMR genes to differentiate the families with hereditary, HNPCC, and sporadic CRC. To accomplish this task, 31 families for which a tumor sample was available were evaluated. All the 31 patients fulfilled the Amsterdam II criteria. MSH6 and PMS2 bind to MSH2 and MLH1, respectively. Moreover, they expressed with their partner but not alone22. Therefore, in the IHC results as expected, loss of MSH6 and MSH2 expression were both detected in 57.1% of the patients. Meanwhile, loss of PMS2 and MLH1 expression were together detected in 28.6% of the patients. CRCs and associated cancers in LS/HNPCC families are reported to occur in younger age than the general population23. Furthermore, the results showed that compared with MMR-proficient CRCs among LS families, MMR-deficient tumorsoccurred in younger patients similar to that in MSI cases24. Deficiency in the DNA MMR pathway can lead to MSI. DNA MMR function is lost because of biallelic inactivation (Knudson two hit theory)25in one of the genes involved in the DNA MMR pathway (MSH2, MLH1, MSH6, PMS2, and PMS1) in HNPCC26. Methylation of the MLH1 gene is an alternative cause of MMR deficiency in sporadic CRCs27. MSI detection can be useful for identifying probands in families with HNPCC because MSI status can be used in clinical management and also in making right decision about treatment approaches28. Different numbers of mononucleotide STRs have been used for MSI status determination and categorization of CRC patients into two main categories, namely, MSS and MSI high. An additional category, although less important, is MSI low8. The methods and criteria to determine MSI in CRC have constantly evolved because its initial discovery was more than a decade ago. Therefore, some investigations have been performed to date to identify an easy to perform and cost-effective assay for MSI status. A panel of markers for detection defects within the MMR system (three dinucleotides and two mononucleotide repeats) was suggested by NCI; however, the usefulness of the panel is argued because of the low sensitivity of dinucleotide repeats14,27,29. To choose the use of current MSI assays is difficult because of economical and application issues in routine laboratory workflow30. We provide evidence that a single PCR assay with only one marker should be the recommended method for MSI evaluation in clinical and research laboratories.
The results presented here support the three important conclusions as follows:
The feature of LS includes cancers occurring at younger ages than that in the general population31,32. In addition, the average age of proficient probands’ group was younger than that of MMR-deficient probands’ group. Although LS is determined as a single condition, the clinical phenotypes can vary significantly depending upon the gene involved32. We showed in this study that 57.1 of CRC patients with MMR deficiency were detected proximal to the splenic flexure (P< 0.01). Furthermore, CRCs are more common than other types of cancers among MMR-deficient families.
High-level MSI results were concordant with MMR protein loss results in MMR defective samples. However, none of the three MSI-L tumors were MMR deficient. All MSI-H cases were in concordance with the MMR protein defects but in MSI-L cases were not. The significance of lowlevel DNA microsatellite instability (MSI-L) is not well understood. In MSI-L, all CRCs were relatively unstable if enough markers were used in the MSI assay. Therefore, detecting MSI-L by more markers, such as Promega kit but not in single marker test, e.g., CAT25 marker that we tested here, is possible. To include MSI-L to MSS category may be beneficial because of their similar response to chemotherapy33,34. The chromosomal instability carcinogenesis pathway is the cause of MSI-L tumors similar to MSS tumors but unlike MSI-H tumors35. High frequency of K-RAS mutations in MSI-L cancers and methylation of methylguanine transferase in MSI-L tumors have been reported in contrast to MSI-H where more mutations occur in MMR pathway; however, this finding is not clear in others36-39. MSI-H tumors exhibit a distinct clinicopathological phenotype in contrast to MSI-L or MSS tumors, including poorly differentiated, right-sided, and mucinous, extendible growth pattern and tumor-infiltrating lymphocytes40. This finding is consistent with our results that the most common pathological phenotype in MSS (31.8%) and MSI-L CRC tumors (66.7%) was “well-differentiated adenocarcinoma”, whereas that in MSI-H CRC tumors (50.0%) was “moderate-differentiated adenocarcinoma”.
To find the best marker as a biomarker tool for diagnosis, many investigations have been conducted on MSI markers because MSI is an important marker to screen for HNPCC as well as a prognostic and predictive marker for sporadic colorectal cancer41-43. Promega kit offered a highly sensitive and specific method in the detection of MSI. However, it is still time consuming and expensive. Therefore, we suggest using single mononucleotide marker, such as CAT25, that can properly detect all the MMR-defective cases alone but without any false positivity in contrast to BAT-26.
Conclusions
A simplified assay for MSI is tested in this study. The one mononucleotide marker (CAT25) exhibited high specificity and sensitivity for identifying tumors with MMR deficiency. The results of studies on distinct markers in different populations are different. Therefore, assessing markers in different ethnics to confirm that the results of these markers are similar to that in different countries with actually different ethnic backgrounds is necessary. The results above lead us to conclude that MSI and IHC results were in concordance with each other. However, MSI typing is more accurate than IHC. The results provided for CAT25 marker suggest that MSI testing for LS would be a cost-effective and convenient method by using only one marker, or at least, it can be included in panel to detect MSI status. Using a single CAT25 marker is fast, easy to perform, cost effective with the least optimization and technically challenging involvement.Sensitivity and specificity data on CAT25 single marker show the success of strategy for excluding the majority of MSS cases.
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.Jemal A, Siegel R, Ward E, Hao YP, Xu JQ, Murray T. Cancer statistics, 2008. CA Cancer J Clin. 2008; 58: 71-96.
2.Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010; 138: 2044-58.
3.KastrinosF, SyngalS. Inherited colorectal cancer syndromes. Cancer J. 2011; 17: 405-15.
4.Pérez-Carbonell L, Ruiz-Ponte C, Guarinos C, Alenda C, Payá A, Brea A. Comparison between universal molecular screening for Lynch syndrome and revised bethesda guidelines in a large population-based cohort of patients with colorectal cancer. Gut. 2011; 61: 865-72.
5.Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009; 76: 1-18.
6.Bonadona V, Bonaïti B, Olschwang S, Grandjouan S, Huiart L, Longy M. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011; 305: 2304-10.
7.Sinicrope FA, Sargent DJ. Molecular pathways: Microsatellite instability in colorectal cancer: Prognostic, predictive, and therapeutic implications. Clin Cancer Res. 2012; 18: 1506-12.
8.Vatandoost N, Ghanbari J, Mojaver M, Avan A, Ghayour-Mobarhan M, Nedaeinia R. Early detection of colorectal cancer: From conventional methods to novel biomarkers. J Cancer Res Clin Oncol. 2015; 142: 341-51.
9.Stone J, Robertson D, Houlston RS. Immunohistochemistry for MSH2 and MHL1: A method for identifying mismatch repair deficient colorectal cancer. J Clin Pathol. 2001; 54: 484-7.
10.McConechy MK, Talhouk A, Li-Chang HH, Leung S, Huntsman DG, Gilks CB. Detection of DNA mismatch repair (MMR) deficiencies by immunohistochemistry can effectively diagnose the microsatellite instability (MSI) phenotype in endometrial carcinomas. Gynecol Oncol. 2015; 137: 306-10.
11.Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome: Part I. The utility of immunohistochemistry. J Mol Diagnost. 2008; 10: 293-300.
12.Desselle F, Verset G, Polus M, Louis E, Van Daele D. [Lynch syndrome and microsatellite instability: A review]. Rev Med Liege. 2012; 67: 638-43. French.
13.Ng K, Schrag D. Microsatellite instability and adjuvant fluorouracil chemotherapy: A mismatch? J Clin Oncol. 2010; 28: 3207-10.
14.Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J. Revised bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004; 96: 261-8.
15.Bacher JW, Flanagan LA, Smalley RL, Nassif NA, Burgart LJ, Halberg RB. Development of a fluorescent multiplex assay for detection of msi-high tumors. Dis Markers. 2004; 20: 237-50.
16.Deschoolmeester V, Baay M, Wuyts W, Van Marck E, Pelckmans P, Lardon F. Comparison of three commonly used PCR-based techniques to analyze msi status in sporadic colorectal cancer. J Clin Lab Anal. 2006; 20: 52-61.
17.Morandi L, de Biase D, Visani M, Monzoni A, Tosi A, Brulatti M. T[20] repeat in the 3'-untranslated region of the MT1X gene: A marker with high sensitivity and specificity to detect microsatellite instability in colorectal cancer. International J Colorectal Dis. 2012; 27: 647-56.
18.Findeisen P, Kloor M, Merx S, Sutter C, Woerner SM, Dostmann N. T25 repeat in the 3' untranslated region of the CASP2 gene: A sensitive and specific marker for microsatellite instability in colorectal cancer. Cancer Res. 2005; 65: 8072-8.
19.Mead LJ, Jenkins MA, Young J, Royce SG, Smith L, St John DJ. Microsatellite instability markers for identifying early-onset colorectal cancers caused by germ-line mutations in DNA mismatch repair genes. Clin Cancer Res. 2007; 13: 2865-9.
20.LeGolvan MP, Taliano RJ, Resnick MB. Application of molecular techniques in the diagnosis, prognosis and management of patients with colorectal cancer: A practical approach. Hum Pathol. 2012; 43: 1157-68.
21.Key M. immunohistochemistry staining methods. Education Guide Immunohistochemical Staining Methods Fourth Edition. 2006; 47
22.SameerAS, NissarS, FatimaK. Mismatch repair pathway: Molecules, functions, and role in colorectal carcinogenesis. Eur J Cancer Prev. 2014; 23: 246-57.
23.Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010; 138: 2044-58.
24.Vilar E, Gruber SB. Microsatellite instability in colorectal cancerthe stable evidence. Nat Rev Clin Oncol. 2010; 7: 153-62.
25.Knudson AG Jr. Mutation and cancer: Statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971; 68: 820-3.
26.Bellacosa A. Genetic hits and mutation rate in colorectal tumorigenesis: Versatility of knudson's theory and implications for cancer prevention. Genes Chromosomes Cancer. 2003; 38: 382-8.
27.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW. A national cancer institute workshop on microsatellite instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998; 58: 5248-57.
28.Moreira L, Balaguer F, Lindor N, de la Chapelle A, Hampel H, Aaltonen LA. Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012; 308: 1555-65.
29.Suraweera N, Duval A, Reperant M, Vaury C, Furlan D, Leroy K. Evaluation of tumor microsatellite instability using fivequasimonomorphic mononucleotide repeats and pentaplex pcr. Gastroenterology. 2002; 123: 1804-11.
30.Laghi L, Bianchi P, Malesci A. Differences and evolution of the methods for the assessment of microsatellite instability. Oncogene. 2008; 27: 6313-21.
31.Ladabaum U, Ford JM, Martel M, Barkun AN. American gastroenterological association technical review on the diagnosis and management of Lynch syndrome. Gastroenterology. 2015; 149: 783-813.e20.
32.Lynch HT, Snyder CL, Shaw TG, Heinen CD, Hitchins MP. Milestones of Lynch syndrome: 1895-2015. Nat Rev Cancer. 2015; 15: 181-94.
33.Laiho P, Launonen V, Lahermo P, Esteller M, Guo MZ, Herman JG. Low-level microsatellite instability in most colorectal carcinomas. Cancer Res. 2002; 62: 1166-70.
34.Halford S, Sasieni P, Rowan A, Wasan H, Bodmer W, Talbot I. Low-level microsatellite instability occurs in most colorectal cancers and is a nonrandomly distributed quantitative trait. Cancer Res. 2002; 62: 53-7.
35.Pawlik TM, Raut CP, Rodriguez-Bigas MA. Colorectal carcinogenesis: MSI-H versus MSI-L. Dis Markers. 2004; 20: 199-206.
36.Greenson JK, Bonner JD, Ben-Yzhak O, Cohen HI, Miselevich I, Resnick MB. Phenotype of microsatellite unstable colorectal carcinomas: Well-differentiated and focally mucinous tumors and the absence of dirty necrosis correlate with microsatellite instability. Am J Surg Pathol. 2003; 27: 563-70.
37.Jass JR. Towards a molecular classification of colorectal cancer. Int J Colorectal Dis. 1999; 14: 194-200.
38.Kim H, Jen J, Vogelstein B, Hamilton SR. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol. 1994; 145: 148-56.
39.Tomlinson I, Halford S, Aaltonen L, Hawkins N, Ward R. Does MSI-low exist? J Pathol. 2002; 197: 6-13.
40.Mojarad EN, Kashfi SMH, Mirtalebi H, Taleghani MY, Azimzadeh P, Savabkar S. Low level of microsatellite instability correlates with poor clinical prognosis in stage II colorectal cancer patients. J Oncol. 2016; 2016: 2196703
41.Walther A, Johnstone E, Swanton C, Midgley R, Tomlinson I, Kerr D. Genetic prognostic and predictive markers in colorectal cancer. Nat Rev Cancer. 2009; 9: 489-99.
42.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005; 23: 609-18.
43.Webber EM, Kauffman TL, O’Connor E, Goddard KAB. Systematic review of the predictive effect of msi status in colorectal cancer patients undergoing 5fu-based chemotherapy. BMC Cancer. 2015; 15: 156
Cite this article as: Babaei H, Zeinalian M, Emami MH, Hashemzadeh M, Farahani N, Salehi R, et al. Simplified microsatellite instability detection protocol provides equivalent sensitivity to robust detection
strategies in Lynch syndrome patients. Cancer Biol Med. 2017; 14: 142-50. doi: 10.20892/j.issn.2095-3941.2016.0091
Rasoul Salehi
E-mail: r_salehi@med.mui.ac.ir
November 10, 2016; accepted December 12, 2016. Available at www.cancerbiomed.org
Copyright © 2017 by Cancer Biology & Medicine
 Cancer Biology & Medicine2017年2期
Cancer Biology & Medicine2017年2期
- Cancer Biology & Medicine的其它文章
- Supplementary materials
- Erratum to Genetic and molecular changes in ovarian cancer
- Littoral-cell angioma of the spleen: a case report
- Prognosis of gestational choriocarcinoma diagnosed incidentally during laparoscopy for a presumed cornual pregnancy: a report of five cases
- Aggressive primary hepatic epithelioid hemangioendothelioma: a case report and literature review
- Rapid response of brain metastasis to crizotinib in a patient with KLC1-ALK fusion and MET gene amplification positive non-small cell lung cancer: a case report
