羊膜上皮细胞旁分泌特点及其在皮肤损伤修复中的作用
付寅生,张怡,张婷婷,芦慧颖,孟庆雪
(1老年性疾病干细胞技术国家地方联合工程技术研究中心,哈尔滨 150028;2黑龙江天晴干细胞股份有限公司,哈尔滨 150028;3哈尔滨富尔斯特生物工程有限责任公司,哈尔滨 150431)
综述
羊膜上皮细胞旁分泌特点及其在皮肤损伤修复中的作用
付寅生2,3,张怡1,2*,张婷婷1,2,芦慧颖1,2,孟庆雪1,2
(1老年性疾病干细胞技术国家地方联合工程技术研究中心,哈尔滨 150028;2黑龙江天晴干细胞股份有限公司,哈尔滨 150028;3哈尔滨富尔斯特生物工程有限责任公司,哈尔滨 150431)
皮肤损伤修复是一个复杂而又高度协同的生物学过程,多种生长因子及白介素、趋化因子等细胞因子参与该过程,并调控皮肤愈合过程中创面的再上皮化、新血管生成以及细胞外基质沉积与重塑3个重要环节。人羊膜上皮细胞是一种类胚胎干细胞,广泛应用于创面损伤修复研究中。许多研究表明,人羊膜上皮细胞能够通过旁分泌作用促进皮肤创面的愈合。这些旁分泌因子不仅能够抑制创面微环境中细胞的凋亡,还能促进新血管生成和上皮再生。在此,本文综述人羊膜上皮细胞旁分泌特点,探讨人羊膜上皮细胞来源的旁分泌因子在创面愈合中的作用机制,并针对人羊膜上皮细胞或人羊膜上皮细胞来源细胞因子溶液应用于皮肤损伤治疗的未来可行性进行详细阐述。
羊膜上皮细胞;细胞因子;旁分泌;创面修复
人羊膜上皮细胞(human amniotic epithelial cell, hAECs)是人羊膜组织中最主要的干细胞之一,因其具有抗炎、抗纤维化、免疫原性低等特点,在再生医学研究领域获得广泛的关注[1,2]。作为一种多潜能干细胞,hAECs不仅能够表达胚胎干细胞相关的表面标记和多种多潜能干细胞转录因子(SSEA-3、SSEA-4、Oct-4、Sox-2、Nanog、Rex-1、FGF-4、CFC-1、DPPA-3等)[2,3],还具有向3个胚层分化的潜能[4-6]。此外,hAECs能够通过旁分泌作用释放多种细胞因子,包括表皮生长因子(EGF)、成纤维生长因子(FGF)、神经生长因子(NGF)、血管内皮生长因子(VEGF)、胰岛素样生长因子(IGF)等生长因子;粒细胞集落刺激因子(G-CSF)、粒细胞-巨噬细胞集落刺激因子(GM-CSF)等集落刺激因子;IL-6、CCL2、CXCL8等白介素和趋化因子[7-9]。这些细胞因子具有不同的亚型,可通过与靶细胞表面受体的特异性结合激活相关的信号通路,调节细胞的增殖、分化、迁移以及炎症反应和细胞外基质(ECM)的产生,在皮肤创伤、角膜烧伤、肺损伤、脑脊髓炎、卵巢损伤、脑出血等组织损伤修复研究中显示了重要的作用[10]。
皮肤创面愈合是一个复杂的生理过程,包括细胞迁移、炎症反应、血管生成、肉芽组织形成、上皮组织再生和细胞外基质重构。这些生理过程由多个信号通路交错形成极其复杂的信号网络调控机制完成,涉及组织、细胞、分子、基因等多个层面。正常皮肤损伤愈合过程一旦受到供血不足、糖尿病、肾病、重创、衰老等因素的影响,其关键愈合机制会被阻断,进而发展成为慢性皮肤创面[11]。目前干细胞技术是治疗慢性皮肤创面最有潜力的方法之一。本文将综述hAECs的旁分泌特点及其在皮肤创面修复中的作用机制。
1 hAECs旁分泌特点
大量研究表明,hAECs能够分泌多种生物活性蛋白,包括生长因子、白介素、趋化因子等(表1),这些蛋白在体内局部形成复杂的分泌蛋白网络,并通过调控分子与分子、分子与细胞、细胞与细胞间的相互作用,激活或阻断相关信号通路,获得免疫调节、调控细胞增殖、分化、迁移以及抑制细胞凋亡等功能[9,37]。早期研究发现,羊膜或羊膜细胞来源的细胞培养液中含有多种促进细胞增殖和组织再生的细胞因子,如EGF、VEGF、bFGF、血管生成素(ANG)、PDGF、TGFβ(1,2)、组织金属蛋白酶抑制剂-1,2(TIMP-1,2)[8,12],并且在动物体内移植后能够显著提高急、慢性大鼠创面皮肤的愈合能力[8,39]。随后Grzywocz等[7]利用抗体阵列技术检测出羊膜细胞条件培养基中含有表皮生长因子、成纤维生长因子、造血生长因子等生长因子家族中的41种生长因子及受体,并发现了7种未报道过的羊膜细胞来源生长因子,包括FGF-6、IGFBP-6、IGFBP-4、VEGFR3、M-CSF-R、PDGF-AB、神经营养因子(NT4)。我们近期的研究也发现,培养条件对hAECs旁分泌能力有较大影响,培养体系中额外加入10ng/ml EGF、显著提高了hAECs的增殖能力的同时造成了PDGF、VEGF的分泌量明显降低和TGFβ1的分泌量明显增高(未发表的数据)。由此分析,采用不同制备工艺获得的hAECs将对炎症细胞浸润、新血管生成、上皮再生以及肉芽组织形成等皮肤愈合过程产生不同影响。
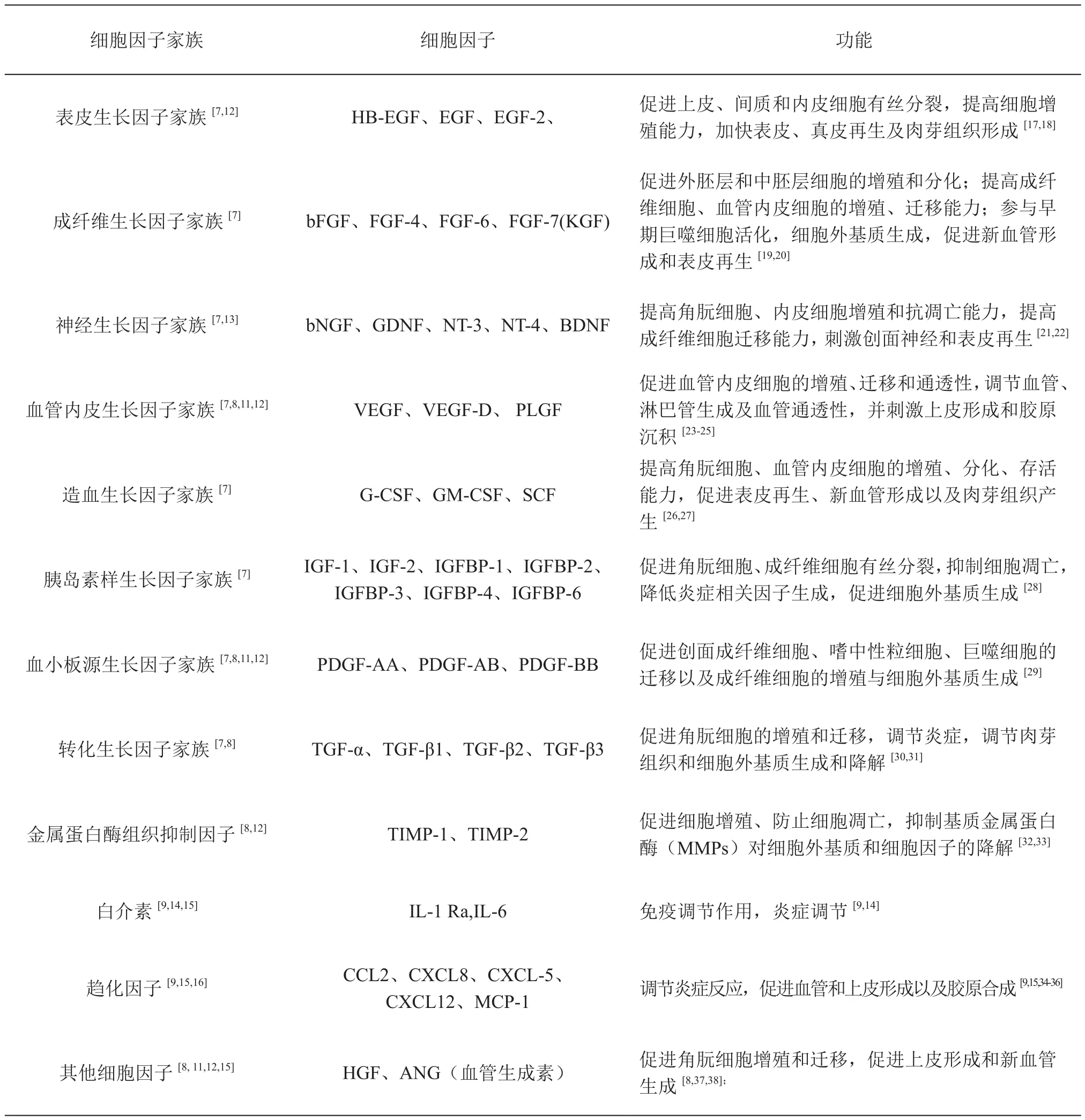
表1 羊膜上皮细胞旁分泌因子Table1 Cytokine secretory profiles of hAECs
白介素也是hAECs旁分泌因子之一,通过ELISA和蛋白芯片检测发现, hAECs在体外培养过程中向无血清培养体系中释放白介素1受体拮抗剂(IL-1Ra)和白介素6(IL-6)[14,15]。IL-1Ra是IL-1家族成员, hAECs可通过分泌IL-1Ra抑制角膜上皮细胞IL-1与IL-1R的结合,从而抑制角膜炎症反应[14]。IL-6与趋化因子CCL2、CXCL8是Th2相关细胞因子,能够介导抗炎症机制[9]、刺激细胞增殖和分化、促血管生成作用[15]、参与表皮角朊细胞的增殖和创面的修复[40]。趋化因子CXCL5、CXCL12同样是能够在hAECs来源的条件培养基中检测出的炎症相关因子[16],可诱导中性粒细胞、巨噬细胞等炎症细胞的募集,并能有效刺激角朊细胞的迁移[36]。此外Song等[15]研究发现,低氧环境能够刺激hAECs在体外培养中对ANG、EGF、IL-6、MCP-1的释放,这些细胞因子分别在损伤组织愈合、免疫因子释放、新血管生成中有重要作用。该团队后续研究结果证实,hAECs注射到心肌梗死大鼠的病灶位置后会持续分泌ANG、EGF、IL-6、MCP-1促进新血管的生成[15]。炎症反应是皮肤损伤愈合的关键时期,在创伤刺激下炎症细胞向伤口床募集,并促进炎症相关因子的分泌促进创面的愈合。hAECs分泌的炎症因子不仅含有炎症促进因子,还包括炎症抑制因子,但在皮肤创面微环境中hAECs的炎症因子分泌特点以及对炎症反应的调节机制仍不十分明确。
2 hAECs旁分泌因子在促进皮肤创面愈合中的作用
皮肤创面愈合过程是一个复杂而又高度协调的生物学过程。创面上皮形成、新血管生成以及细胞外基质的产生与重塑是皮肤创面愈合过程中重要的3个环节,因此与这些生物学过程相关的表皮角朊细胞、真皮成纤维细胞以及血管内皮细胞的增殖和迁移作用是皮肤创面快速愈合的关键。皮肤受损会刺激创周细胞迁移与分化,并释放大量细胞因子调控炎症的发生、细胞外基质的沉积、血管的生成、表皮的再生以及组织重塑[41,42]。研究表明,干细胞或干细胞细胞因子溶液移植到皮肤损伤位置能够通过旁分泌因子激活局部信号网络,提高细胞有丝分裂能力,刺激靶细胞迁移,调控细胞外基质的沉积与降解,促进皮肤创面的愈合[43,44]。体外和体内实验研究表明,hAECs能够通过旁分泌作用调控皮肤损伤部位多种细胞的生物学功能,在提高创面愈合能力以及皮肤附属器的再生中具有显著作用[16]。皮肤创面的再上皮化是皮肤愈合的重要环节之一,细胞因子及相关信号网络参与上皮形成的完整过程。皮肤发生损伤时会造成皮肤表层屏障的缺失,此时中性粒细胞、单核细胞和巨噬细胞会募集于伤口床与创面邻近组织中细胞,如角朊细胞、成纤维细胞、内皮细胞、间质细胞、黑素细胞等大量释放并交换细胞因子,同时角朊细胞在多种细胞因子调控下被激活。激活后的角朊细胞的细胞骨架和表面受体发生改变,引起细胞与细胞、细胞与细胞外基质间连接(桥粒、半桥粒)发生解离,刺激角朊细胞向伤口床迁移及分化,促进再上皮化过程(图1)[37,45]。表皮角朊细胞的增殖、分化和迁移能力直接关系着皮肤创面再上皮化能力。在hAECs的旁分泌因子中,EGF、HB-EGF、TGF-α、IGF-1、FGF-2(bFGF)、FGF-7(KGF)、HGF、VEGF-A、GM-CSF、TGFβ1以及转录因子CXCL8等细胞因子在调控角朊细胞增殖和迁移中具有重要的促进作用[36,46],并通过刺激细胞外基质的合成与重塑以及新血管生成过程协同提高表皮再生能力[46,47]。最新的一项研究结果发现,hAECs来源条件培养基能够活化下游信号通路中ERK、JNK以及AKT的磷酸化作用,通过调节MAPK、AKT信号途径促进角朊细胞的体外迁移能力[16]。此外,在共培养条件下,hAECs能够诱导角朊细胞中细胞周期蛋白(cyclin D1和cyclin D1)以及Mdm2表达水平的增加,并通过AKT途径促进角朊细胞DNA的合成(S期细胞数增加),提高角朊细胞的存活与增殖能力[16]。随后的体内实验进一步发现,hAECs来源条件培养基能够显著促进ERK、JNK、AKT抑制因子作用下C57BL/5小鼠的皮肤创面愈合速度,证明hAECs来源旁分泌因子能够通过调控MAPK、AKT途径促进上皮形成,加快创面的闭合[16]。
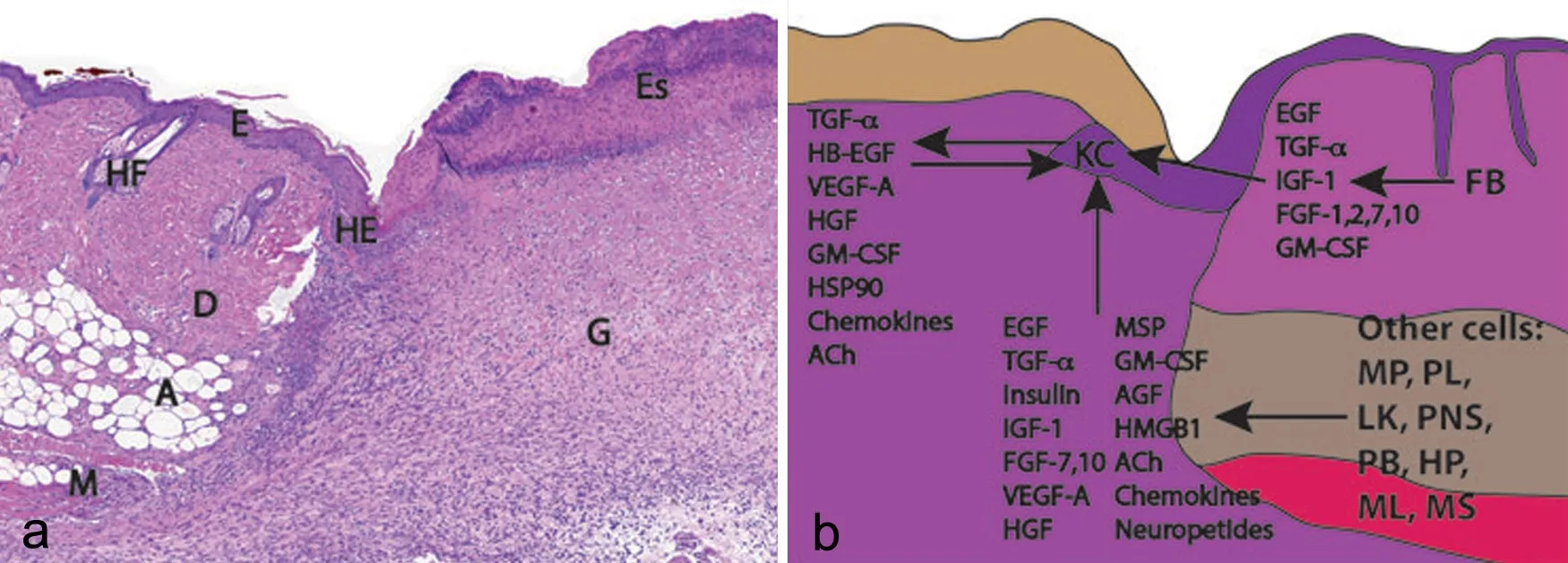
图1 全层皮肤创面愈合示意图。a,小鼠皮肤疮面愈合HE染色观察;b,模式图[48];HE, 增生表皮; E, 表皮; HF, 毛囊; D, 真皮; A, 脂肪组织; M, 肌肉; Es, 血痂; G, 肉芽组织; KC, 角朊细胞; FB, 成纤维细胞; MP, 巨噬细胞; PL, 血小板; LK, 白细胞; PNS, 周围神经系统细胞; PB, 胰岛β细胞; HP, 肝细胞; ML, 黑素细胞; MS, 间充质干细胞Fig. 1 Histologic (a) and schematic (b) representations of a healing full-thickness excisional wound in mouse skin. HE, hyperproliferative epidermis; E, epidermis; HF, hair follicle; D, dermis; A, adipose tissue; M, muscle; Es, eschar; G, granulation tissue; KC, keratinocytes; FB, fbroblasts; MP, macrophages; PL, platelets; LK, leukocytes; PNS, peripheral nervous system cells; PB, pancreatic β-cells; HP, hepatocytes; ML, melanocytes; MS, mesenchymal stem cells.
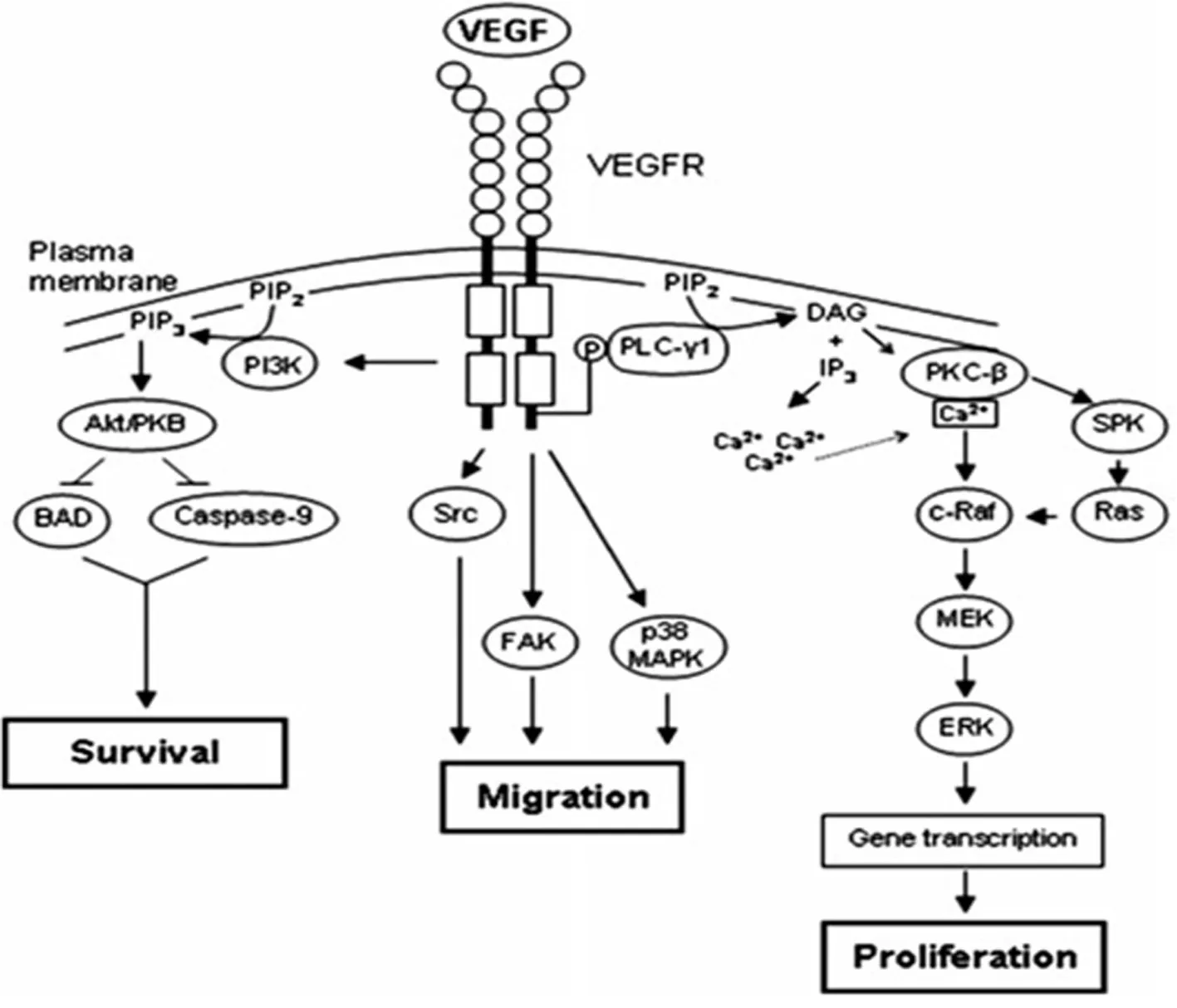
图2 VEGFR信号通路调控血管内皮细胞增殖与迁移[49]Fig. 2 VEGFR signaling regulates the proliferation and migration of vascular endothelial cells
新血管的生成同样在创面愈合中具有重要作用。这一过程在慢性、难愈合性皮肤创面中新血管的生长尤为重要,血管化能力的降低不仅影响肉芽组织的形成,还影响着上皮的再生[48-50]。而新血管形成过程是通过多种生长因子、趋化因子、细胞亚群之间相互复杂的作用关系调控的[61]。EGF、PDGF、ANG、VEGF、IL-6、bFGF等细胞因子是hAECs主要的旁分泌因子[51,15,49,52],具有提高血管内皮细胞增殖和迁移能力、促进新生血管的生成作用。其中VEGF是皮肤创面新血管生成过程中核心调控因子之一。VEGF家族通过结合并激活细胞表面的酪氨酸激酶受体VEGFR2,刺激下游信号通路级联反应的发生,以此调控血管内皮细胞增殖与迁移(图2)[49]。经体外培养后,hAECs培养液中VEGF含量显著高于原培养液,并随培养时间的延长而累积(未发表的数据)。近期研究也发现,炎性因子TNFα、IFNβ的刺激下hAECs能够进一步提高促血管生成因子VEGFA、PDGFB、ANGPT1以及转录因子FOXC1的表达,促进新血管的生成,加快创伤组织的修复[51]。此外,在患有Ⅲ期压疮的大鼠模型研究中进一步证实,hAECs体内移植能够通过上调VEGF的表达,促进皮肤创面的愈合[53]。因此,hAECs分泌的VEGF可能是早期创面加快愈合的重要因子,并通过VEGF途径促进皮肤创面内新血管的形成。
细胞外基质形成与组织重塑是皮肤创面完整愈合进程中的重要生物学过程,在组织损伤炎症期、增生期完成细胞外基质的合成、分泌与沉积,并于皮肤创面修复后期完成细胞外基质的重塑[54]。创面损伤初期嗜中性粒细胞、单核细胞、淋巴细胞由周围血管向创面浸润,其中嗜中性粒细胞清除病原体及病变组织,并通过TGF-β、PDGF、IL-1等细胞因子调控淋巴细胞的迁移和巨噬细胞的生成[55,56]。巨噬细胞释放大量细胞因子,如FGF、TGF-β、PDGF、EGF等调控成纤维细胞、血管内皮细胞的增殖、迁移以及细胞外基质的合成,诱导肉芽组织形成[57,58]。TGFβ是创面愈合过程中调控细胞外基质沉积与重塑的关键因子,是hAECs可分泌的生长因子之一[7]。TGF-β家族包括TGF-β1、TGF-β2和TGF-β3,在皮肤组织内由巨噬细胞、成纤维细胞、角朊细胞和血小板合成、分泌[55]。TGFβ1和TGFβ2在创面早期愈合过程中募集成纤维细胞和炎症细胞进入伤口床,可通过抑制由成纤维细胞、巨噬细胞和嗜中性粒细胞所释放的金属蛋白酶(MMPs),防止细胞外基质的降解,促进肉芽组织形成,血管生成和胶原的合成和产生[54,55]。TGFβ3与TGFβ1和TGFβ2作用相反,在疤痕形成中对TGFβ1有拮抗作用[59]。在小疤痕或无疤痕组织中,如口腔黏膜,TGFβ1的水平下降,同时TGFβ3/TGFβ1比率显著上升[60]。本实验室体外研究曾发现,生长状态下hAECs培养液中TGFβ1的含量随培养时间的延长而增加,而TGFβ3含量无明显变化,表明hAECs在营养交换过程中能够增加TGFβ1在周围环境中的释放(未发表的数据)。另一项最新的研究结果发现TGFβ1诱导活化的真皮成纤维细胞在hAECs条件培养基处理下降低了向成肌纤维转化能力,并通过降低TGFβ1/Smad3信号通路中Smad2和Smad3磷酸化作用抑制成纤维细胞中胶原蛋白的生成,同时,hAECs培养液中的活性因子对活化状态下真皮成纤维细胞中纤维化相关蛋白的表达具有负反馈调节作用,以此抑制瘢痕的形成[61]。以上研究表明hAECs的旁分泌因子可能参与调节皮肤创面愈合过程中胶原的沉积与重塑。
3 展望
最近的基础研究表明,hAECs主要通过旁分泌作用影响皮肤创面的愈合速度,主要体现为促新血管生成以及表皮再生[51,16]。这些研究虽然在一定程度上阐述了hAECs旁分泌作用对皮肤损伤修复中的作用机制,但仍然缺少充足的证据揭示hAECs在皮肤创面微环境中的具体作用机理。EGF、TGFβ、CXCL8、VEGF、TIMPs等因子虽然在创面愈合过程中对血管生成、细胞外基质沉积具有重要的促进作用,但需要注意的是在不同愈合阶段,这些关键蛋白分泌量的异常仍存在降低创面愈合质量的可能性[55,66-69],如胶原蛋白过度增生导致肥厚性疤痕的产生[69],炎症期延长以及血管、肉芽组织过度增殖引起上皮化减慢[56,66,67],而每个蛋白家族中不同亚型也同样存在着双向调控作用(如TGFβ)[59],这些因素为hAECs在创面愈合中的具体作用机制的探索增加了较大的困难。体外培养研究发现,hAECs具有较低的增殖能力以及培养过程中易发生上皮-间质转化现象[70],对未来大规模临床转化有一定限制,这些关键性问题给hAECs的临床转化研究带来了新的课题,如体内移植的hAECs处于皮肤创面愈合不同阶段微环境下的细胞因子分泌特点及分子调控机制;hAECs和hAECs来源细胞因子溶液在规模化生产、质量控制以及有效性、安全性等方面相对比,哪种更适合作为终端产品用于创伤治疗。
虽然hAECs或hAECs来源细胞因子溶液在皮肤组织损伤修复中的具体作用机制仍不十分明确,但hAECs旁分泌作用具有明显的促组织修复、再生以及抗炎症作用已得到证实[37,38,48,62,63],表明hAECs或hAECs来源细胞因子溶液是未来可应用于难愈合性皮肤损伤的潜在治疗方法之一。目前clinicaltrials.gov上登记的hAECs或羊膜细胞来源细胞因子溶液(amnion-derived cell cytokine solution,ACCS)相关临床研究项目包括了眼表损伤、支气管瘘、原发性卵巢功能障碍、难愈合性糖尿病足等多种难治性疾病[65]。皮肤损伤修复临床研究用的hAECs主要以hAECs悬液、hAECs来源细胞因子溶液以及负载hAECs的羊膜组织产品形式存在,其中已有多款富含活性hAECs的羊膜产品在欧美批准上市,用于难治性皮肤损伤及眼科疾病的治疗,如AmnioGenix公司的AmnioMTMTM;BioTissue公司的AmnioGraft®;Osiric Therapeutics公司的Grafx®等,这些成功的产品化案例证实了hAECs在皮肤创面治疗中的应用前景。
[1] Vosdoganes P, Wallace EM, Chan ST, et al. Human amnion epithelial cells repair established lung injury. Cell Transplant, 2013, 22(8)∶ 1337-1349.
[2] Bilic G, Zeisberger SM, Mallik AS, et al. Comparative characterization of cultured human term amnion epithelial and mesenchymal stromal cells for application in cell therapy. Cell Transplant, 2008, 17(8)∶ 955-968.
[3] Miki T, Lehmann T, Cai H, et al. Stem cell characteristics of amniotic epithelial cells. Stem Cell, 2005, 23(10)∶ 1549-1559.
[4] Ilancheran S, Michalska A, Peh G, et al. Stem cells derived from human fetal membranes display multilineage diferentiation potential. Biol Reprod, 2007, 77(3)∶ 577-588.
[5] Pratama G, Vaghjiani V, Tee JY, et al. Changes in cultur expanded human amniotic epithelial cells∶ implications for potential therapeutic applications. PLoS One, 2011, 6(11)∶ e26136.
[6] García-Castro IL, García-López G, Ávila-González D, et al. Markers of pluripotency in human amniotic epithelial cells and their differentiation to progenitor of cortical neurons. PLoS One, 2015, 10(12)∶ e0146082.
[7] Grzywocz Z, Pius-Sadowska E, Klos P, et al. Growth factors and their receptors derived from human amniotic cells in vitro. Folia Histochem Cytobiol, 2014, 52(3)∶ 163-170.
[8] Steed DL, Trumpower C, Duffy D, et al. Amnion-derived cellular cytokine solution a physiological combination of cytokines for wound healing. Eplasty, 2008, 8∶ e18.
[9] Insausti CL, Blanquer M, García-Hernández AM, et al. Amniotic membrane-derived stem cells∶ immunomodulatory properties and potential clinical application. Stem Cells Cloning, 2014, 7∶ 53-63.
[10] Hodge A, Lourensz D, Vaghjiani V, et al. Soluble factors derived from human amniotic epithelial cells suppress collagen production in human hepatic stellate cells. Cytotherapy, 2014, 16(8)∶ 1132-1144.
[11] Ojeh N, Pastar I, Tomic-Canic M, et al. Stem cells in skin regeneration, wound healing and their clinical applications. Int J Mol Sci, 2015, 16(10)∶ 25476-254501.
[12] Wolbank S, Hildner F, RedL H, et al. Impact of human amniotic membrane preparation on release of angiogenic factors. J Tissue Eng Regen Med, 2009, 3(8)∶ 651-654.
[13] 张晓明, 孙海梅, 杨慧, 等. 人羊膜上皮细胞分泌神经营养因子诱导人脐血间充质干细胞向神经元样细胞的分化∶ 可能性验证. 中国组织工程研究与临床康复, 2010, 14(6)∶ 973-978.
[14] 李彬斌, 周清, 姚敏, 等. 人羊膜上皮细胞培养液抑制角膜炎症的实验研究. 器官移植, 2013, 4(1)∶ 12-18.
[15] Song YS, Joo HW, Park IH, et al. Transplanted human amniotic epithelial cells secrete paracrine proangiogenic cytokines in rat model of myocardial infarction. Cell Transplant, 2015, 24(10)∶ 2055-2064.
[16] Zhao B, Liu JQ, Zheng Z, et al. Human amniotic epithelial stem cells promote wound healing by facilitating migration and proliferation of keratinocytes via ERK, JNK and AKT signaling pathways. Cell Tissue Res, 2016, 365(1)∶ 85-99.
[17] Alemdaroğlu C, Degim Z, Celebi N, et al. Investigation of epidermal growth factor containing liposome formulation effects on burn wound healing. J Biomed Mater Res A, 2008, 85(1)∶ 271-283.
[18] Alemdaroğlu C, Değim Z, Celebi N, et al. An investigation on burn wound healing in rats with chitosan gel formulation containing epidermal grow factor. Burns, 2006, 32(3)∶ 319-327.
[19] Thilagar S, Jothi NA, Omar AR, ET al. Effect of keratin-gelatin and bFGF-gelatin composite flm as a sandwich layer for full-thickness skin mesh graft in experimental dogs. J Biomed Mater Res B Appl Biomater, 2009, 88(1)∶12-16.
[20] Akita S, Akino K, Imaizumi T, et al. Basic fbroblast growth factor accelerates and improves seconddegree burn wound healing. Wound Repair Regen, 2008, 16(5)∶ 635-641.
[21] Harsum S, Clarke JD, Martin P. A reciprocal relationship between cutaneous nerves and repairing skin wounds in the developing chick embryo. Dev Biol, 2001, 238(1)∶ 27-39.
[22] Raychaudhuri SK, Raychaudhuri SP, Weltman H, et al. Effect of nerve growth factor on endothelial cell biology∶proliferation and adherence molecule expression on human dermal microvascular endothelial cells. Arch Dematol Res, 2001, 293(6)∶ 291-295.
[23] Paavonen K, Puolakkainen P, Jussila L, et al. Vascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healing. Am J Pathol, 2000, 156(5)∶ 1499-1504.
[24] Papapetropoulos A, Pyriochou A, Altaany Z, et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci USA, 2009, 106(51)∶ 21972-21977.
[25] Bao P, Kodra A, Tomic-Canic M, et al. The role of vascular endothelial growth factor in wound healing. J Surg Res, 2009, 153(2)∶ 347-358.
[26] Mann A, Breuhahn K, Schirmacher P, et al. Keratinocyte-derived granulocyte-macrophage colony stimulating factor accelerates wound healing∶ stimulation of keratinocyte proliferation, granulation tissue formation, and vasculariztion. J Invest Dermatol, 2001, 117(6)∶ 1382-1390.
[27] Mann A, Niekisch K, Schirmacher P, et al. Granulocyte-macrophage colony-stimulation factor is essential for normal wound healing. J Invest Dermatol Symp Proc, 2006, 11(1)∶ 87-92.
[28] Dasu MR, Herndon DN, Nesic O, et al. IGF-1 gene transfer efects on infammatory elements present after thermal trauma. Am J Physiol Regul Integr Comp Physiol, 2003, 285(4)∶ R741-746.
[29] Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev, 2003, 83(3)∶ 835-870.
[30] Singer AJ, Huang SS, Huang JS, et al. A novel TGF-beta antagonist speeds reepithelialization and reduces scarring of partial thickness porcine burns. J Burn Care Res, 2009, 30(2)∶ 329-334.
[31] Chen W, Fu X, Ge S, et al. Ontogeny of expression of transforming growth factor-beta and its receptors and their possible relationship with scarless healing in human fetal skin. Wound Repair Regen, 2005, 13(1)∶ 68-75.
[32] Ladwig GP, Robson MC, Liu R, et al. Ratios of activated matrix metalloproteinase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fuids are inversely correlated with healing of pressure ulcers. Wound Repair Regen, 2002, 10(1)∶ 26-37.
[33] Ching YH, Sutton TL, Pierpont YN, et al. The use of growth factors and other humoral agents to accelerate and enhance burn wound healing. Eplasty, 2011, 11∶ e41.
[34] 陈金安, 柳岚, 蒋克春, 等. 糖尿病足溃疡中趋化因子的作用及机制. 国际内分泌代谢杂志, 2015, 35(5)∶ 357-359.
[35] Kroeze KL, Boink MA, Sampat-Sardjoepersad SC, et al. Autocrine regulation of re-epithelialization after wounding by chemokine receptors CCR1, CCR10, CXCR1, CXCR2, and CXCR3. J Invest Dermatol, 2012, 132(1)∶ 216-225.
[36] Mishra PJ, Mishra PJ, Banerjee D. Cell-free derivatives from mesenchymal stem cells are efective in wound therapy. World J Stem Cell, 2012, 4(5)∶ 35-43.
[37] Seeger MA, Paller AS. The roles of growth factors in keratinocyte migration. Adv Wound Care (New Rochelle), 2015, 4(4)∶ 213-224.
[38] Hisadome M, Ohnishi T, Kakimoto K, et al. Hepatocyte growth factor reduces CXCL10 expression in keratinocytes. FEBS Lett, 2016, 590(20)∶ 3595-3605.
[39] Franz MG, Payne WG, Xing L, et al. The use of amnion-derived cellular cytokine solution to improve healing in acute and chronic wound models. Eplasty, 2008, 8∶ e21.
[40] Bhatia A, O’Brien K, Chen M, et al. Keratinocyte-secreted heat shock protein-90alpha∶ leading wound reepithelialization and closure. Adv Wound Care (New Rochelle), 2016, 5(4)∶ 176-184.
[41] Whittam AJ, Maan ZN, Duscher D, et al. Challenges and opportunities in drug delivery for wound healing. Adv Wound Care (New Rochelle), 2016, 5(2)∶ 78-88.
[42] Ghatak S, Maytin EV, Mack JA, et al. Roles of proteoglycans and glycosaminoglycans in wound healing and fbrosis. Int Cell Biol, 2015, 2015∶ 834893.
[43] Ching YH, Suttin TL, Pierpont YN, et al. The use of growth factors and other humoral agents to accelerate and enhance burn wound healing. Eplasty, 2011, 11∶ e41.
[44] Chen L, Xu Y, Zhao J, et al. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS One, 2014, 9(4)∶e96161.
[45] Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in wound healing∶ a comprehensive review. Adv Wound Care(New Rochelle), 2014, 3(7)∶ 445-464.
[46] Ponugoti B, Xu F, Zhang C, et al. FOXO1 promotes wound healing through the up-regulation of TGF-β1 and prevention of oxidative stress. J Cell Biol, 2013, 203(2)∶ 327-343.
[47] Li W, Henry G, Fan J, et al. Signal that initiate, augment, and provide directionality for human keratinocyte motility. J Invest Dermatol, 2004, 23(4)∶ 622-633.
[48] McDonald CA, Payne NL, Sun G, et al. Immunosuppressive potential of human amnion epithelial cells in the treatment of experimental autoimmune encephalomyelitis. J Neuroinfamm, 2015, 12∶ 112.
[49] Johnson KE, Wilqus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care (New Rochelle), 2014, 3(10)∶ 647-661.
[50] Nunes QM, Li Y, Sun C, et al. Fibroblast growth factors as tissue repair and regeneration therapeutics. PeerJ, 2016, 4∶e1535.
[51] Zhu D, Muljadi R, Chan ST, et al. Evaluating the impact of human amnion epithelial cells on angiogenesis. Stem Cells Int, 2016, 2016∶ 4565612.
[52] Salgado AJ, Reis RL, Sousa NJ, et al. Adipose tissue derived stem cells secretome∶ solube factors and their roles in regenerative medicine. Curr Stem Cell Res Ther, 2010, 5(2)∶ 103-110.
[53] Zhou A, Zheng X, Yu L, et al. Mechanisms of human amniotic epithelial cell transplantation in treating stage Ⅲ pressure ulcer in a rat model. Exp Ther Med, 2015, 10(6)∶ 2161-2168.
[54] Pakyari M, Farrokhi A, Maharlooei MK, et al. Critical role of transforming growth factor beta in different phases of wound healing. Adv Wound Care (New Rochelle), 2013, 2(5)∶ 215-224.
[55] Barrientos S, Stojadinovic O, Golinko MS, et al. Growth factors and cytokines in wound healing. Wound Repair Regen, 2008, 16(5)∶ 585-601.
[56] Zaja-milatovic S, Richmond A. CXC chemokines and their receptors∶ a case for a signifcant biological role in cutaneous wound healing. Histol Histopathol, 2008, 23(11)∶ 1399-1407.
[57] Behm B, Babilas P, Landthaler M, et al. Cytokines, chemokines and growth factors in wound healing. J Eur Acad Dermatol Venereol, 2012, 26(7)∶ 812-820.
[58] Xu F, Zhang C, Graves DT. Abnormal cell responses and role of TNF-α in impaired diabetic wound healing. Biomed Res Int, 2013, 2013∶ 754802.
[59] Waddington SN, Crossley R, Sheard V, et al. Gene delivery of a mutant TGFβ3 reduces markers of scar tissue formation after cutaneous wounding. Mol Ther, 2010, 18(12)∶2104-2111.
[60] Schrementi ME, Ferreira AM, Zender C, et al. Site-specific production of TGF-beta in oral mucosal and cutaneous wounds. Wound Repair Regen, 2008, 16(1)∶ 80-86.
[61] Zhao B, Liu JQ, Yang C, et al. Human amniotic epithelial cells attenuate TGF-β1-induced human dermal fibroblast transformation to myofbroblasts via TGF-β1/Smad3 pathway. Cytotherapy, 2016, 18(8)∶ 1012-1024.
[62] Khanna S, Biswas S, Shang Y, et al. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One, 2010, 5(3)∶ e9539.
[63] Salonurmi T, Parikka M, Kontusaari S, et al. Overexpression of TIMP-1 under the MMP-9 promoter interferes with wound healing in transgenic mice. Cell Tissue Res, 2004, 315(1)∶ 27-37.
[64] Tredget EB, Demare J, Chandran G, et al. Transforming growth factor-beta and its effect on reepithelialization of partial-thickness ear wounds in transgenic mice. Wound Repair Regen, 2005, 13(1)∶ 61-67.
[65] Pratama G, Vaghjiani V, Tee JY, et al. Changes in culture expanded human amniotic epithelial cells∶ implications for potential therapeutic applications. PLoS One, 2011, 6(11)∶e26136.
[66] Yao X, Guo Y, Wang Q, et al. The paracrine efect of transplanted human amniotic epithelial cells on ovarian function improvement in a mouse model of chemotherapy-induced primary ovarian insufciency. Stem Cells Int, 2016, 2016∶4148923.
[67] Moodley Y, Vaghjiani V, Chan J, et al. Anti-infammatory efects of adult stem cells in sustained lung injury∶ a comparative study. PLoS One, 2013, 8(8)∶ e69299.
[68] https∶//www.clinicaltrials.gov/ct2/results?term=Amniotic+-cell&pg=1
[69] Iocono JA, Colleran KR, Remick DG, et al. Interleukin-8 levels and activity in delayed-healing human thermal wounds. Wound Repair Regen, 2000, 8(3)∶ 216-225.
The paracrine effect of human amniotic epithelial cells and their roles in wound healing
Fu Yinsheng2,3, Zhang Yi1,2*, Zhang Tingting1,2, Lu Huiying1,2, Meng Qingxue1,2,
(1National and local joint stem cell research & engineering center for aging diseases, Harbin 150028, China ;2Heilongjiang Tian Qing Stem Cell Co., Ltd, Harbin 150028, China ;3Harbin First Bio-Engineering Co., Ltd, Harbin 150431, China)
Human skin wound healing is a complex and highly coordinated biological process in which growth factors, interleukins and chemokines play a part and regulate three critical steps namely re-epithelialization, neoangiogenesis, and extracellular matrix (ECM) deposition and remodeling. As primitive stem cells, human amniotic epithelial cells (hAECs) are widely applied in wound healing research. Recent evidence reveals that hAECs facilitate wound healing by secreting soluble bioactive factors via paracrine to protect recipient cells from apoptosis, stimulate neovascularization and promote re-epithelialization. This review will focus on the secretory profles of hAECs and their roles in wound healing. The therapeutic potential of hAECs or hAECs-derived cytokine solution in wound healing is also discussed.
Human amniotic epithelial cells; cytokine; paracrine; wound healing
R458
A
10.16705/ j. cnki. 1004-1850.2017.02.015
2016-12-13
2017-03-30
黑龙江省自然科学基金项目(C201430)
付寅生,男(1986年),汉族,工程师
*通讯作者(To whom correspondence should be addressed):neo_yi_zhang@163.com

