脓毒症患者内皮细胞蛋白C受体基因rs867186A/G位点多态性观察
梁燕冰,覃月秋,蒋玉洁,黄霞,李军 ,彭丁伟, 黄雨晴, 廖品琥
(右江民族医学院附属医院,广西百色533000)
脓毒症患者内皮细胞蛋白C受体基因rs867186A/G位点多态性观察
梁燕冰,覃月秋,蒋玉洁,黄霞,李军 ,彭丁伟, 黄雨晴, 廖品琥
(右江民族医学院附属医院,广西百色533000)
目的 观察脓毒症患者外周血内皮细胞蛋白C受体(EPCR)基因rs867186A/G位点多态性。方法 64例脓毒症患者(其中男38例、女26例)为脓毒症组,113例健康体检者为对照组。采集观察组、对照组5 mL外周静脉血,检测两组外周血EPCR基因rs867186A/G位点的多态性,采用ELISA法检测两组血浆可溶型EPCR(sEPCR)。分析EPCR基因rs867186A/G位点多态性对脓毒症患者血浆sEPCR表达及病情严重程度的影响 结果 脓毒症组AA、AG、GG三种基因型和A、G等位基因的分布频率分别为81.2%、17.2%、1.6%、89.8%、10.2%,对照组分别为83.2%、16.8%、0、91.6%、8.4%,两组间EPCR基因型及等位基因频率间差异均无统计学意义(P均>0.05)。EPCR基因 rs867186A/G位点携带等位基因A的男性较携带相同等位基因的女性患脓毒症的风险高(OR=1.735;95%CI: 1.063~2.833,P<0.05)。脓毒症组血浆sEPCR水平高于对照组但差异无统计学意义(P均>0.05)。不同rs867186A/G位点基因型脓毒症患者血浆sEPCR水平、病情严重程度间差异均无统计学意义(P均>0.05)。结论 脓毒症患者外周血EPCR基因rs867186A/G位点上多为AA基因型或A等位基因。EPCR基因rs867186A/G位点多态性可能与脓毒症发病风险升高有关。
脓毒症;内皮细胞蛋白C受体;基因多态性
脓毒症是由感染引起的全身炎症反应综合征[1],发病率高,一旦发生病情较重,严重时可导致感染性休克、多器官功能障碍综合征等,患者病死率高[2]。目前脓毒症已成为ICU危重患者最常见的死亡原因。深入研究脓毒症的发病原因和机制对其治疗具有重要意义。单核苷酸基因多态性作为人群、地域的一种标志,对人类疾病的发生发展有着重要影响。研究表明脓毒症的发生发展与遗传因素密切相关[3],内皮细胞蛋白C受体(EPCR)基因多态性与脓毒症[4]、血栓性疾病[5]、乳腺癌[6]、疟疾[7]等疾病的临床预后有关。最新研究发现,EPCR在内皮损伤和炎症病变的发生过程中起重要作用[3]。但目前关于EPCR基因多态性与脓毒症相关性方面报道较少。我们观察了脓毒症患者外周血EPCR基因rs867186A/G位点的多态性,分析其与血浆可溶型EPCR(sEPCR)水平的关系,旨在为脓毒症患者发病风险评估提供理论依据。
1 资料与方法
1.1 临床资料 选取右江民族医学院附属医院重症医学科2014年7月~2015年12月入院的脓毒症患者(脓毒症组)64例,其中男38例、女26例,年龄(57.45±15.80)岁,均符合2012年国际脓毒症诊断标准[8],其中严重脓毒症17例、脓毒性休克41例。排除标准为:①年龄<18岁或>80岁;②心脏停搏者;③需行急诊手术者;④不愿接受中心静脉置管或有置管禁忌者。另选取同期门诊的健康体检人群(对照组)113例,其中男82例、女31例,年龄(55.3±11.7)岁,全部体检人群临床和实验室检查指标均在正常范围内。所有纳入对象间均无血缘关系,两组性别、年龄比例间差异无统计学意义。本研究经本院伦理委员会批准,纳入者均知情同意并签署知情同意书。
1.2 两组外周血EPCR基因rs867186A/G位点多态性观察 于观察组入院24 h内用EDTA-K2抗凝采血管收集5 mL外周静脉血,对照组采集清晨空腹静脉血。①采用离心柱法提取两组外周血DNA,-70 ℃保存备用,试剂盒为DNeasy Blood & Tissue Kit(Qiagen, Germany),所有操作均严格按照使用说明书进行;②采用单碱基延伸PCR技术扩增目的基因片段,EPCR基因rs867186A/G 的序列来自NCBI的核苷酸序列(Gene ID: 10544),采用Primer3 软件设计(http://bioinfo.ut.ee/primer3-0.4.0/)引物,并由上海天昊生物科技有限公司合成。rs867186上游引物:5′-ATGGACTCCTTGGGGGCCTATT-3′;下游引物:5′-GTGGGCAGATGTGGGAGAAGAA-3′, 20 μLPCR反应体系包括:1×GC-I buffer(TaKaRa)、3.0 mmol/L Mg2+、0.3 mmol/L dNTP、 1 μmmol/L HotStarTaq polymerase (Qiagen Inc.)、1 μL 样本DNA、1 μL多重PCR 引物。PCR循环程序:95 ℃ 预变性2 min,94 ℃ 变性 20 s, 65 ℃ 退火 40 s, 72 ℃ 延伸 1.5 min,重复 11 个循环; 然后 94 ℃ 变性20 s,59 ℃ 退火30 s,72 ℃ 延伸 1.5 min,重复 24 个循环; 72 ℃ 延伸2 min,产物4 ℃保存备用;③使用ABI3730XL测序仪对纯化后的PCR产物(SNaPshot Multiplex Kit, ABI, USA)进行测序,所得的原始数据使用GeneMapper 4.1(Applied Biosystems Co, Ltd, USA)进行分析。
1.3 两组血浆可溶型EPCR(sEPCR)检测 于观察组入院24 h内用采血管收集5 mL外周静脉血,对照组采集清晨空腹静脉血。自然凝固后,离心取上清,-70 ℃保存备用。采用ELISA法检测两组sEPCR,所有操作均严格按照使用说明书进行。
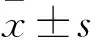
2 结果
2.1 两组外周血EPCR基因 rs867186A/G位点多态性 两组外周血EPCR基因 rs867186A/G位点基因型分布符合Hardy-Weinberg遗传平衡定律,说明实验样本具有群体代表性。基因分型结果表明,rs867186A/G位点存在AA、AG、GG三种基因型。两组外周血EPCR基因 rs867186A/G位点多态性比较见表1。不同性别两组外周血EPCR基因 rs867186A/G位点多态性比较见表2。由表2可见,EPCR基因 rs867186A/G位点携带等位基因A的男性较携带相同等位基因的女性患脓毒症的风险高(OR=1.735;95%CI: 1.063~2.833,P<0.05)。
2.2 两组血浆sEPCR水平比较 脓毒症组、对照组血浆sEPCR水平分别为(100.52±95.60)、(81.84±49.19)ng/mL,二者比较,P>0.05。脓毒症患者男性、女性血浆sEPCR水平分别为(110.35±103.89)、(86.16±81.85)ng/mL,二者比较,P>0.05。
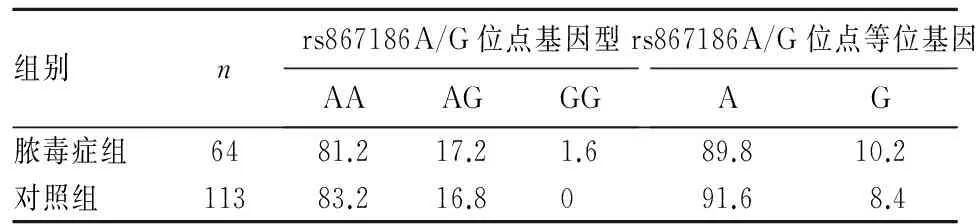
表1 两组外周血EPCR基因 rs867186A/G位点基因型和等位基因比较(%)
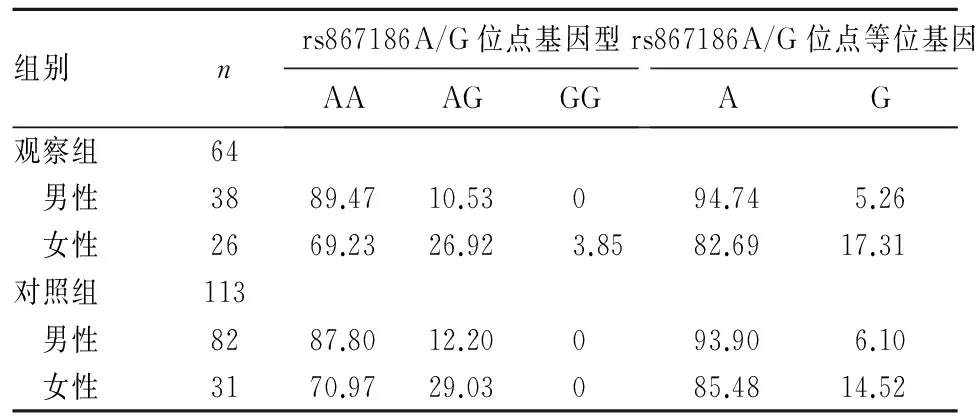
表2 不同性别两组外周血EPCR基因 rs867186A/G位点基因型和等位基因比较(%)
2.3 脓毒症患者外周血EPCR基因rs867186A/G位点多态性与血浆sEPCR水平、病情严重程度的相关性 不同rs867186A/G位点基因型脓毒症患者血浆sEPCR水平、病情严重程度间差异均无统计学意义(P均>0.05),见表3、4。

表3 不同rs867186A/G位点基因型脓毒症患者血浆sEPCR水平比较
注:因rs867186位点女性GG基因型仅1例。

表4 不同病情脓毒症患者rs867186A/G位点基因型比较(例)
注:因GG基因型例数较少,与AG基因型合并。
3 讨论
不同地区、种族的人群基因多态性之间存在明显差异,基因多态性与疾病之间的关系是目前研究的热点[9~11]。EPCR可通过激活蛋白C(PC)发挥抗炎、抗凋亡、保护内皮细胞屏障等作用[12,13]。人类EPCR基因(PROCR)位于20号染色体q11.2,全长约8 kb,含4个外显子和3个内含子。膜联型EPCR(mEPCR)在金属蛋白酶的作用下从内皮细胞表面脱落形成可溶型EPCR(sEPCR),脱落入血的sEPCR仍保留与PC/活化蛋白C(APC)结合的能力,其可通过与mEPCR竞争结合PC/APC,从而抑制mEPCR的功能[14]。研究发现EPCR存在多种基因突变,变异的EPCR基因可以通过调节APC的细胞保护和抗凝血作用从而影响膜联型EPCR(mEPCR)及sEPCR的表达[4,15]。
人类EPCR基因型可分为三种:A1、A2和A3[16]。携带A3单倍体型虽然与冠心病、脑卒中的发病风险没有影响[17],但与深静脉血栓形成[5]、女性特发性复发性流产[18]等疾病有关。本实验所选的EPCR rs867186A/G属于A3单倍体型,携带rs867186-AG基因型的患者更易发生静脉血栓形成[19], rs867186-GG基因型是重症疟疾患者的保护因素[20]。
本研究结果表明,两组EPCR基因 rs867186位点的基因型和等位基因分布趋势相同,携带rs867186 A等位基因的男性患脓毒症风险较高,提示EPCR基因多态性可能与脓毒症发病风险升高有关;对照组及脓毒症组间EPCR基因多态性与血浆sEPCR的表达水平无关,提示脓毒症的发生发展过程与sEPCR水平的高低可能无明显的关联,与其他研究结果意见相似[21,22]。也有的研究表明患者血浆sEPCR水平在脓毒症中升高[4],或者减少[23]。这种差异的形成可能是因为检测血浆sEPCR浓度的方法不同,或实验研究对象所处的种族、区域不同。同时,我们的研究结果也提示了脓毒症的病情轻重并不受rs867186A/G基因多态性的影响。
综上所述,脓毒症患者外周血EPCR基因rs867186A/G位点上多为AA型基因或携带等位基因A。EPCR基因rs867186A/G位点多态性可能与脓毒症发病风险升高有关。
[1] Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)[J]. JAMA, 2016,315(8):801-810.
[2] Gaieski DF, Edwards JM, Kallan MJ, et al. Benchmarking the incidence and mortality of severe sepsis in the United States[J]. Crit Care Med, 2013,41(5):1167-1174.
[3] Gupta DL, Nagar PK, Kamal VK, et al. Clinical relevance of single nucleotide polymorphisms within the 13 cytokine genes in North Indian trauma hemorrhagic shock patients[J]. Scand J Trauma Resusc Emerg Med, 2015,23:96.
[4] Vassiliou AG, Maniatis NA, Kotanidou A, et al. Endothelial protein C receptor polymorphisms and risk of severe sepsis in critically ill patients[J]. Intensive Care Med, 2013,39(10):1752-1759.
[5] Zoheir N, Eldanasouri N, Abdel-Aal AA, et al. Endothelial cell protein C receptor gene 6936A/G and 4678G/C polymorphisms as risk factors for deep venous thrombosis[J]. Blood Coagul Fibrinolysis, 2016,27(3):259-265.
[6] Tinholt M, Viken MK, Dahm AE, et al. Increased coagulation activity and genetic polymorphisms in the F5, F10 and EPCR genes are associated with breast cancer: a case-control study[J]. BMC Cancer, 2014,14:845.
[7] Hansson HH, Turner L, Miller L, et al. Haplotypes of the endothelial protein C receptor (EPCR) gene are not associated with severe malaria in Tanzania[J]. Malar J, 2015, 14:474.
[8] Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012[J]. Intensive Care Med, 2013,39(2):165-228.
[9] 田虹,魏殿军,何新飙. 肿瘤坏死因子α基因多态性与脓毒症易感性及感染程度的关系[J]. 山东医药,2015,55(24):17-19.
[10] 王海彦,廖品琥,熊滨,等.TLR4基因内含子区rs12377632T/C位点多态性研究[J].广西医科大学学报,2013,30(6):887-889.
[11] 胡东海, 廖品琥, 熊滨, 等. IL-8基因启动子区rs4073A/T和rs2227306C/T的多态性分析[J]. 临床与实验病理学杂志, 2013,29(12):1306-1309.
[12] Antón I, Molina E, Luis-Ravelo D, et al. Receptor of activated protein C promotes metastasis and correlates with clinical outcome in lung adenocarcinoma[J]. Am J Respir Crit Care Med, 2012,186(1):96-105.
[13] Bouwens EA, Stavenuiter F, Mosnier LO. Mechanisms of anticoagulant and cytoprotective actions of the protein C pathway[J]. J Thromb Haemost, 2013,11 (Suppl 1):242-253.
[14] Regan LM, Stearns-Kurosawa DJ, Kurosawa S, et al. The endothelial cell protein C receptor. Inhibition of activated protein C anticoagulant function without modulation of reaction with proteinase inhibitors[J]. J Biol Chem, 1996,271(29):17499-17503.
[15] Wu C, Dwivedi DJ, Pepler L, et al. Targeted gene sequencing identifies variants in the protein C and endothelial protein C receptor genes in patients with unprovoked venous thromboembolism[J]. Arterioscler Thromb Vasc Biol, 2013,33(11):2674-2681.
[16] Saposnik B, Reny JL, Gaussem P, et al. A haplotype of the EPCR gene is associated with increased plasma levels of sEPCR and is a candidate risk factor for thrombosis[J]. Blood, 2004,103(4):1311-1318.
[17] Reiner AP, Carty CL, Jenny NS, et al. PROC, PROCR and PROS1 polymorphisms, plasma anticoagulant phenotypes, and risk of cardiovascular disease and mortality in older adults: the Cardiovascular Health Study[J]. J Thromb Haemost, 2008,6(10):1625-1632.
[18] Dendana M, Messaoudi S, Hizem S, et al. Endothelial protein C receptor 1651C/G polymorphism and soluble endothelial protein C receptor levels in women with idiopathic recurrent miscarriage[J]. Blood Coagul Fibrinolysis, 2012,23(1):30-34.
[19] Yin G, Jin X, Ming H, et al. Endothelial cell protein C receptor gene 6936A/G polymorphism is associated with venous thromboembolism[J]. Exp Ther Med, 2012,3(6):989-992.
[20] Naka I, Patarapotikul J, Hananantachai H, et al. Association of the endothelial protein C receptor (PROCR) rs867186-G allele with protection from severe malaria[J]. Malar J, 2014,13:105.
[21] Kallel C, Cohen W, Saut N, et al. Association of soluble endothelial protein C receptor plasma levels and PROCR rs867186 with cardiovascular risk factors and cardiovascular events in coronary artery disease patients: the Athero Gene study[J]. BMC Med Genet, 2012,13:103.
[22] Yamagishi K, Cushman M, Heckbert SR, et al. Lack of association of soluble endothelial protein C receptor and PROCR 6936A/G polymorphism with the risk of venous thromboembolism in a prospective study[J]. Br J Haematol, 2009,145(2): 221-226.
[23] Mosnier LO, Yang XV, Griffin JH. Activated protein C mutant with minimal anticoagulant activity, normal cytoprotective activity, and preservation of thrombin activable fibrinolysis inhibitor-dependent cytoprotective functions[J]. J Biol Chem, 2007,282(45):33022-33033.
Polymorphism of endothelial protein C receptor gene rs867186A/G in patients with sepsis
LIANGYanbing,QINYueqiu,JIANGYujie,HUANGXia,LIJun,PENGDingwei,HUANGYuqing,LIAOPinhu
(AffiliatedHospitalofYoujiangMedicalUniversityforNationalities,Baise533000,China)
Objective To observe the polymorphism of endothelial protein C receptor (EPCR) gene rs867186A/G in the peripheral blood of patients with sepsis. Methods Sixty-four patients with sepsis (sepsis group, male 38, female 26) and 113 healthy subjects (control group) were selected. We collected 5 mL peripheral blood samples from both the observation group and the control group. The polymorphisms of EPCR gene rs867186A/G in the peripheral blood were observed, and serum soluble EPCR (sEPCR) of two groups was measured by ELISA. Meanwhile, we investigated the effect of EPCR gene rs867186A/G polymorphisms on plasma sEPCR level and sepsis severity. Results The frequencies of AA, AG and GG genotypes and A ,G alleles were 81.2%, 17.2%, 1.6%, 89.8% and 10.2% respectively, in sepsis group, versus 83.2%, 16.8%, 0, 91.6% and 8.4% in control group, and there was no significant difference in frequency distribution of the above genotypes and alleles of two groups (allP>0.05). Males carrying rs867186 A allele of EPCR gene were associated with a significantly increased risk of sepsis than women carrying the same alleles (OR=1.735, 95%CI: 1.063-2.833,P<0.05). The plasma sEPCR level in the sepsis group was slightly higher than that in the control group but no significant difference was found (allP>0.05). There was no significant difference in plasma sEPCR level and severity of sepsis between patients with different rs867186A/G genotypes (allP>0.05). Conclusions The AA genotype or A allele is more frequently found in EPCR gene rs867186A/G in the peripheral blood of patients with sepsis. The polymorphism of EPCR gene rs867186A/G may be associated with the increased risk of sepsis.
sepsis; endothelial protein C receptor; gene polymorphism
国家自然科学基金资助项目(81560321);2014年广西医学高层次骨干人才培养"139"计划培养人选项目;广西高校急重症分子免疫研究重点实验室项目(yy2015ky002);广西研究生教育创新计划项目(YCSZ2015222)。
梁燕冰(1993-),女,硕士研究生在读,主要研究方向为脓毒症基因学机制。E-mail: 1067532076@qq.com
廖品琥(1967-),男,教授,主任医师,博士生导师,主要研究方向为脓毒症和ARDS。E-mail: liaopinhu@163.com
10.3969/j.issn.1002-266X.2017.19.007
R446.11
A
1002-266X(2017)19-0025-04
2016-12-15)

