NMDA受体在匹罗卡品癫痫小鼠海马星形胶质细胞活化中的作用
杨展能,姜靓婧,顾仕红,陈磊,黎虹薇,朱新建
(东南大学 医学院,江苏 南京 210009)
·论 著·
NMDA受体在匹罗卡品癫痫小鼠海马星形胶质细胞活化中的作用
杨展能,姜靓婧,顾仕红,陈磊,黎虹薇,朱新建
(东南大学 医学院,江苏 南京 210009)
目的:探讨在匹罗卡品诱导的小鼠癫痫持续状态(SE)模型中,N- 甲基- D- 天门冬氨酸(NMDA)受体在海马星形胶质细胞增生中的作用及其可能的分子机制。方法:将32只6周龄雄性C57/BL6小鼠随机分为对照组、SE组、SE后注射NMDA受体拮抗剂MK- 801组和单纯注射MK- 801组,每组8只。腹腔注射300 mg·kg-1匹罗卡品建立SE模型。免疫组化方法检测各组小鼠海马脑区星形胶质细胞的形态差异,同时利用western Blot方法检测海马脑区神经胶质纤维酸性蛋白(GFAP)的表达和转录因子cAMP反应元件结合蛋白(CREB)的磷酸化水平。结果:SE组小鼠海马星形胶质细胞出现显著活化现象,免疫组化结果显示SE组小鼠海马GFAP免疫反应强度显著高于对照组(P<0.01),而在SE后给予MK- 801阻断NMDA受体则显著抑制了SE诱导的海马星形胶质细胞活化。与免疫组化结果一致,Western Blot结果显示SE组小鼠海马GFAP蛋白表达水平显著高于对照组(P<0.05),而在SE后给予MK- 801阻断NMDA受体则显著抑制了SE诱导的海马GFAP蛋白表达水平的升高。同时我们的研究结果发现,SE组小鼠海马CREB蛋白磷酸化水平显著高于对照组,而阻断NMDA受体则显著抑制了SE诱导的海马CREB蛋白磷酸化。结论:匹罗卡品癫痫小鼠持续状态7 d后海马星形胶质细胞被显著活化,而这一活化过程依赖于NMDA受体的激活。同时,海马星形胶质细胞活化的过程伴随CREB的磷酸化,提示海马CREB磷酸化参与了NMDA受体介导的匹罗卡品癫痫小鼠星形胶质细胞活化过程。
N- 甲基- D- 天门冬氨酸受体; 匹罗卡品; 癫痫; 海马; 星形胶质细胞活化; 小鼠
N- 甲基- D- 天门冬氨酸(N- methy- D- asparate, NMDA)受体一直是基础和临床研究的焦点。生理状态下,NMDA受体在突触传递、突触可塑性以及学习记忆等过程中起着重要作用[1- 4],然而当其被过度激活后所致的兴奋性毒性可导致某些神经系统疾病[5- 7]。研究发现,癫痫发作时神经元兴奋性增强使兴奋性谷氨酸递质大量释放,大量的谷氨酸递质过度激活神经元膜上的NMDA受体引起Ca2+内流,导致细胞内信号过度激活和神经元兴奋性异常增高,并最终引起中枢神经系统内某些特定神经环路结构和功能的异常改变从而引发癫痫疾病[8- 9]。癫痫发作引起的脑内特殊的病理改变,尤其是海马结构的损伤,如海马神经元丢失(neuron loss)、星形胶质细胞活化(astrocytes activation)、神经元再生(neurogenesis)、苔藓纤维发芽(mossy fiber sprouting)、炎症反应(inflammation)等[10- 15],而这些海马结构损伤是异常兴奋性突触环路形成的重要原因,可促进形成难治性癫痫疾病[16- 17]。本研究通过建立匹罗卡品(pilocarpine)小鼠癫痫持续状态(status epilepticus, SE)模型,探讨小鼠SE后海马星形胶质细胞活化的情况并揭示NMDA受体在SE后海马星形胶质细胞活化中的作用及其可能的分子机制。
1 材料和方法
1.1 动物和分组
32只6周龄雄性成年C57/BL6小鼠,体重18~22 g,由扬州大学比较实验动物中心提供。小鼠于室温18~25 ℃、人工12 h昼夜循环照明条件下饲养,自由摄食与饮水。小鼠随机分为对照组、SE组、SE后注射NMDA受体拮抗剂MK- 801组和单纯注射MK- 801组,每组8只。
1.2 主要试剂和仪器
匹罗卡品、东莨菪碱、MK- 801(Sigma Aldrich),神经胶质纤维酸性蛋白(GFAP)抗体(武汉博士得生物),磷酸化cAMP反应元件结合蛋白(pCREB)、CREB抗体(Millipore, Billerica, MA, USA),β- actin抗体(华安生物),辣根过氧化物酶偶联羊抗兔和羊抗小鼠二抗(武汉博士得生物),FITC偶联山羊抗小鼠荧光二抗(世纪康为),RIPA蛋白裂解液(碧云天),BCA蛋白定量分析试剂盒(Pierce,Rockford, IL, USA),增强型ECL发光液(Super Signal West Pico Trial Kit,Pierce),5%脱脂奶粉(BD- Difco,USA),DAPI- 抗猝灭封片剂(SouthernBiotech, Birmingham, AL, USA),硝酸纤维素膜(Amersham, LittleChalfont, UK),冷冻切片机(Leica Microsystems, Wetzlar, Germany),激光共聚焦显微镜(Olympus LSM- GB200, Japan),Western Blot化学发光图像分析系统(DNR Bio- imaging Systems, Jerusalem, Israel),蛋白电泳转移设备(Bio- Rad, Hercules, CA, USA),Immage J软件(NIH, Bethesda, MD, USA)。
1.3 动物处理
SE组、SE后注射NMDA受体拮抗剂MK- 801组小鼠建立癫痫模型:给予匹罗卡品300 mg·kg-1腹腔注射诱导SE,注射匹罗卡品前30 min给予1 mg·kg-1东莨菪碱腹腔注射以阻断外周胆碱能作用,观察动物癫痫发作,根据Racine标准[18]判定癫痫发作级别。0级:无抽搐发作;1级:耳、面部抽搐;2级:肌阵挛,但无直立位;3级:肌阵挛,伴直立位;4级:全身强直阵挛发作;5级:强直阵挛发作并失去体位控制。达到癫痫发作3~5级的存活小鼠入选。SE后注射NMDA受体拮抗剂MK- 801组小鼠在SE结束后腹腔注射1 mg·kg-1MK- 801(连续注射7 d,每天1次);对照组腹腔注射等量用于配置匹罗卡品的生理盐水;单纯注射MK- 801组小鼠腹腔注射1 mg·kg-1MK- 801(连续注射7 d,每天1次)。
1.4 Western Blot检测
取小鼠双侧海马置于RIPA蛋白裂解液中,冰浴上迅速匀浆,匀浆完毕后将样品置于冰浴上裂解15 min,2 000 r·min-1离心10 min,小心吸取上清即为蛋白提取液,-80 ℃保存。Brad法测定蛋白浓度,根据所测定的的蛋白浓度将所有蛋白样品调至等浓度,然后于待测蛋白样品中加入0.2体积的6×蛋白加样缓冲液 [300 mmo1·L-1Tris(pH 6.8)、600 mmo1·L-1DTT、12% SDS、0.6%澳酚蓝、60%甘油]100 ℃煮沸3 min, 12 000 r·min-1离心10 min,取上清至-20 ℃保存待分析。灌制12%的SDS- PAGE胶,恒压100 V电泳分离海马组织蛋白样品。电泳结束后,组织蛋白样品在转移液(9 mmol·L-1甘氨酸、48 mmo1·L-1Tris碱、0.037% SDS、20%甲醇)中300 mA恒流转移2.5 h至硝酸纤维素膜。5%脱脂奶粉封闭1 h,TBST洗膜10 min×3次,小鼠GFAP抗体(1∶2 000)和兔p-CREB/CREB抗体(1∶2 000)孵育过夜。次日,弃去一抗,TBST洗膜10min×3次,辣根过氧化物酶标记的羊抗小鼠(1∶5 000)和羊抗兔二抗(1∶10 000)孵育2h,TBST洗膜10min×3次,末次洗膜后迅速以ECL发光液试剂A液和B液各500μl混合后于膜上反应0.5~1.0min,WesternBlot化学发光系统采集信号,ImmageJ软件进行数据分析。
1.5 免疫组化检测
腹腔注射过量的乌拉坦麻醉小鼠,经左心室依次灌入200ml生理盐水和4%多聚甲醛(PA)200ml,小心将鼠脑取出并将其置于4%多聚甲醛中固定过夜。取出固定后的鼠脑,选择海马部位,用震荡切片机进行冠状连续切片,脑片厚度为25μm,每隔200μm取脑片1张,每只小鼠取12张脑片,收集于PBS(pH7.4)中。切片于50%甲酰胺(formamide,Sigma)/2×SSC中65 ℃加热2h,PBS(pH=7.4)洗片5min×3次,2mol·L-1HCl37 ℃孵育30min,0.1mol·L-1硼酸(pH8.5)洗片10min,PBS洗片1次,3%H2O2(PBS稀释)室温孵育30min,PBS洗片5min×3次,封闭血清室温孵育60min,弃去封闭血清但不洗片,加入小鼠GFAP抗体(1∶200,稀释于山羊血清)4 ℃孵育过夜。次日弃去一抗并用PBS洗片5min×3次,加入FITC偶联山羊抗小鼠荧光二抗(1∶200)室温孵育2h,弃去二抗并用PBS洗片5min×3次。染好的脑片爬片,晾干后用中DAPI抗猝灭封片剂封片。普通荧光显微镜下观察海马GFAP阳性细胞,激光共聚焦显微镜拍照。
1.6 统计学处理
采用SigmaPlot(11.0)软件进行统计学分析,数据以平均数±标准误表示,组间多样本均数比较采用ANOVA方差分析。P<0.05为差异具有统计学意义。
2 结 果
2.1 匹罗卡品癫痫小鼠持续状态行为学观察
小鼠腹腔注射匹罗卡品(300 mg·kg-1)10~15 min后出现运动减少、凝视不动、咀嚼、流涎、须动、湿狗样抖动、耳面部抽搐和平衡失调等行为学变化。随后进一步出现前肢阵挛及(或)肢体强直阵挛伴直立、跌倒或翻转等边缘系统运动性发作,呈持续性3~5级发作即为SE。
2.2 匹罗卡品癫痫小鼠海马星形胶质细胞活化情况
给予匹罗卡品300 mg·kg-1腹腔注射诱导SE,注射匹罗卡品前30 min给予1 mg·kg-1东莨菪碱腹腔注射以阻断外周胆碱能作用,观察动物癫痫发作,根据Racine标准[18]判定癫痫发作级别。癫痫发作未达3级、超过5级或死亡者均剔除。SE后7 d分别利用免疫组化方法和Western Blot方法检测海马GFAP表达情况(图1)。免疫组化结果显示,匹罗卡品癫痫小鼠海马GFAP标记的阳性细胞数量较对照组显著增加,并且出现细胞分支增多的现象(图2A)。数据统计分析显示,匹罗卡品癫痫小鼠的GFAP免疫荧光强度显著高于对照组小鼠(图2B)。Western Blot结果显示匹罗卡品癫痫小鼠海马GFAP的蛋白水平较对照组有显著升高(图3A、B),提示匹罗卡品癫痫小鼠海马星形胶质细胞被显著活化。
2.3 匹罗卡品癫痫小鼠海马星形胶质细胞活化与NMDA受体激活的关系
为了进一步探索匹罗卡品癫痫小鼠海马星形胶质细胞被显著活化是否依赖于NMDA受体的激活,我们在SE后给小鼠持续腹腔注射NMDA受体拮抗剂MK- 801后分别利用免疫荧光和Western Blot检测海马GFAP表达情况。免疫组织化学结果显示,SE后注射NMDA受体拮抗剂MK- 801显著抑制了诱导的GFAP阳性细胞数量的增加,而单独给予小鼠MK- 801后GFAP阳性细胞数量与对照组比较未见显著差异。与免疫组织化学结果一致,Western Blot结果显示SE后注射NMDA受体拮抗剂MK- 801显著抑制了癫痫持续状态诱导的GFAP蛋白表达的增加,而单独给予MK- 801后GFAP蛋白表达水平较对照组比较未见显著差异。以上结果证实,匹罗卡品癫痫小鼠海马星形胶质细胞被显著活化依赖于NMDA受体的激活。
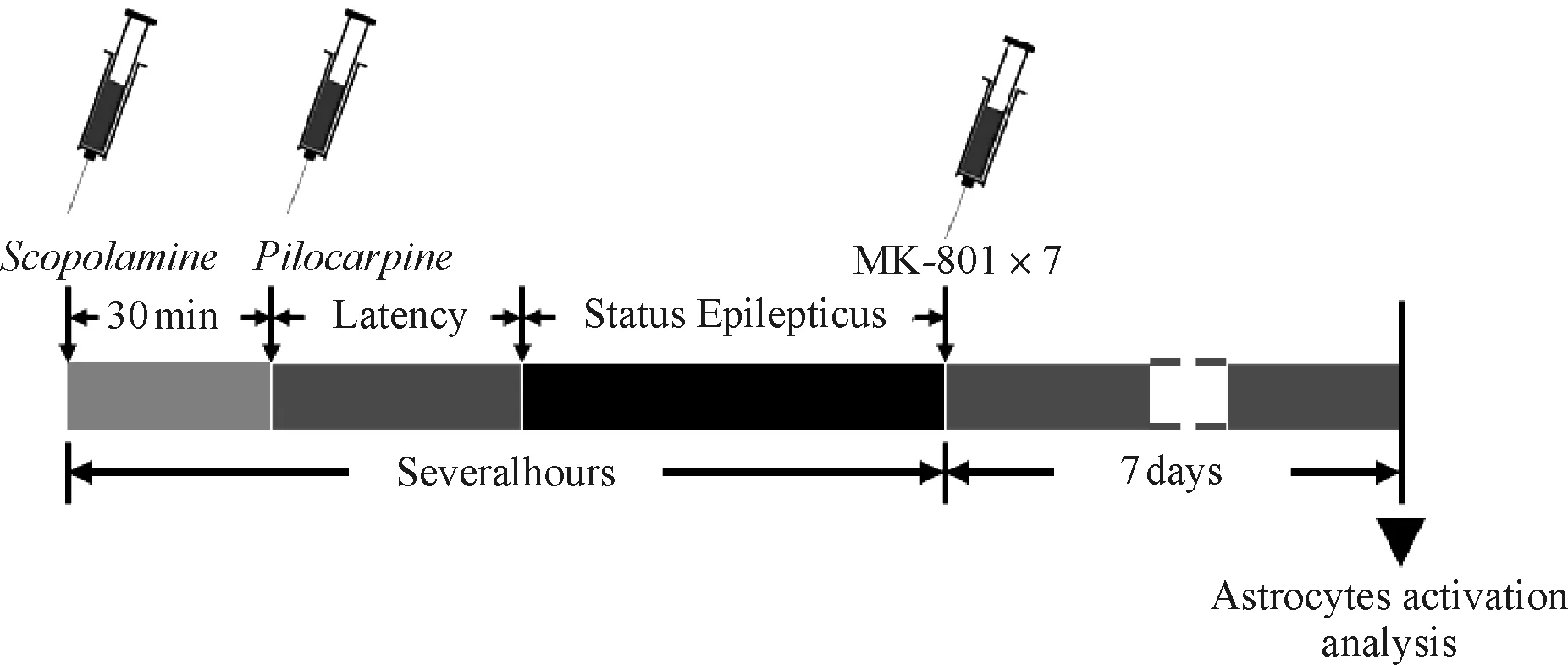
图1 小鼠匹罗卡品癫痫持续状态模型的建立
Fig 1 Schematic representation of pilocarpine- induced SE mice model
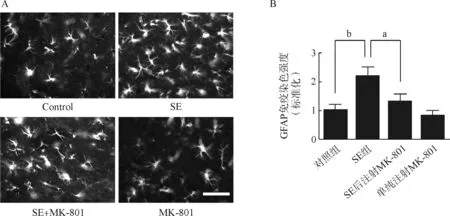
A.对照组、SE组、SE后注射NMDA受体拮抗剂MK- 801组和单纯注射MK- 801组小鼠海马GFAP免疫荧光标记;B.各组小鼠海马GFAP免疫荧光强度定量分析结果(n=5)
aP<0.05,bP<0.01,比例尺=100 μm
A.Representative immunofluorescence images of GFAP-staining in the hippocampus of Control, SE, SE+MK- 801 and MK- 801- treated mice;B.Bar graph showed the quantification of GFAP immunostaining intensity in the hippocampus of Control, SE, SE+MK- 801 and MK- 801- treated mice(n=5)
aP<0.05,bP<0.01, scale bar=100 μm
图2 免疫组化检测匹罗卡品癫痫小鼠海马星形胶质细胞活化
Fig 2 Hippocampal astrocytes were activated in SE mice
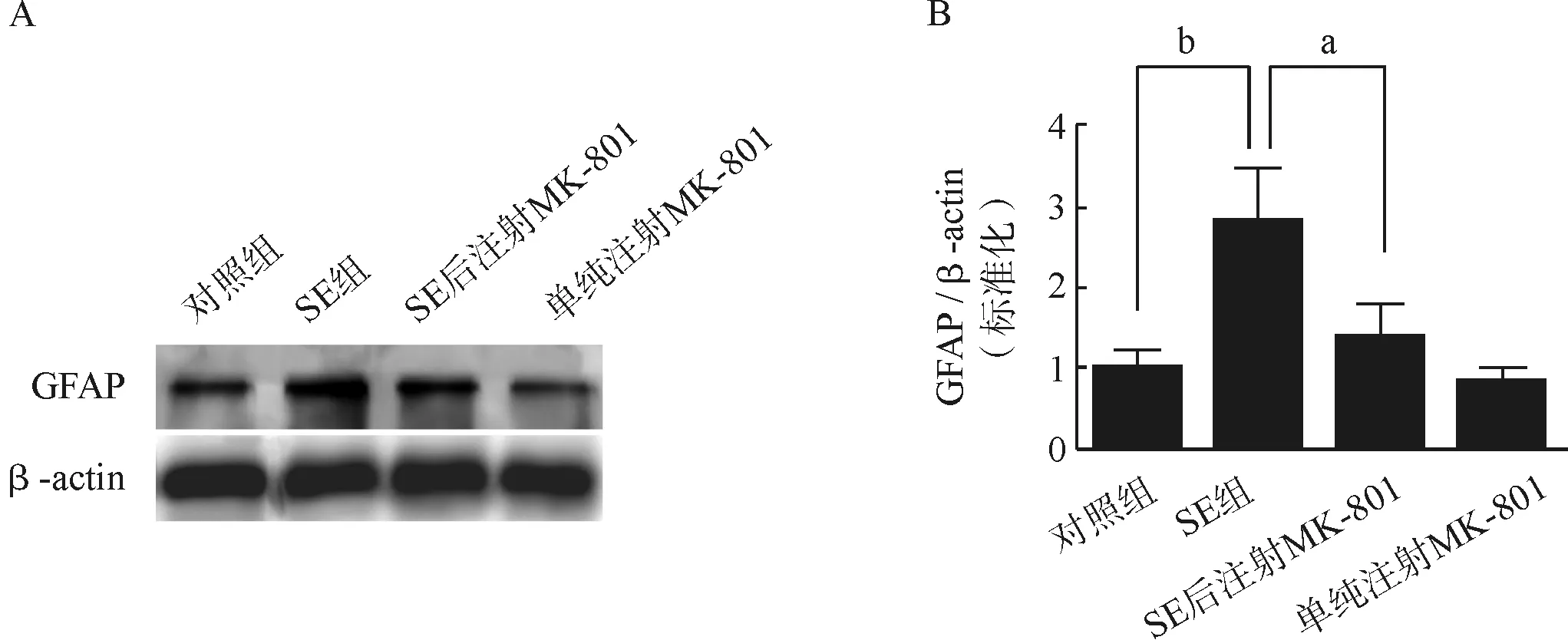
A.对照组、SE组、SE后注射NMDA受体拮抗剂MK- 801组和单纯注射MK- 801组小鼠海马GFAP Western Blot检测结果;B.上述各组小鼠海马GFAP Western Blot定量分析结果(n=5)
aP<0.05,bP<0.01
A.Western Blot assays of GFAP expression in the hippocampus of Control, SE, SE+MK- 801 and MK- 801- treated mice; B.Bar graph showed the quantification of hippocampal GFAP protein levels, which were represented as the intensity ratio of GFAP to β- actin in the hippocampus of Control, SE, SE+MK- 801 and MK- 801- treated mice(n=5)
aP<0.05,bP<0.01
图3 Western Blot检测匹罗卡品癫痫小鼠海马星形胶质细胞活化
Fig 3 Western Blot assays of astrocytes activation in the hippocampus of SE mice
2.4 匹罗卡品癫痫小鼠海马CREB磷酸化水平与NMDA受体激活的关系
CREB作为一种刺激诱导型转录因子广泛分布于中枢神经系统,有研究发现CREB与抑郁症密切相关[19],我们的早期研究结果显示,SE发生后皮层和海马中的pCREB水平显著提高,提示CREB在癫痫发生的病理过程中具有重要作用。在本研究中,我们利用匹罗卡品腹腔注射诱导SE 7 d后,利用Western Blot方法检测海马总的CREB以及pCREB水平,并计算分析海马CREB磷酸化水平。我们的研究结果显示,匹罗卡品癫痫小鼠海马CREB的磷酸化水平较对照组有显著升高,SE后注射NMDA受体拮抗剂MK- 801则显著抑制了SE诱导的CREB磷酸化水平的增加,而单独给予小鼠MK- 801后CREB的蛋白磷酸化水平与对照组比较未见显著差异(图4)。以上结果说明匹罗卡品诱导的SE诱导了转录因子CREB磷酸化,且这一过程依赖于NMDA受体的激活。
3 讨 论
神经胶质细胞是广泛分布于中枢神经系统内的支持细胞,具有支持、滋养神经元的作用。其中星形胶质细胞具有支持和引导神经元迁移,并为神经元运输营养物质和排除代谢产物[20- 21]的作用。新近的研究表明,星形胶质细胞在中枢神经系统除了具有支持和营养作用,同时还参与神经元的突触传递活动[22]。星形胶质细胞与神经元细胞膜紧紧相邻,而且表面分布有大量的受体、转运蛋白和离子通道,这为星形胶质细胞和神经元之间的交换作用创造了必要条件。大量的研究表明,在一些病理状态下星形胶质细胞从静止态向活化态转变,其结构和功能均发生改变,并通过神经递质和离子通道进一步调控神经元的活动。
GFAP是星形胶质细胞的骨架蛋白,被公认为是星形胶质细胞的特征标志物。我们先前的研究发现戊四唑(pentylenetetrazole,PTZ)诱导的点燃癫痫模型中海马组织发生星形胶质细胞活化的现象,表现为GFAP标记的星形胶质细胞数量增加、分支增多[23]。本研究在匹罗卡品诱导的SE模型小鼠中观察到海马GFAP标记的星形胶质细胞数量显著增加,并且GFAP阳性细胞分支增多的现象,Western Blot结果显示匹罗卡品癫痫小鼠海马GFAP的蛋白水平较对照组有显著升高,提示匹罗卡品SE小鼠海马星形胶质细胞被显著活化。
尽管目前已知星形胶质细胞活化是癫痫疾病中的一个典型的病理性损伤,然而在癫痫疾病中介导星形胶质细胞活化的确切的细胞分子机制仍然不是很清楚。NMDA受体在癫痫疾病的病理生理学过程具有重要的作用。我们先前的研究发现NMDA受体在PTZ诱导的点燃癫痫模型中被激活,并在海马星形胶质细胞活化和氧化应激过程中发挥了重要作用[23]。本研究在SE后给予MK- 801阻断NMDA受体,发现其显著抑制了SE诱导的海马GFAP蛋白表达水平的升高,提示匹罗卡品癫痫小鼠海马星形胶质细胞活化依赖于NMDA受体的激活。
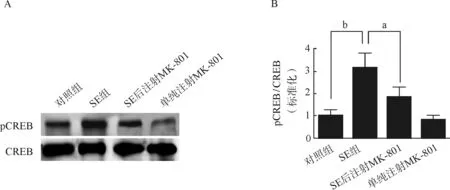
A.对照组、SE组、SE后注射NMDA受体拮抗剂MK- 801组和单纯注射MK- 801组小鼠海马CREB、pCREB Western Blot检测结果;B.上述各组小鼠海马CREB磷酸化(以pCREB/CREB表示)定量分析结果(n=5)
aP<0.05,bP<0.01
A.Western Blot assays of phosphorylated CREB(pCREB) and CREB expression in the hippocampus of Control, SE, SE+MK- 801 and MK- 801- treated mice; B.Bar graph showed the quantification of hippocampal CREB phosphorylation level, which were represented as the intensity ratio of pCREB to CREB in the hippocampus of Control, SE, SE+MK- 801 and MK- 801- treated mice(n=5)
aP<0.05,bP<0.01
图4 Western Blot检测匹罗卡品癫痫小鼠海马CREB磷酸化水平
Fig 4 Western Blot assays of CREB phosphorylation in the hippocampus of SE mice
CREB作为一种刺激诱导型转录因子广泛分布于中枢神经系统[24],在神经元中大量的细胞外刺激因子通过磷酸化激活CREB启动CREB依赖的下游基因的转录[25- 27],从而参与许多生理及病理机制的调节。生理状态下,CREB在中枢神经系统中的主要功能包括调控神经元的增殖、分化、生长的过程[24,28]以及调节学习、记忆的功能和突触可塑性[29- 30]。新近的研究发现,CREB转录因子介导的转录过程参与癫痫发生过程并起到重要作用[31- 32]。有研究发现,SE发生后,皮层和海马中的pCREB水平显著提高[33]。CREB不仅在癫痫发生的过程中被激活,它还主动参与调控癫痫的发生过程。我们先前的研究发现通过遗传学手段抑制CREB显著遏制了匹罗卡品诱导的持续癫痫以及自发性癫痫的发生[34- 35]。本研究中我们在匹罗卡品诱导的SE小鼠中观察到CREB磷酸化水平显著增高,且这一过程依赖于NMDA受体的激活,提示CREB可能参与了海马星形胶质细胞活化的过程。SE后NMDA受体被激活进而导致大量的Ca2+内流,Ca2+作为第二信使激活了其下游一系列的信号通路参与癫痫发生的病理过程调控,而CREB作为一种诱导型的转录因子,在癫痫发作后可被上游的分子信号所激活。由此我们推测,SE后被激活的NMDA受体通过CREB转录因子调控了星形胶质细胞的活化过程。
[1] LEE I,KESNER R P.Differential contribution of NMDA receptors in hippocampal subregions to spatial working memory[J].Nat Neurosci,2002,5(2):162- 168.
[2] von ENGELHARDT J,DOGANCI B,JENSEN V,et al.Contribution of hippocampal and extra- hippocampal NR2B- containing NMDA receptors to performance on spatial learning tasks[J].Neuron,2008,60(5):846- 860.
[3] HEPP Y,SALLES A,CARBO- TANO M,et al.Surface expression of NMDA receptor changes during memory consolidation in the crab Neohelice granulata[J].Learn Mem,2016,23(8):427- 434.
[4] KUNZ P A,ROBERTS A C,PHILPOT B D.Presynaptic NMDA receptor mechanisms for enhancing spontaneous neurotransmitter release[J].J Neurosci,2013,33(18):7762- 7769.
[5] HARA M R,SNYDER S H.Cell signaling and neuronal death[J].Annu Rev Pharmacol Toxicol,2007,47:117- 141.
[6] STANIKA R I,PIVOVAROVA N B,BRANTNER C A,et al.Coupling diverse routes of calcium entry to mitochondrial dysfunction and glutamate excitotoxicity[J].Proc Natl Acad Sci U S A,2009,106(24):9854- 9859.
[7] OKAMOTO S,POULADI M A,TALANTOVA M,et al.Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin[J].Nat Med,2009,15(12):1407- 1413.
[8] MCNAMARA J O,HUANG Y Z,LEONARD A S.Molecular signaling mechanisms underlying epileptogenesis[J].Sci STKE,2006,2006(356):re12.
[9] GHASEMI M,SCHACHTER S C.The NMDA receptor complex as a therapeutic target in epilepsy:a review[J].Epilepsy Behav,2011,22(4):617- 640.
[10] BUCKMASTER P S,LEW F H.Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy[J].J Neurosci,2011,31(6):2337- 2347.
[11] PEIXOTO- SANTOS J E,VELASCO T R,GALVIS- ALONSO O Y,et al.Temporal lobe epilepsy patients with severe hippocampal neuron loss but normal hippocampal volume:Extracellular matrix molecules are important for the maintenance of hippocampal volume[J].Epilepsia,2015,56(10):1562- 1570.
[12] GOUBRAN M,BERNHARDT B C,CANTOR- RIVERA D,et al.InvivoMRI signatures of hippocampal subfield pathology in intractable epilepsy[J].Hum Brain Mapp,2016,37(3):1103- 1119.
[13] CHO K O,LYBRAND Z R,ITO N,et al.Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline[J].Nat Commun,2015,6(6606):1- 13.
[14] ALSHARAFI W A,XIAO B,ABUHAMED M M,et al.Correlation between IL- 10 and microRNA- 187 expression in epileptic rat hippocampus and patients with temporal lobe epilepsy[J].Front Cell Neurosci,2015,9(466):1- 9.
[15] GERSHEN L D,ZANOTTI- FREGONARA P,DUSTIN I H,et al.Neuroinflammation in temporal lobe epilepsy measured using positron emission tomographic imaging of translocator protein[J].JAMA Neurol,2015,72(8):882- 888.
[16] SCHARFMAN H E,SOLLAS A L,BERGER R E,et al.Electrophysiological evidence of monosynaptic excitatory transmission between granule cells after seizure- induced mossy fiber sprouting[J].J Neurophysiol,2003,90(4):2536- 2347.
[17] GABRIEL S,NJUNTING M,POMPER J K,et al.Stimulus and potassium- induced epileptiform activity in the human dentate gyrus from patients with and without hippocampal sclerosis[J].J Neurosci,2004,24(46):10416- 10430.
[18] RACINE R J.Modification of seizure activity by electrical stimulation.Ⅱ.Motor seizure[J].Electroencephalography and Clinical Neurophysiology,1972,32(3):281- 294.
[19] 董琼,赵雪梅,袁天荣,等.CREB及其与抗抑郁症相关性的研究进展[J].现代医学,2014,42(6):710- 712.
[20] GARCIA- MARIN V,GARCIA- LOPEZ P,FREIRE M.Cajal’s contributions to glia research[J].Trends Neurosci,2007,30(9):479- 487.
[21] VERKHRATSKY A.Patching the glia reveals the functional organisation of the brain[J].Pflugers Arch,2006,453(3):411- 420.
[22] HALASSA M M,FELLIN T,HAYDON P G.The tripartite synapse:roles for gliotransmission in health and disease[J].Trends in Molecular Medicine,2007,13(2):54- 63.
[23] ZHU X,DONG J,SHEN K,et al.NMDA receptor NR2B subunits contribute to PTZ- kindling- induced hippocampal astrocytosis and oxidative stress[J].Brain Research Bulletin,2015,114:70- 78.
[24] LONZE B E,GINTY D D.Function and regulation of CREB family transcription factors in the nervous system[J].Neuron,2002,35(4):605- 623.
[25] WIGGIN G R,SOLOAGA A,FOSTER J M,et al.MSK1 and MSK2 are required for the mitogen- and stress- induced phosphorylation of CREB and ATF1 in fibroblasts[J].Molecular and Cellular Biology,2002,22(8):2871- 2881.
[26] LIN C H,YEH S H,LIN C H,et al.A role for the PI- 3 kinase signaling pathway in fear conditioning and synaptic plasticity in the amygdala[J].Neuron,2001,31(5):841- 851.
[27] PERKINTON M S,IP J K,WOOD G L,et al.Phosphatidylinositol 3- kinase is a central mediator of NMDA receptor signalling to MAP kinase(Erk1/2),Akt/PKB and CREB in striatal neurones[J].J Neurochem,2002,80(2):239- 254.
[28] STEVENS B,FIELDS R D.Response of Schwann cells to action potentials in development[J].Science,2000,287(5461):2267- 2271.
[29] KIDA S,JOSSELYN S A,PENA DE ORTIZ S,et al.CREB required for the stability of new and reactivated fear memories[J].Nat Neurosci,2002,5(4):348- 355.
[30] ZHOU Y,WON J,KARLSSON M G,et al.CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala[J].Nat Neurosci,2009,12(11):1438- 1443.
[31] BEAUMONT T L,YAO B,SHAH A,et al.Layer- specific CREB target gene induction in human neocortical epilepsy[J].J Neurosci,2012,32(41):14389- 14401.
[32] LOPEZ DE ARMENTIA M,JANCIC D,OLIVARES R,et al.cAMP response element- binding protein- mediated gene expression increases the intrinsic excitability of CA1 pyramidal neurons[J].J Neurosci,2007,27(50):13909- 13918.
[33] LUND I V,HU Y,RAOL Y H,et al.BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT pathway[J].Science Signaling,2008,1(41):ra9.
[34] ZHU X,HAN X,BLENDY J A,et al.Decreased CREB levels suppress epilepsy[J].Neurobiol Dis,2012,45(1):253- 263.
[35] ZHU X,DUBEY D,BERMUDEZ C,et al.Suppressing cAMP response element- binding protein transcription shortens the duration of status epilepticus and decreases the number of spontaneous seizures in the pilocarpine model of epilepsy[J].Epilepsia,2015,56(12):1870- 1878.
(本文编辑:周兰波)
Effect of NMDA receptor on hippocampal astrocyte activation in pilocarpine- induced epileptic mice
YANG Zhan- neng,JIANG Jing- jing,GU Shi- hong,CHEN Lei,LI Hong- wei,ZHU Xin- jian
(MedicalSchoolofSoutheastUniversity,Nanjing210009,China)
Objective: To study the effect of N- methy- D- asparate(NMDA) receptor on hippocampal astrocyte activation in pilocarpine- induced epileptic mice and the possible mechanism involved. Methods: Thirty two C57/BL6 mice were randomly divided into control group, status epilepticu(SE) group, NMDA receptor antagonist MK- 801 treated SE group and MK- 801 alone treated group, with 8 mice in each group. SE epilepsy model was established by intraperitoneal injection of 300 mg·kg-1pilocarpine. Immunohistochemistry was employed to determine hippocampal astrocyte activation in different group of mice. Western Blot was used to detect hippocampal glial fibrillary acidic protein(GFAP) content and transcription factor cAMP response element binding protein(CREB) phosphorylation level. Results: Hippocampal astrocytes were largely activated in SE mice. Immunohistochemistry results showed that GFAP immunostaining intensities in the hippocampus of SE mice were significantly increased as compared to those of control mice. NMDA receptor antagonist MK- 801 treatment after SE, however, suppressed SE- induced hippocampal astrocyte activation. Consistent with the immunohistochemistry results, our Western Blot showed that GFAP level in SE mice was significantly higher than that of control mice. MK- 801 treatment after SE, however, suppressed SE- induced increase of GFAP level. Meanwhile, our Western Blot result showed that phosphorylation level of cAMP response element binding protein(CREB) was significantly increased in SE mice as compared to that of control mice. Blocking NMDA receptor by MK- 801, however, suppressed the SE- induced increase of CREB phosphorylation. Conclusion: Pilocarpine- induced SE can activate hippocampal astrocytes, which are dependent on the activation of NMDA receptors. Meanwhile, hippocampal astrocyte activation in pilocarpine- induced SE mice is accompanied with CREB phosphorylation, suggesting CREB phosphorylation is involved in NMDA receptor- mediated hippocampal astrocytes activation in pilocarpine induced epileptic mice.
N- methy- D- asparate receptors; pilocarpine; epilepsy; hippocampus; astrocyte activation; mice
2016- 09- 24
2016- 12- 05
国家自然科学基金资助项目(81673413);江苏省自然科学基金资助项目(BK20141335);高等学校博士学科点专项科研基金新教师类资助项目(20130092120043);国家本科生科研训练计划(SRTP)项目(201610286134)
杨展能(1994-),男,江苏淮安人,东南大学医学院临床医学专业在读学生。E- mail:875211500@qq.com
朱新建 E- mail:xinjianzhu@seu.edu.cn
杨展能,姜靓婧,顾仕红,等.NMDA受体在匹罗卡品癫痫小鼠海马星形胶质细胞活化中的作用[J].东南大学学报:医学版,2017,36(2):129- 136.
R- 33; R742.1
A
1671- 6264(2017)02- 0129- 08
10.3969/j.issn.1671- 6264.2017.02.001

