High-efficiency Flexible Organic Solar Cells with UV-ozone Treated Silver Modification ITO Electrode
YAN Min-nan, ZHENG Shuang, WANG Dan-bei, LIU-Huan, ZHANG Hong-mei*
(1. Key Laboratory for Organic Electronics and Information Displays & Institute of Advanced Materials, Institute of The Materials Science and Technology, Nanjing University of Posts & Telecommunications, Nanjing 210023, China; 2. Jiangsu National Synergistic Innovation Center for Advanced Materials (SICAM), Nanjing University of Posts & Telecommunications, Nanjing 210023, China)
High-efficiency Flexible Organic Solar Cells with UV-ozone Treated Silver Modification ITO Electrode
YAN Min-nan1,2, ZHENG Shuang1,2, WANG Dan-bei1,2, LIU-Huan1,2, ZHANG Hong-mei1,2*
(1. Key Laboratory for Organic Electronics and Information Displays & Institute of Advanced Materials, Institute of The Materials Science and Technology, Nanjing University of Posts & Telecommunications, Nanjing 210023, China; 2. Jiangsu National Synergistic Innovation Center for Advanced Materials (SICAM), Nanjing University of Posts & Telecommunications, Nanjing 210023, China)
Highly efficient heterojunction polymer solar cells based on ITO flexible electrode were developed with an UV-ozone-treated ultrathin silver interlayer combined with MoO3/PEDOT∶PSS as modification materials. By optimizing UV-ozone-treated time of silver thin films, it could improve the power conversion efficiency (PCE) of the device based on the blend of poly(3-hexylthiophene) (P3HT)∶[6,6]-phenyl C61-butyric acidmethyl ester (PC61BM) from 1.68% (without UV-ozone treatment) to 2.57% (Ag with 1 min UV-ozone treatment), which might be an AgOxlayer formation that improves hole extraction and several promising characteristics, including high optical transparency, low sheet resistance and superior surface work function. Meanwhile, the devices with UV-ozone treated thin Ag layer together with MoO3or PEDOT∶ PSS improves PCEs from 2.02% for the device with PET/ITO/MoO3to 2.97% for the device with PET/ITO/AgOx/MoO3and from 2.01% to 2.93% for device with PEDOT∶PSS. In addition, the PCE of 6.21% of the flexible polymer solar cells based on poly[4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)-benzo[1,2-b;4,5-b′] dithiophene-2,6-diyl-alt-(4-(2-ethylhe-xyl)-3-fluorothieno[3,4-b]thiophene-)-2-carboxylate-2-6-diyl(PBDTTT-EFT)∶[6,6]-phenyl C71-butyric acid methyl ester (PC71BM) as an photoactive layer was obtained. The efficiency improvement of the flexible OPV based on ITO is due to the increase of the work function of ITO by ITO/Ag/PEDOT∶PSS or MoO3composition.
flexible organic solar cell; electrode modification; UV-ozone treatment; ultra-thin Ag
1 Introduction
Organic solar cells (OSCs) have attracted increased attention due to their potential in high mechanical flexibility, simple fabrication process, low cost and light weight[1-5]. Moreover, the innately bendy characteristics of flexible organic solar cells are beneficial to their applications in outerwear, communications, and sustainable energy technology. Although their power conversion efficiency (PCE) has exceeded 10% with rapid progress in device optimization and new materials, the devices with flexible substrates have improved more slowly because of the longstanding drawbacks of flexible transparent electrodes[6]. Indium tin oxide (ITO) is the most used material for transparent electrodes, but its high sheet resistance on plastic substrates (typically >35 Ω·□-1, which is 3-4 times higher than on glass)[7-8], the chemically ill-defined nature of the surface[9]and the relatively high surface roughness[10-11]adversely impact the interface between ITO electrode and the photoactive layer. It is widely recognized that the electrode interfaces are critical determinants of the performance of OPVs[12-14]. Therefore, in order to achieve high device efficiencies on flexible ITO, it should choose the appropriate materials and preparation processing used for the flexible ITO electrode modification.
Poly (3, 4-ethylenedioxythiophene)∶poly (sty-renesulfonate) (PEDOT∶PSS) is frequently used as an anode interfacial layer for improving anode contact and increasing hole collection[15-18]. Moreover, transition metal oxides, such as NiO[19], MoO3[20-22], V2O5[23]or WO3[24], have also been reported to be effective anode buffer layers due to their good hole-transporting and electron-blocking behavior. Due to intrinsic flexibility and high conductivity, metal thin films(such as Au[25], Ag[26]) are also suitable for modifying electrode in organic solar cells. The application of metal thin film avoids the problem of etching ITO by PEDOT∶PSS, the semi-transmittance in the visible region and appropriate treatment of metal thin film benefit the formation of Ohmic contact at the anode/donor interface to improve the PCEs of devices[27-29]. And appropriate UV-ozone treatment is employed to further efficient hole collection,which improves the performance of the device significantly[30-31]. Besides, a composited anode buffer layer including transition metal oxide (MoO3, V2O5, WO3etc.) and thin high work function metal (Au, Ag, Al) has also been proved to improve device efficiency[32-34].
In this paper,UV-ozone-treated ultrathin Ag with PEDOT∶PSS or MoO3composited anode buffer layer is used to modify ITO electrode respectively. In order to further understand the effect of the modification onto the ITO, ultraviolet photoemission spectroscopy (UPS), X-ray photoelectron spectroscopy (XPS) and atomic force microscope (AFM) are analyzed in details to explain the achievements.
2 Experiments
Poly (3-hexylthiophene) (P3HT), [6,6]-phenyl C61butyric acid methyl ester (PCBM), poly [4,8-bis(5-(2-ethylhexyl) thiophen-2-yl)-benzo[1,2-b;4,5-b0]dithiophene-2,6-diyl-alt-(4-(2- ethylh- exyl)-3- fluorothieno[3,4-b] thiophene-)-2-carboxylate-2-6-diyl](PBDTTT-EFT), [6,6]-phenyl C71-butyric acid methyl ester (PC71BM), and PEDOT∶PSS (Levios P VP. AL 4083), were purchased from Organtec Material, Inc., Rieke Metals Inc., Nichem-(Fine Technology Co. Ltd.), Inc. and Heraeus-(Precious Metals GmbH & Co.KG) Inc., respectively and used as received without further purification. P3HT and PC61BM were dissolved together in 1, 2-dichlorobenzene (1∶1 weight ratio with a total concentration of 34 mg/mL) and stirred and heat 60 ℃ for 12 h before use. The polymer PBDTTT-EFT and PC71BM with a weight ratio of 1∶1.5 were dissolved in 1, 2-dichlorobenzene with a total concentration of 30 mg/mL. The mixed solution was stirred with a magnetic stirrer at 70 ℃ for 12 h, and then 3%(volume ratio) 1,8-diiodooctane additive was added to form the final PBDTTTEFT∶PC71BM solution.
The structure of the devices in this study is illustrated in Fig.1(a). ITO-coated PET substrates are cleaned sequentially in an ultrasonic bath with detergent, deionized water (DI water), acetone and alcohol for 30 s. After being dried with nitrogen gas, the ITO surface was treated with UV-ozone (the power density was 28-32 mW/cm2) for 10 min. The substrates were then transferred to vacuum chamber immediately for Ag evaporation. Thin Ag films were deposited onto PET/ITO by thermal evaporation at 3×10-4Pa, and evaporated at a rate of 0.5-0.1 nm/s. Then PEDOT∶PSS (AL4083 or PH1000) aqueous solution or MoO3was added to UV-ozone-treated thin Ag film. The corresponding device groups are as follows:
Group A: PET/ITO (UV-ozoneXmin)/Ag (1 nm without UV-ozone)/P3HT∶PCBM/LiF/Al.
Group B: PET/ITO (UV-ozone 10 min)/Ag (1 nm, UV-OzoneYmin)/P3HT∶PCBM/LiF/Al.
Device C, D: PET/ITO/Ag (1 nm, UV-ozone 1 min)/PEDOT∶PSS (MoO3)/P3HT∶PCBM/LiF/Al.
PEDOT∶PSS layer was spin-coated at 3 500 r/min for 1 min, followed by baking at 120 ℃ for 30 min in air. When high conductivity PEDOT∶PSS (PH1000) was spin-coated on the PET/ITO/Ag (1 nm, UV-ozone 1 min) substrate, PEDOT∶PSS (PH1000) on the half side of the substrate without ITO was erased by a little cotton-bud immediately before baking in air. And this will not overestimate the PCE of the device[35-36]. MoO3layer was thermally evaporated on the substrates at a deposition rate of 0.05 nm/s under vacuum of 5×10-4Pa. After that, the PEDOT∶PSS (or MoO3)-coated substrates were immediately transferred into a nitrogen-filled dry glove box with oxygen and moisture levels below 10-7. Subsequently, blend films of photoactive layers were spin-coated on top of PEDOT∶PSS or MoO3layer. P3HT∶PC61BM solution was then spin-coated at 800 r/min for 60 s followed by slowly dried in covered petri dishes at room temperature for 1 h, and annealing at 120 ℃ for 10 min. The blend PBDTTT-EFT∶PC71BM solution was spin-coated at 1 500 r/min for 40 s to prepare the active layer. After being dried for 30 min, the active layers were treated with methanol. Finally, the devices were completed by thermally depositing a 0.8 nm LiF layer and a 100 nm-thick Al layer cathodes in vacuum chamber (5×104Pa). The evaporation rates were monitored by a quartz oscillator system, and the film thickness was calibrated by a surface profiler (Veeco Dektak 6M). The active area of each device is 0.11 cm2. The OPVs were encapsulated in the glovebox with a UV-curable epoxy and glass sheets for the electrical measurement. The current density-voltage (J-V) curves of the devices were measured with a computer-programmed Keithley 2400 source/meter under 100 mW/cm2illumination of simulated AM 1.5 G sunlight (Newport’s Oriel class A), which was calibrated by the JIS C 8912 standard. In addition, the ultraviolet photoemission spectroscopy (UPS) was carried out using a He discharged lamp (He I 21.22 eV, Kratos Analytical). The interface electronic structure was performed by X-ray photoelectron spectroscopy (XPS) with Al Kα X-ray source (1 486.6 eV). Transmittance measurement was obtained with a Shimadzu UV-1800 spectrometer. The morphology of different treatments of ITO surface was measured by atomic force microscope (AFM) (Bruker FastScan). All the measurements were carried out in ambient atmosphere at room temperature. The date listed of each different PSCs was the average date based on 20 samples for each.

Fig.1 (a) Device architecture of the flexible OPVs with the configuration of PET/ITO/Ag (1 nm)/PEDOT∶PSS (50 nm) or MoO3(5 nm)/P3HT∶PCBM (80 nm)/LiF (0.8 nm)/Al (100 nm). (b) Energy band diagram of the device.
3 Results and Discussion
The current density-voltage (J-V) curves of the devices Group A and B with different UV-ozone treated ITO and Ag were present in the Fig.2(a) and (b) as well as their corresponding photovoltaic performances are summarized in Tab.1. From Fig.2 (a), it could be seen that the UV-ozone treatment time of ITO has an impact on the performance of the devices. The result shows that the device with 10 min UV-ozone treated achieved highest power conversion efficiency (PCE) of 1.68%. The device with 10 min UV-ozone treated ITO anode could achieve the best photovoltaic performance. The effect of thin Ag layer with different UV-ozone treatment time was further studied based on the device with 10 min UV-ozone treated ITO anodes. Compared with the devices without UV-ozone treatment, the performance of the devices modified by UV-ozone treated thin Ag improved significantly, with improvement in PCE (from 1.68% to over 2.0%),Voc(higher than 0.57 V) and FF (all exceed 0.50). As a result, the highest PCE of 2.57% withJscof 8.15 mA/cm2,Vocof 0.59 V, and FF of 0.536 was achieved for the device with 1 min UV-ozone treated 1 nm Ag layer. For comparison, the reference device with PET/ITO/PEDOT∶PSS as anode exhibited PCE of 2.01% withJscof 8.21 mA/cm2,Vocof 0.56 V, and FF of 0.44. It is known that PET/ITO has poor flexibility due to its fragile nature[32].
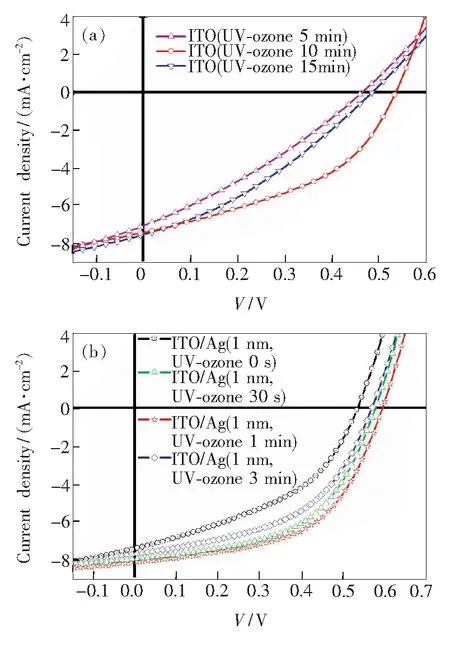
Fig.2 Current-density (J-V) characteristics of the devices with different time UV-ozone treated ITO (a) and different time treated Ag by UV-ozone (b) based on 10 min UV-ozone treated ITO
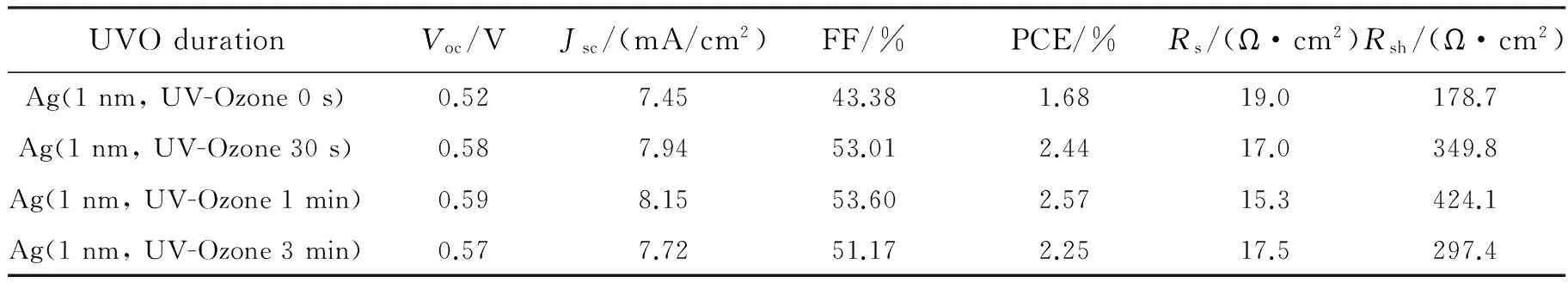
Tab.1 Performance parameters of the devices modified by 1 nm Ag with different UV-ozone time
The structure is PET/ITO/Ag (1 nm, UV-ozoneYmin)/P3HT∶PCBM/LiF/Al.
However, it can been seen from the AFM images of bare ITO, UV-ozone-treated Ag (1 nm)/ITO in the Fig.3 (a) and (b) that the root-mean-square (rms) roughness of ITO and UV-ozone-treated Ag (1 nm)/ITO was measured as 0.8 nm and 1.86 nm, respectively. Inserting the anode interfacial layer increased the surface roughness slightly, which is associated with the fact that ITO layer was not fully covered by AgOxdue to the formation of Ag islands during evaporation[37]. Therefore the increment of device efficiency can be ascribed to the oxidation of Ag by UV-ozone treatment.
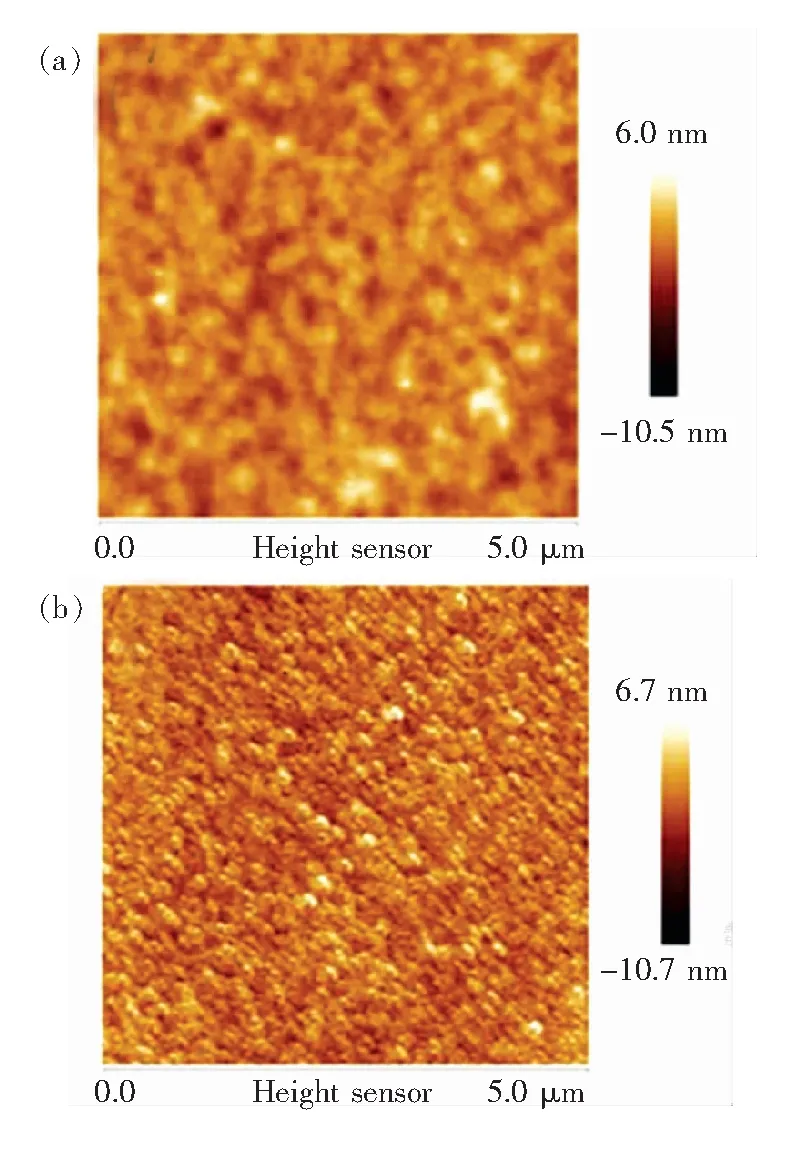
Fig.3 AFM surface images of bare ITO (a) and ITO/UV-ozone treated Ag (1 nm) (b)
X-ray photoelectron spectroscopy (XPS) measurements were carried out. Fig.4 shows the XPS spectra of Ag 3d core levels for the pristine Ag (1 nm)/ITO, 1 min UV-ozone treated Ag (1 nm)/ITO. For the thermally deposited pristine Ag (1 nm)/ITO film, the Ag 3d peak located at 368.2 eV and it is in good agreement with former literature data[38-39]. When Ag was UV-ozone treated for 1 min, the binding energy of Ag 3d peaks shifted to 367.4 eV, suggesting that Ag0was reduced to Ag+in this cases[40]. The low binding shift of the Ag 3d spectra indicates the transition from Ag to Ag2O after 1 min UV-ozone treatment. Therefore, it is clear that the UV-ozone treatment is an effective method to change Ag into AgOxwhich is a p-type semiconductor with high work function. The work function of the UV-ozone-treated Ag was determined by UPS, which is presented in Fig.7.
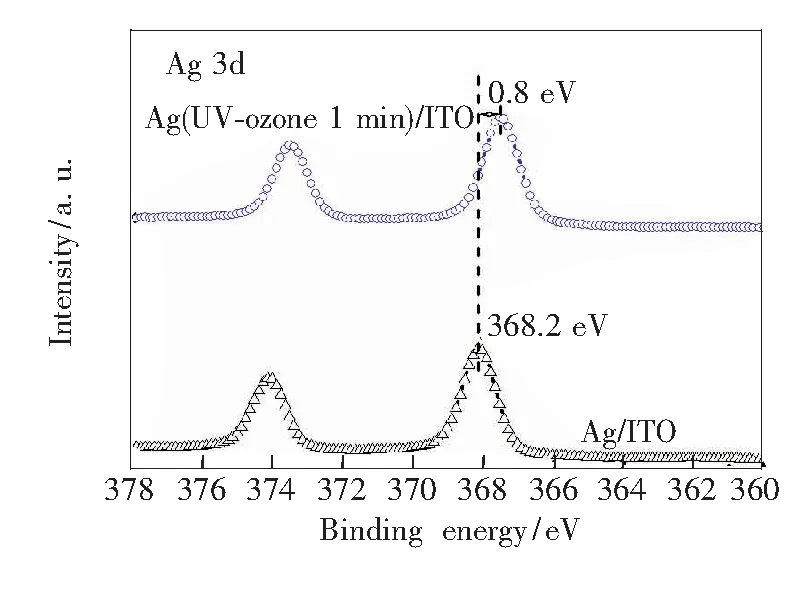
Fig.4 XPS spectra of Ag 3d of ITO coated with ultrathin Ag treated by different UV-ozone time
Besides the reduction of energy barrier at the anode/polymer interface due to the formation of Ag2O, the transmittance of the Ag (1 nm)/ITO film also slightly enhances after UV-ozone treatment shown in Fig.5. Therefore, the improvement ofJscis associated with the decrease of the contact resistance and the improvement of the film transmittance.
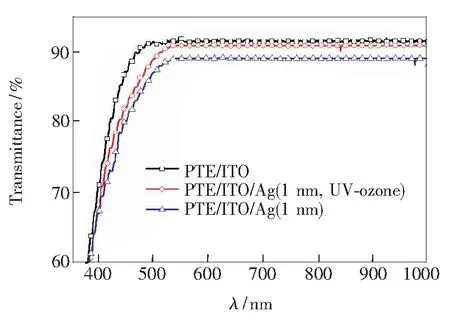
Fig.5 Optical transmittance of bare ITO, ITO/Ag (1 nm, UV-ozone) and ITO/Ag (1 nm) films, respectively.
Although the performance of the devices with Ag2O interfacial layer was improved, the PCE of the devices was still lower because the AgOxcan not fully covered the ITO surface which will influence free charges collection. Based on this reason, PEDOT∶PSS or MoO3was spin-coated/deposited on top of Ag2O/ITO substrates to form a continuous electrode modification layer. The current density-voltage (J-V) characteristics of the devices with the structure of ITO (UV-ozone 10 min)/Ag (1 nm, UV-ozone 1 min)/PEDOT∶PSS or MoO3are shown in Fig.6 and their performance data are listed in Tab.2, respectively. As can be seen from Fig.6, the device with PEDOT∶PSS exhibits an optimal PCE of 2.93%. TheVockeeps 0.59 V,Jscincreases from 8.21 to 8.77 mA/cm2and FF from 0.44 to 0.57, resulting in the notable increase of PCE from 2.01% based on ITO (UV-ozone 10 min)/PEDOT∶PSS to 2.93%. Although theJscincreased very slightly, there was a moderate increase in FF of these devices which led to an improvement in the PCE. The enhancement of FF is related to both of the shunt resistance (Rsh) and the series resistance (Rs) (seen from Tab.2) that stems from the smooth effect of UV-ozone treated Ag together with PEDOT∶PSS layer. It is also noted that devices with MoO3exhibit similar efficiency improvement and the maximum PCE is 2.97%. In addition to the formation of Ag2O after UV-ozone treatment, the increment of the efficiency of the device with UV-ozone treated Ag together with PEDOT∶PSS or MoO3as compound anode buffer layer can also be ascribed to the incorporation of Ag2O with PEDOT∶PSS or MoO3, which will increase the work function of ITO anode.

Fig.6 Current-density (J-V) characteristics of the devices with UV-ozone treated Ag anode interfacial layer together with PEDOT∶PSS or MoO3
Tab.2 Summary of the performance of devices with UV-ozone treated Ag anode interfacial layer together with PEDOT∶PSS or MoO3

UVOdurationVoc/VJsc/(mA·cm-2)FF/%PCE/%Rs/(Ω·cm2)Rsh/(Ω·cm2)ITO/PEDOT0.568.2144.052.0140322ITO/Ag(1nm,1min)/PEDOT0.598.7756.532.9316757ITO/MoO30.568.2843.582.0240323.6ITO/Ag(1nm,1min)/MoO30.598.7657.562.9713.5610.7
The device structure is ITO/Ag(1 nm)/PEDOT∶PSS (MoO3)/P3HT∶PCBM/LiF/Al.
The UPS spectra of UV-ozone treated Ag for 1 min, Ag/PEDOT∶PSS, Ag/MoO3on ITO, and bare ITO are shown in Fig.7. From the UPS spectra, the work function of ITO is determined to be 4.7 eV. This value is consistent with the commonly accepted values[41]. The work functions of the other ITO surfaces modified by 1 nm Ag with UV-ozone treatment, Ag (1 nm, UV-ozone 1 min)/ PEDOT∶PSS, and Ag (1 nm, UV-ozone 1 min)/MoO3, are measured to be 5.0, 5.2, 5.3 eV, respectively. From these measured data, we observe that there are about 0.3-0.6 eV increase in the work function of ITO from ITO/Ag surface treated by UV-ozone to Ag (1 nm, UV-ozone 1 min)/MoO3, respectively. The results indicate that the barrier at the anode/polymer interface was reduced, and thus leads to better device performance.

Fig.7 UPS spectra of various samples: A: ITO only, B: ITO/Ag (1 nm) with 1min UV-ozone treatment, C: ITO/Ag (1 nm, UV-ozone 1 min)/PEDOT∶PSS, D: ITO/Ag (1 nm, UV-ozone 1 min)/MoO3.
The energy barrier decreased significantly, the metal/polymer interface avoids the exciton recombination on the Ag islands from the photoactive layer and extracts holes efficiently, which results in the increasement ofJscand FF. Additionally, it was also found that the performance of the devices with 3 min UV-ozone treated Ag interfacial film degraded, which arose from over oxidation of ultrathin Ag film and thus degrade interface properties and increase sheet resistance[42].
Recently, the polymer poly[[2,6′-4,8-di(5-ethylhexylthienyl) benzo[1,2-b;3,3-b] dithiophene]3- fluoro-2[(2- ethylhexyl)carbonyl] thieno[3,4-b]thiophenediyl] (PBDTTT-EFT) (also called PTB7-Th) has ttracted intense attention due to the excellent device performance in both polymer/fullerene and polymer/polymer solar cells[43]. In this article, we use the high efficiency blend of the polymer PBDTTT-EFT with PC71BM as active layer materials to achieve higher device efficiency. As shown in Fig.8, the higher PCE of 6.21% of flexible OPVs based on PBDTTT-EFT∶PC71BM as an active layer using 1min-UV-ozone-treated Ag/PEDOT∶PSS composited layer as anode modification was obtained. The parameters of this device areJscof 13.7 mA/cm2,Vocof 0.79 V, and FF of 0.574, respectively. The performance of the device could compare with the reported OSCs with PBDTTT-EFT∶PC71BM as active layer based on glass substrate (Jscof 14.88 mA/cm2,Vocof 0.79 V, FF of 0.645 and PCE of 7.58%)[44]. This results indicated that the method of modification the ITO flexible electrode can be applied in the high efficiency polymer solar cells.

Fig.8 Current density-voltage (J-V) characteristics of the device with ultrathin UV-ozone treated Ag/PEEOT∶PSS based on PBDTTT-EFT∶PC71BM photoactive layer under illumination
4 Conclusion
In this paper, we show two kinds of flexible electrode modification approaches and study their effects on the performance of organic photovoltaics. By inserting ultra-thin Ag with UV-ozone treatment, it shows that the device with 1 nm Ag thin layer of 1 min-UV-ozone treatment together with MoO3or PEDOT∶PSS improves power conversion efficiency from 2.02% to 2.97% for device with MoO3and 2.01% to 2.93% for device with PEDOT∶PSS. The efficiency improvement of device with UV-ozone treated Ag lies in the increment of work function of AgOxdemonstrated by UPS and XPS. The device with Ag/PEDOT∶PSS or Ag/MoO3composited buffer layer between ITO and photoactive layer can further improve smooth of the ITO surface, and then significantly enhance the efficiency of the devices.
[1] LI G, ZHU R, YANG Y. Polymer solar cells [J].Nat.Photon., 2012, 6:153-161.
[2] LIN Q F, HUANG H T, JING Y,etal.. Flexible photovoltaic technologies [J].J.Mater.Chem. C, 2014, 2:1233-1247.
[3] CHEN S L, DAI Y J, ZHAO D W,etal.. ITO-free flexible organic photovoltaics with multilayer MoO3/LiF/MoO3/Ag/MoO3as transparent electrode [J].Semicond.Sci.Technol., 2016, 31(5):055013.
[4] HU T, LI F, YUAN K,etal.. Efficiency and air-stability improvement of flexible inverted polymer solar cells using ZnO/poly (ethylene glycol) hybrids as cathode buffer layers [J].ACSAppl.Mater.Interf., 2013, 5 5763-5770.
[5] 吴晓晓, 李福山, 吴薇, 等. 基于石墨烯/PEDOT∶PSS叠层薄膜的柔性OLED器件 [J]. 发光学报, 2014, 35(4):486-490. WU X X, LI F S, WU W,etal.. Flexible organic light emitting diodes based on double-layered graphene/PEDOT∶PSS conductive film [J].Chin.J.Lumin., 2014, 35(4):486-490. (in Chinese)
[6] YAO K, XIN X K, CHUEN C C,etal.. Enhanced light-harvesting by integrating synergetic microcavity and plasmonic effects for high performance ITO-free flexible polymer solar cells [J].Adv.Funct.Mater., 2015, 25:567-574.
[7] SONG S M, YANG T L, LIU J J,etal.. Rapid thermal annealing of ITO films [J].Appl.Surf.Sci., 2011, 257:7061-7064.
[8] CHOI K H, JEONG J A, KANNG J W,etal.. Characteristics of flexible indium tin oxide electrode grown by continuous roll-to-roll sputtering process for flexible organic solar cells [J].Sol.Energy.Mater.Sol.Cells, 2009, 93:1248-1255.
[9] STEC H M, HATTON R A. Widely applicable coinage metal window electrodes on flexible polyester substrates applied to organic photovoltaics [J].ACSAppl.Mater.Interf., 2012, 4:6013-6020.
[10] HAMASHA M M, DHAKAL T, ALZOUBI K,etal.. Stability of ITO thin film on flexible substrate under thermal aging and thermal cycling conditions [J].J.Disp.Technol., 2012, 8:383-388.
[11] ARMSTRONG N R, VENEMAN P A, RATCLIFF E,etal.. Oxide contacts in organic photovoltaics: characterization and control of near-surface composition in indium-tin oxide (ITO) electrodes [J].Acc.Chem.Res., 2009, 42:1748-1757.
[12] WANG D B, ZENG W J, CHEN S L,etal.. Effect of a cathode buffer layer on the stability of organic solar cells [J].Semicond.Sci.Technol., 2015, 30 (6):085017.
[13] JOUANE Y, COLIS S, SCHMERBER G,etal.. Influence of flexible substrates on inverted organic solar cells using sputtered ZnO as cathode interfacial layer [J].Org.Electron., 2013, 14:1861-1868.
[14] LU Z, CHEN X H, ZHOU J P,etal.. Performance enhancement in inverted polymer solar cells incorporating ultrathin Au and LiF modified ZnO electron transporting interlayer [J].Org.Electron., 2015, 17:364-370.
[15] ZENG W J, BI R, ZHANG H M,etal.. The effect of the hole injection layer on the performance of single layer organic light-emitting diodes [J].J.Appl.Phys., 2014, 116:224502.
[16] 胡雪花, 李福山, 徐胜, 等. 稀释溶剂对PEDOT∶PSS薄膜和有机太阳能电池性能的影响 [J]. 发光学报, 2014, 35(3):322-326. HU X H, LI F S, XU S,etal.. Effect of solvent dilution on preparation of PEDOT∶PSS transparent conductive films and device performance of organic solar cells [J].Chin.J.Lumin., 2014, 35(3):322-326. (in Chinese)
[17] 赵丹, 徐登辉, 杨在发, 等. 旋涂法酸处理PEDOT∶PSS薄膜对OLED性能的影响 [J]. 发光学报, 2016, 37(2):174-180. ZHAO D, XU D H, YANG Z F,etal.. Optical and electrical properties of PEDOT∶PSS films treated by spin coating with acid for organic light-emitting diodes [J].Chin.J.Lumin., 2016, 37(2):174-180. (in Chinese)
[18] ZHANG R C, WANG M Y, YANG L Y,etal.. Polymer solar cells using a PEDOT∶PSS/Cu nanowires/PEDOT∶PSS multilayer as the anode interlayer [J].Chin.Phys.Lett., 2015, 32(7):077202.
[19] MANDERS J R, TSANG S W, HARTEL M J,etal.. Solution-processed nickel oxide hole transport layers in high efficiency polymer photovoltaic cells [J].Adv.Funct.Mater., 2013, 23:2993-3001.
[20] JASIENIAK J J, SEIFTER J, JO J,etal.. A solution-processed MoOxanodex interlayer for use within organic photovoltaic devices [J].Adv.Funct.Mater., 2012, 22:2594-2605.
[21] WU NA, LUO Q, WU Z W,etal.. Influence of electrode interfacial buffer layers on thermal stability of P3HT∶PC61BM solar cells [J].ActaPhys.-Chim.Sinica, 2015, 31(7):1413-1420.
[22] YANG Q Q, YANG D B, ZHAO S L,etal.. UV-ozone-treated MoO3as the hole-collecting buffer layer for high-efficiency solution-processed SQ∶PC71BM photovoltaic devices [J].Chin.Phys. B, 2014, 23(3):038405.
[23] CHO S P, YEO J S, KIM D Y,etal.. Brush painted V2O5hole transport layer for efficient and air-stable polymer solar cells [J].Sol.EnergyMater.Sol.Cells, 2015, 132:196-203.
[24] CHEN L, XIE C, CHEN Y W. Optimization of the power conversion efficiency of room temperature- fabricated polymer solar cells utilizing solution processed tungsten oxide and conjugated polyelectrolyte as electrode interlayer [J].Adv.Funct.Mater., 2012, 4:3986-3995.
[25] KOUSKOUSSA B, MORSLI M, BENCHOUK K,etal.. On the improvement of the anode/organic material interface in organic solar cells by the presence of an ultra-thin gold layer [J].Phys.Stat.Sol. (a), 2009, 206:311-315.
[26] YAMBEMN S D, LIAO K S, CURRAN S A. Flexible Ag electrode for use in organic photovoltaics [J].Sol.EnergyMater.Sol.Cells, 2011, 95:3060-3064.
[27] YAMBEMN S D, LIAO K S, ALLEY N J,etal.. Stable organic photovoltaics using Ag thin film anodes [J].J.Mater.Chem., 2012, 22:6894-6898.
[28] HALDAR A, YAMBEM S D, LIAO K S,etal.. Organic photovoltaics using thin gold film as an alternative anode to indium tin oxide [J].ThinSolidFilms, 2011, 519:6169-6173.
[29] CHEN C W, HSIEH P Y, CHIANG H H,etal.. Top-emitting organic light-emitting devices using surface-modified Ag anode [J].Appl.Phys.Lett., 2003, 83:5127-5129.
[30] WANG C D, CHOY W C H. Efficient hole collection by introducing ultra-thin UV-ozone treated Au in polymer solar cells [J].Sol.EnergyMater.Sol.Cells, 2011, 95:904-908.
[31] DAS S, ALFORD T L. Improved efficiency of P3HT∶PCBM solar cells by incorporation of silver oxide interfacial layer [J].J.Appl.Phys., 2014, 116:044905.
[32] ZHANG H M, CHOY W C H. Indium tin oxide modified by Au and vanadium pentoxide as an efficient anode for organic light-emitting devices [J].IEEETrans.ElectronDev., 2008, 55:2517-2520.
[33] ZHU X L, SUN J X, YU X M,etal.. Investigation of Al-and Ag-based top-emitting organic light-emitting diodes with metal oxides as hole-injection layer [J].Jpn.J.Appl.Phys., 2007, 46:1033-1036.
[34] WANG Z K, LOU Y H, NAKA S G,etal.. Bias and temperature dependent charge transport in solution-processed small molecular mixed single layer organic light emitting devices [J].Appl.Phys.Lett., 2011, 98:063302.
[35] JEONG W, LEE J, PARK S Y,etal.. Reduction of collection efficiency of charge carriers with increasing cell size in polymer bulk heterojunction solar cells [J].Adv.Funct.Mater., 2011, 21(2):343-347.
[36] SNAITH H J. How should you measure your excitonic solar cells [J].EnergyEnviron.Sci., 2012, 5(4):6513-6520.
[37] CATTIN L, LARE Y, MAKHA M,etal.. Effect of the Ag deposition rate on the properties of conductive transparent MoO3/Ag/MoO3multilayers [J].Sol.EnergyMater.Sol.Cells, 2013, 117:103-109.
[38] WEAVER J F, HOFLUND G B. Surface characterization study of the thermal decomposition of AgO [J].J.Phys.Chem., 1994, 98:8519-8524.
[39] GAARENSTROOM S W, WINOGRAD N. Initial and final state effects in the ESCA spectra of cadmium and silver oxides [J].J.Chem.Phys., 1977, 67:3500.
[40] WATERHOUSE G I N, BOWMAKER G A, METSON J B. Oxidation of a polycrystalline silver foil by reaction with ozone [J].Appl.Surf.Sci., 2001, 183:191-204.
[41] LEE T W, CHUNG Y. Control of the surface composition of a conducting polymer complex film to tune the work function [J].Adv.Funct.Mater., 2008, 18:2246-2252.
[42] LIU C H, YU X. Silver nanowire-based transparent, flexible, and conductive thin film [J].NanoscaleRes.Lett., 2011, 6:75-82.
[43] CUI C, WONG W Y, LI Y. Improvement of open-circuit voltage and photovoltaic properties of 2D-conjugated polymers by alkylthio substitution [J].EnergyEnviron.Sci., 2014, 7:2276-2284.
[44] WANG D, ZHANG F J, LI L L,etal.. Tuning nanoscale morphology using mixed solvents and solvent vapor treatment for high performance polymer solar cells [J].RSCAdv., 2014, 4:48724-48733.

闫敏楠(1992-),女,宁夏固原人,硕士研究生,2014年于杭州电子科技大学获得学士学位,主要从事有机发光二极管的研究。
E-mail: 1214063415@njupt.edu.cn

张宏梅(1966-),女,吉林松原人,博士,教授,博士生导师,2006年于吉林大学获得博士学位,主要从事半导体光电子器件与器件物理的研究。
E-mail: iamhmzhang@njupt.edu.cn
2016-12-07;
2017-03-03
973国家重大科学研究计划(2015CB932203); 国家自然科学基金(91233117, 51333007); 江苏省自然科学基金(BK2012834)资助项目 Supported by 973 National Basic Research Program of China (2015CB932203); National Natural Science Foundation of China (91233117,51333007) ; Natural Science Fund of Jiangsu Province (BK2012834)
紫外臭氧处理超薄银修饰ITO电极的高效柔性有机太阳能电池
闫敏楠1,2, 郑 爽1,2, 王丹蓓1,2, 刘 缓1,2, 张宏梅1,2*
(1. 南京邮电大学 有机电子与信息显示国家重点实验室培育基地信息材料与纳米技术研究院, 江苏 南京 210023; 2. 南京邮电大学 江苏国家先进材料协同创新中心, 江苏 南京 210023)
以紫外臭氧处理超薄Ag复合MoO3或PEDOT∶PSS修饰ITO电极的高效柔性有机太阳能电池。通过优化紫外臭氧处理Ag薄膜的时间,提高了以P3HT∶PCBM为有源层的器件的功率转换效率,从1.68%(未经过紫外臭氧处理)提高到2.57%(紫外臭氧处理Ag 1 min)。提高的原因推测是紫外臭氧处理形成了AgOx薄膜,提高了电荷提取并使器件具有高光学透明度、低串联电阻和优异的表面功函数等一些性能。并且,紫外臭氧处理Ag薄膜与MoO3或者PEDOT∶PSS复合修饰ITO的器件效率分别得到提高,Ag薄膜与MoO3复合修饰ITO的器件效率从2.02%(PET/ITO/MoO3)提高到2.97%(PET/ITO/AgOx/MoO3),Ag薄膜与PEDOT∶PSS复合修饰ITO的器件效率从2.01%(PET/ITO/PEDOT∶PSS)提高到2.93%(PET/ITO/AgOx/PEDOT∶PSS)。此外,以PBDTTT-EFT∶PC71BM为有源层的柔性聚合物太阳能电池效率可达6.21%。基于ITO的柔性光电器件效率的提高主要归于ITO被Ag/PEDOT∶PSS 或 Ag/MoO3修饰后功函数的提高。
柔性有机太阳能电池; 电极修饰; 紫外臭氧处理; 超薄Ag
1000-7032(2017)07-0882-09
TP384.1 Document code: A
10.3788/fgxb20173807.0882
*Corresponding Author, E-mail: iamhmzhang@njupt.edu.cn

