Cardiovascular Immunology Research in Wuhan Union Hospital: Over the Past 25 years
Yuhua Liao and Yiyi Wang
1Laboratory of Cardiovascular Immunology, Key Laboratory of Molecular Targeted Therapies of the Ministry of Education,Department of Cardiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, China
Introduction
Most research on the cardiovascular system has focused on its blood circulation function. It was rarely considered to be related to in flammation and immunity. The origin of cardiovascular immunology research may date back to 30 years ago.The first autoantibody targeting the myocardial molecule adenine nucleotide translocator was found by Schultheiss and Bolte [1] in 1985; it acts against the adenine nucleotide translocator of myocardial mitochondria in patients with dilated cardiomyopathy (DCM). One after another, more autoantibodies were defined, including the autoantibodies against β1-adrenergic receptor, myosin heavy chain, M2cholinergic receptor, and L-type calcium channel in patients with DCM and viral myocarditis. The pathogenesis of chronic heart failure (CHF) was mainly considered to be the imbalance of hemodynamics and neuroendocrine function until 1990. Then Levine et al. [2] found that circulating levels of tumor necrosis factor (TNF) are related to the aggravation of CHF. It was more interesting that autoantibodies were found in patients with hypertension. Fu et al. [3] foundautoantibodies against α1-adrenoceptor in patients with malignant hypertension in 1994. Autoantibodies against α1-adrenoceptor and angiotensin II receptor type 1 (AT1 receptor) were found in patients with preeclampsia and refractory hypertension [4]. In 1999, Ross [5] proposed the theory that atherosclerosis is a kind of in fl ammatory disease. Therefore cardiovascular immunology research is accompanied by investigation of the autoantibodies, cytokines, and in fl ammatory cells in cardiovascular diseases such as DCM, hypertension, atherosclerosis, acute myocardial infarction (AMI), and CHF. This cardiovascular immunology study was conducted in Wuhan Union Hospital from 1991, and it mainly covered (1) the autoantibodies associated with cardiomyopathy and hypertension, (2) T-cell subsets and cytokines related to heart failure, and (3) a therapeutic vaccine for the prevention and treatment of hypertension.
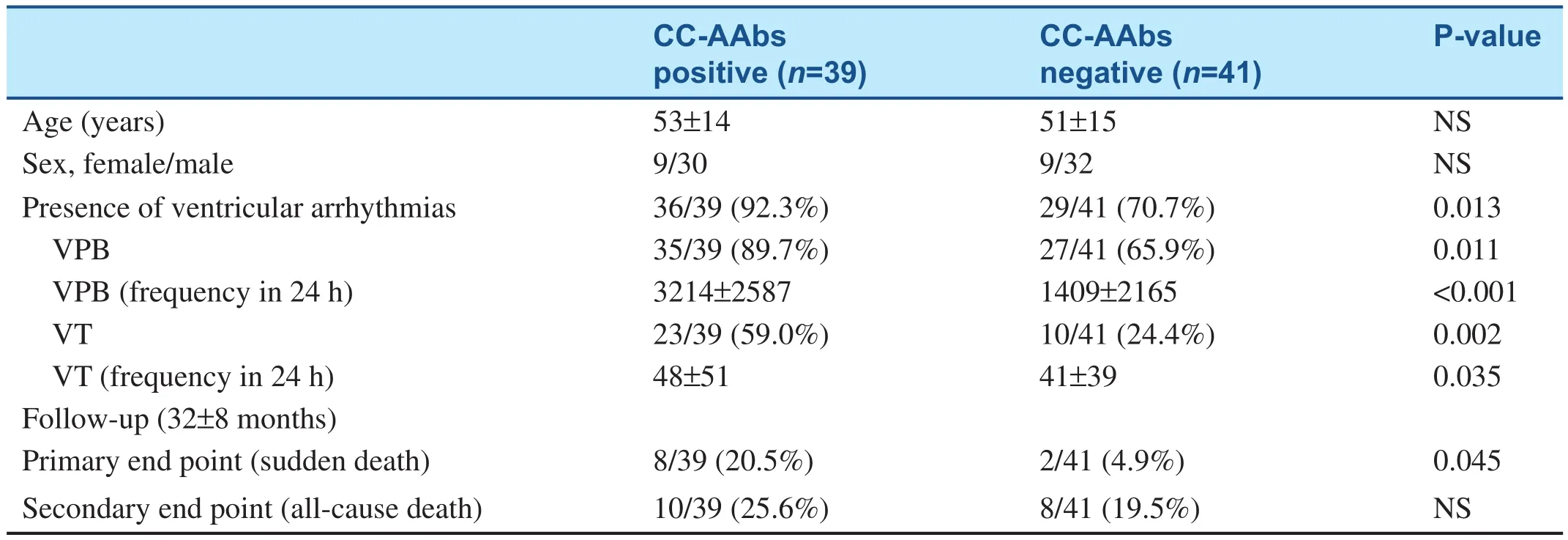
Table 1 Antibodies Against L-type Calcium Channel Induce Ventricular Arrhythmias and Sudden Death in Dilated Cardiomyopathy Patients.
Autoantibodies Associated with Cardiomyopathy and Hypertension
The molecular mimicry analysis of viral proteins with myocardial proteins, applied by Hoebeke [6],showed high homology in 1996, which prompted the study of anti-myocardial antibodies in patients with acute viral myocarditis and DCM. The molecular mimicry theory greatly drove the progress of cardiovascular immunology research. Liao et al.[7–9] began to report anti-myocardial antibodies in DCM patients and their pathogenic mechanisms from 1993. Yuan et al. [10] found that Th17 cells are hyperactive in patients with acute viral myocarditis, assisting B cells to produce anti-heart adenine nucleotide translocator antibodies, while Th2 cells facilitate the activation of B cells in DCM.Sudden death is very common in patients with DCM. This was suspected to be related to some antibodies from DCM. Xiao et al. [11] found antibodies against L-type calcium channel in sera of patients with DCM in 2011 (see Table 1). The affinity purified autoantibody against L-type calcium channel induces ventricular arrhythmias and action potential changes, prolongation of action potential duration and early afterdepolarization, and causes ventricular tachycardia and sudden death.
DCM is often a fatal cause of heart failure. It has recently been con firmed that the pathogenesis of DCM is related to autoimmune reaction. DCM usually worsens gradually. Some patients are asymptomatic; however, their left ventricle may have been dilated for months or even years. Myocardial damage mediated by autoantibodies is the main cause of DCM, and the damage may be inhibited by a calcium antagonist. The Intervention Study of Diltiazem in Dilated Cardiomyopathy (ISDDC), a multicenter, randomized and placebo-controlled clinical trial in 16 hospitals of China was reported by Liao [12] in 1998. From the analysis of the clinical findings of ISDDC, it was concluded that the calcium antagonist diltiazem significantly reducesmortality and the heart failure hospitalization rate in patients with DCM (see Table 2). The purpose of the trial was to study the clinical changes with DCM stage and the effects of intervention at different stages of DCM (see Figure 1).
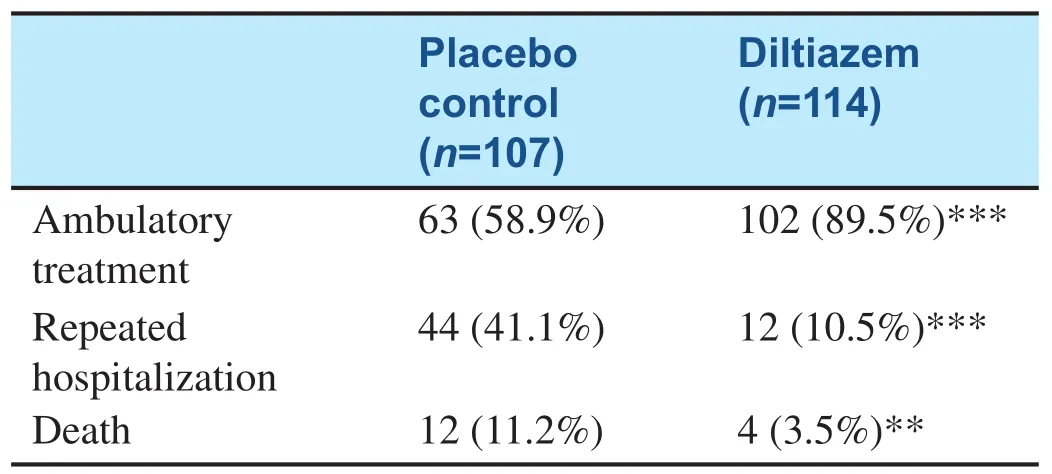
Table 2 Prognosis Analysis of Dilated Cardiomyopathy Patients in the Intervention Study of Diltiazem in Dilated Cardiomyopathy.
Higher levels of autoantibodies against AT1 receptor and α1-adrenoceptor in patients with hypertension were detected, particularly in those with refractory hypertension, in 2002. Meanwhile,abnormal activation of the neural-humoral-immune system in refractory hypertension was found [13].The autoantibodies were con firmed to cause cardiac and arterial remodeling [14, 15], and they were associated with the increased recurrence of stroke and death from stroke in hypertensive stroke patients [16,17]. A multicenter, randomized, parallel-group comparison clinical trial, Study of Optimal Treatment in Hypertensive Patients with Anti-AT1-Receptor Autoantibody (SOT-AT1), was conducted in 2011,with regimens based on candesartan (an angiotension receptor blocker) or imidapril (an angiotensinconverting enzyme inhibitor). From the clinical trial it was con firmed that the antihypertensive effect of the angiotension receptor blocker is better than that of the angiotensin-converting enzyme inhibitor, suggesting that anti-AT1 receptor autoantibodies can induce hypertension [18] (see Figure 2). SOT-AT1 provided a basis for targeted therapy of hypertension.
T-Cell Subsets and Cytokines Related to Heart Failure
The relationship between circulating levels of TNF and heart failure was worth studying. Lymphocyte in filtration was observed in the infarct area of AMI rats in 2002 [19]. It can also be present in the myocardial interstitium of normal rats after adoptive transfer. Anti–myosin heavy chainantibodies can be detected in both AMI rats and rats that have undergone transfer [20]. This suggested that the immune system is activated after AMI.
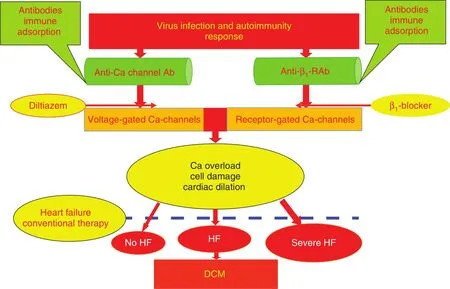
Figure 1 Immunopathogenesis of and Immunotherapy for Dilated Cardiomyopathy (DCM).Ab, antibody; HF, heart failure; anti-β1-RAb, anti-β1-adrenergic receptor antibody.
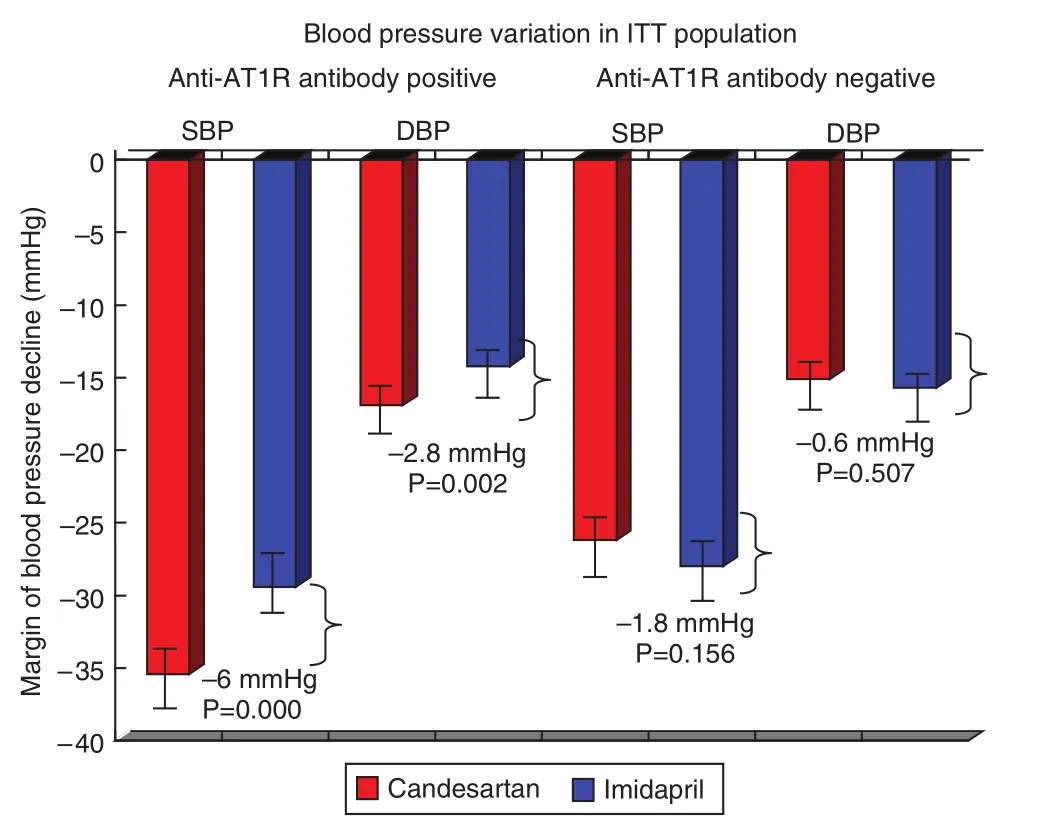
Figure 2 Blood Pressure Variation in the Intent-to-Treat(ITT) Population According to Anti-Angiotensin II Receptor Type 1 (anti-AT1R) Antibody Strati fi cation Analysis.
The anti-TNF therapy against congestive heart failure (ATTACH) trial indicated that the TNF-α monoclonal antibody in fl iximab increased mortality and the hospitalization rate for heart failure deterioration in 2003 [21]. Cheng et al. [22] found that the level of TNF-α was elevated markedly in cardiomyocytes of AMI rats in 2005. This observation suggested that in fliximab targets TNF-α on the myocardial membrane. However, they did not determine whether the TNF-α expressed in cardiomyocytes was from the circulation or myocardium; therefore they hypothesized the autocrine effects of TNF-α after AMI. In 2012, Yu et al. [23]showed that hypoxia promotes the stable expression of hypoxia-inducible factor 1α and its transfer into the nucleus. Hypoxia-inducible factor 1α combined with HRE-8, a hypoxia response element in the TNF-α gene promoter region, to initiate the transcription of TNF-α. At the translational level,TNF-α protein occurs in two forms. It is initially expressed as a 26-kDa transmembrane protein(mTNF), and after cleavage by a TNF-converting enzyme, a 17-kDa soluble protein (sTNF) is released. mTNF was found the main form of TNF-α released from hypoxic cardiomyocytes. It was carried out of hypoxic cardiomyocytes by exosomes,and then transported to target cells to induce the apoptosis of themselves or other cardiomyocytes.This study clarified the mechanism of TNF-α autocrine effects after AMI (see Figure 3). In 2013, Yu et al. [24] firstly suggested that TNF-α-secreting B cells contribute to myocardial fibrosis, which was a new DCM pathogenesis mechanism.
Cheng et al. [25] found that the functional imbalance of T-cell subsets mediates myocardial injury and CHF, and showed the hyperfunction of Th1/Th17 in AMI and acute viral myocarditis through clinical and experimental studies. They found elevation of local IL-17A levels in myocardial ischemia–reperfusion(I/R)-injured cardiomyocytes mainly derives from γδ T cells and that IL-17A accelerates cardiac function harm of I/R-injured mice [26]. In CHF patients,the number of circulating T regulatory cells (Tregs)decreased, as did their inhibitory effects. Tang et al.[27–29] con firmed the causes of Treg defects, including thymic dysfunction, increased apoptosis of Tregs,and responsiveness decrease of responder T cells.Adoptively transferred Tregs alleviated myocardial in fl ammation after AMI; the mechanism included the myocardial in flammation restrained by adoptively transferred Tregs and the apoptosis and fibrosis of cardiomyocytes [30]. It was found that statins, beta blockers, andqiliqiangxincan reduce the proportion of Th1 cells and increase the proportion of Tregs. In addition,renin-angiotensin system inhibitors such as captopril, irbesartan, and olmesartan also reduced TNF-α,IL-1, and IL-6 expression in the hearts of AMI rats,and they can also significantly improve survival, cardiac function, and ventricular remodeling after AMI[31–33]. These cardiovascular drugs can also regulate the imbalance of the expression of the in flammatory cytokines TNF-α and IL-10 in lymphocytes and cardiomyocytes, and prevent ventricular remodeling [22,34–36]. O the basis of the results of the clinical and experimental studies mentioned above, we proposed the immunopathogenesis of heart failure, which provided a new theoretical basis for immunoregulation therapy for heart failure (see Figure 4).
Therapeutic Vaccine in Prevention and Treatment of Hypertension
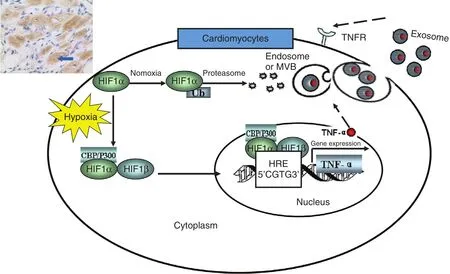
Figure 3 Mechanisms of Tumor Necrosis Factor α (TNF-α) Autocrine Effects in Acute Myocardial Ischemia, HypoxiaInduces Accumulation and Expression of Hypoxia-Inducible Factor 1α (HIF-1α) in Cardiomyocytes.
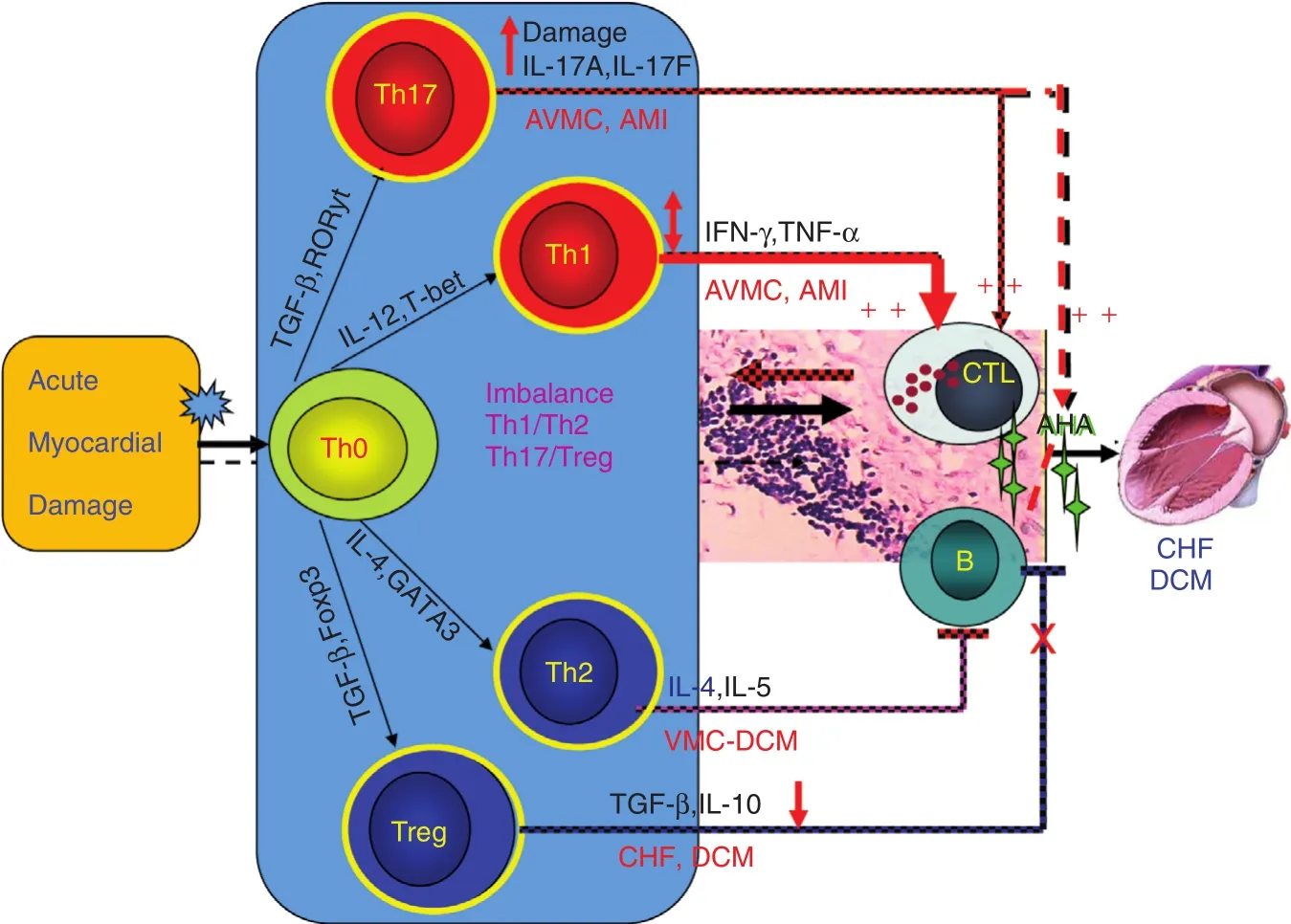
Figure 4 Mechanism of T-Cell Subset Imbalance After Acute Myocardial Damage.
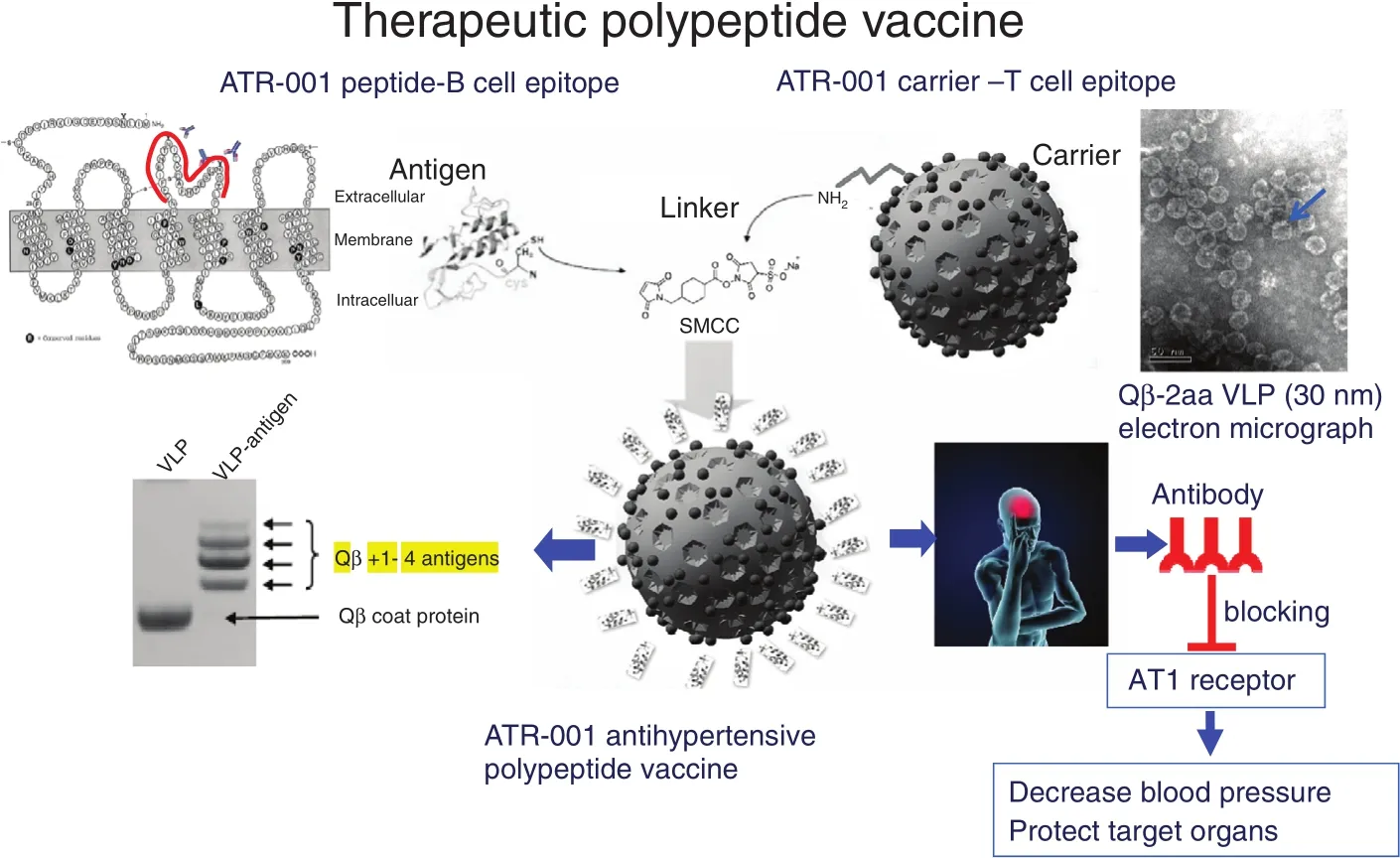
Figure 5 Construction and Antihypertensive Effect of the ATRQβ-001 Therapeutic Polypeptide Vaccine.
Vaccines have the characteristics of precision and long-term effect, and may be used in the treatment of chronic diseases. Zhu at al. [37] and Li et al.[38] invented the first antihypertensive polypeptide vaccine ATR12181 against AT1 receptor in 2005[37, 38]. In 2010, Chen et al. [39] constructed the technical platform for polypeptide vaccines and the improved ATRQβ-001 antihypertensive vaccine(see Figure 5). ATRQβ-001 vaccine could not only effectively reduce blood pressure and protect target organs but could also significantly inhibit the progression of atherosclerotic plaques and reduce renal pathological damage in diabetic nephropathy rats[39–41]. ATRQβ-001 vaccine is different from angiotensin receptor blockers in the mechanism of action.It does not activate the renin-angiotensin system by a feedback loop. It was verified to downregulate the angiotensin-converting enzyme–angiotensin II–AT1 receptor axis and upregulate angiotensin I-converting enzyme 2–angiotensin 1-7–Mas receptor axis at the same time [41]. Therefore, the ATRQβ-001 antihypertensive vaccine is a novel renin-angiotensin system regulator. ATRQβ-001 vaccine should be injected subcutaneously once a month, making it convenient to manage systematically. A successful transnational study of antihypertensive vaccines will improve adherence and increase the control rate in the long-term treatment of cardiovascular diseases.It will also improve the precise management of chronic diseases.
Conclusion
In flammation and immunity play an important role in the pathogenesis of DCM, hypertension,atherosclerosis, AMI, and CHF. Autoantibodies,cytokines, and T-cell subsets play a major role in mediating cardiovascular diseases. In the past 25 years, cardiovascular immunology research in Wuhan Union Hospital found autoantibodies against L-type calcium channel that result in ventricular tachycardia and sudden death in DCM, explained the mechanism of functional imbalance of T-cell subsets and TNF-α autocrine effects in AMI, and invented the first antihypertensive polypeptide vaccine (ATRQβ-001). The bookCardiovascular Immunologywas published in 2008 and introduced the basic theories of cardiovascular immunology,immunopathogenesis, and targeted therapy of clinical diseases, and experimental methods of cardiovascular diseases research [42]. In the future, there will be more cardiovascular immunology research in Wuhan Union Hospital.
Con fl ict of Interest
The authors declare that they have no con flict of interest.
REFERENCES
1. Schultheiss HP, Bolte HD.Immunological analysis of autoantibodies against the adenine nucleotide translocator in dilated cardiomyopathy. J Mol Cell Cardiol 1985;17(6):603–17.
2. Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 1990;323(4):236–41.
3. Fu ML, Herlitz H, Wallukat G,Hilme E, Hedner T, Hoebeke J, et al.,Functional autoimmune epitope on α1-adrenergic receptors in patients with malignant hypertension.Lancet 1994;344(8938):1660–3.
4. Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B,Jupner A, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor.J Clin Invest 1999;103(7):945–52.
5. Ross R. Atherosclerosis–an in flammatory disease. N Engl J Med 1999;340(2):115–26.
6. Hoebeke J. Structural basis of autoimmunity against G protein coupled membrane receptors. Int J Cardiol 1996;54(2):103–11.
7. Liao YH, Tu YS, Li SL. A preliminary study of autoantibodies against cardiomyocyte membrane in dilated cardiomyopathy.J Clin Cardiol 1993;9(3):131–3.
8. Liao YH, Tu YS, Li SL, Zou AR. Study on autoantibodies against myocardial mitochondrial ADP/ATP carrier in dilated cardiomyopathy.Chin J Cardiol 1994;10(1):43–5+79.
9. Liao YH, Tu YS, Peng YH, Li SL, Cheng LX, Wang ZH, et al.Observation of the cardiac cytotoxicity of anti-β receptor antibody in patients with dilated cardiomyopathy.Natl Med J China 1994;10(6):375–6.
10. Yuan J, Cao AL, Yu M, Lin QW,Yu X, Zhang JH, et al. Th17 cells facilitate the humoral immune response in patients with acute viral myocarditis.J Clin Immunol 2010;30(2):226–34.
11. Xiao H, Wang M, Du Y, Yuan J, Cheng X, Chen Z, et al.Arrhythmogenic autoantibodies against calcium channel lead to sudden death in idiopathic dilated cardiomyopathy.Eur J Heart Fail 2011;13(3):264v70.
12. Liao YH. Interventional study of diltiazem in dilated cardiomyopathy: a report of multiple centre clinical trial in China. Chinese Cooperative Group of Diltiazem Intervention Trial in Dilated Cardiomyopathy.Int J Cardiol 1998;64(1):25–30.
13. Liao YH, Wei YM, Wang M,Wang ZH, Yuan HT, Cheng LX.Autoantibodies against AT1-receptor and α1-adrenergic receptor in patients with hypertension.Hypertens Res 2002;25(4):641–6.
14. Zhou Z, Liao YH, Wei Y, Wei F, Wang B, Li L, et al. Cardiac remodeling after long-term stimulation by antibodies against the α1-adrenergic receptor in rats.Clin Immunol 2005;114(2):164–73.
15. Wang B, Liao YH, Zhou Z, Li L,Wei F, Wang M, et al. Arterial structural changes in rats immunized by AT1-receptor peptide.Heart Vessels 2005;20(4):153–8.
16. Liao YH, Zhou ZH, Wang M, Cheng LX, Wang ZH.Autoantibodies against the α1-adrenoceptor related with the increased stroke recurrence in hypertensive patients.Circulation 2005;112 Suppl II:346.
17. Zhou ZH, Liao YH, Li LD, Wei F, Wang B, Wang M, et al. The autoantibodies against AT1-receptors had relation with stroke but no effects on the recurrence of stroke in hypertensive patients.Eur J Heart 2005;26 Suppl:750.
18. Wei F, Jia XJ, Yu SQ, Gu Y, Wang L, Guo XM, et al. Candesartan versus imidapril in hypertension: a randomised study to assess effects of anti-AT1 receptor autoantibodies.Heart 2011;97(6):479–84.
19. Liao YH, Tao R, Cheng X, Tong D,Hu Y, Wang M, et al. Myocardial in flammatory response after acute myocardial infarction and its significance.Mol Cardiol China 2002;2(3):8–11.
20. Tao R, Liao YH, Cheng X, Hu Y, Wang M, Wang ZH. Adoptive transfer of splenocytes from acute myocardial infarction rats mediated myocardial injury.Chin J Immun 2003;19(9):642–4.
21. Chung ES, Packer M, Lo KH,Fasanmade AA, Willerson JT.Randomized, double-blind, placebo-controlled, pilot trial of in fliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure:results of the anti-TNF Therapy Against Congestive Heart Failure(ATTACH) trial.Circulation 2003;107(25):3133–40.
22. Cheng X, Liao YH, Li B, Yang YL, Zhang JY, Lu BJ, et al.[Effects of early treatment with metoprolol on myocardial in flammatory cytokine expression and heart function in rats with acute myocardial infarction].Chin J Cardiol 2005;33(5):448–52.
23. Yu X, Deng L, Wang D, Li N, Chen X, Cheng X, et al. Mechanism of TNF-α autocrine effects in hypoxic cardiomyocytes: initiated by hypoxia inducible factor 1α,presented by exosomes.J Mol Cell Cardiol 2012;53(6):848–57.
24. Yu M, Wen S, Wang M, Liang W, Li HH, Long Q, et al. TNF-α-secreting B cells contribute to myocardial fibrosis in dilated cardiomyopathy. J Clin Immunol 2013;33(5):1002–8.
25. Cheng X, Liao YH, Ge H, Li B,Zhang J, Yuan J, et al. Th1/Th2 functional imbalance after acute myocardial infarction: coronary arterial in flammation or myocardial in flammation.J Clin Immunol 2005;25(3):246–53.
26. Liao YH, Xia N, Zhou SF, Tang TT,Yan XX, Lv BJ, et al. Interleukin-17A contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and neutrophil in filtration.J Am Coll Cardiol 2012;59(4):420–9.
27. Tang TT, Ding YJ, Liao YH, Yu X,Xiao H, Xie JJ, et al. Defective circulating CD4+CD25+Foxp3+CD127lowregulatory T-cells in patients with chronic heart failure.Cell Physiol Biochem 2010;25(4–5):451–8.
28. Tang TT, Zhu ZF, Wang J, Zhang WC, Tu X, Xiao H, et al. Impaired thymic export and apoptosis contribute to regulatory T-cell defects in patients with chronic heart failure.PLoS One 2011;6(9):e24272.
29. Tang H, Zhong Y, Zhu Y, Zhao F,Cui X, Wang Z. Low responder T cell susceptibility to the suppressive function of regulatory T cells in patients with dilated cardiomyopathy. Heart 2010;96(10):765–71.
30. Tang TT, Yuan J, Zhu ZF, Zhang WC, Xiao H, Xia N, et al.Regulatory T cells ameliorate cardiac remodeling after myocardial infarction.Basic Res Cardiol 2012;107(1):232.
31. Lapointe N, Blais Jr. C, Adam A,Parker T, Sirois MG, Gosselin H,et al. Comparison of the effects of an angiotensin-converting enzyme inhibitor and a vasopeptidase inhibitor after myocardial infarction in the rat.J Am Coll Cardiol 2002;39(10):1692–8.
32. Berthonneche C, Sulpice T,Tanguy S, O’Connor S, Herbert JM, Janiak P, et al. AT1 receptor blockade prevents cardiac dysfunction after myocardial infarction in rats.Cardiovasc Drugs Ther 2005;19(4):251–9.
33. Sandmann S, Li J, Fritzenkotter C, Spormann J, Tiede K, Fischer JW, et al. Differential effects of olmesartan and ramipril on in flammatory response after myocardial infarction in rats. Blood Press 2006;15(2):116–28.
34. Cheng X, Liao YH, Zhang J, Li B, Ge H, Yuan J, et al. Effects of atorvastatin on Th polarization in patients with acute myocardial infarction. Eur J Heart Fail 2005;7(7):1099–104.
35. Xu Y, Tang T, Ding Y, Yao R, Xie J, Liao M, et al. Improved cardiac performance by rosuvastatin is associated with attenuations in both myocardial tumor necrosis factor-α and p38 MAP kinase activity in rats after myocardial infarction. Am J Med Sci 2010;340(2):121–7.
36. Xiao H, Song Y, Li Y, Liao YH,Chen J.Qiliqiangxinregulates the balance between tumor necrosis factor-α and interleukin-10 and improves cardiac function in rats with myocardial infarction.Cell Immunol 2009;260(1):51–5.
37. Zhu F, Liao YH, Li LD, Cheng M,Wei F, Wei YM, et al. Target organ protection from a novel angiotensin II receptor (AT1) vaccine ATR12181 in spontaneously hypertensive rats. Cell Mol Immunol 2006;3(2):107–14.
38. Li LD, Liao YH, Zhou ZH, Wei F,Wang M, Wang B, et al. Effects of AT1 receptor short peptides active immunization on blood pressure and cardiovascular remodeling in spontaneously hypertensive rats.Chin J Immun 2005;21(4):372–6.
39. Chen X, Qiu Z, Yang S, Ding D,Chen F, Zhou Y, et al. Effectiveness and safety of a therapeutic vaccine against angiotensin II receptor type 1 in hypertensive animals.Hypertension 2013;61(2):408–16.
40. Zhou Y, Wang S, Qiu Z, Song X,Pan Y, Hu X, et al. ATRQβ-001 vaccine prevents atherosclerosis in apolipoprotein E-null mice.J Hypertens 2016;34(3):474–85; discussion 485.
41. Ding D, Du Y, Qiu Z, Yan S, Chen F, Wang M, et al. Vaccination against type 1 angiotensin receptor prevents streptozotocin-induced diabetic nephropathy.J Mol Med(Berl) 2016;94(2):207–18.
42. Liao YH, Shen GX, Gong FL.Cardiovascular immunology. 1st ed.China: Science Press, 2008: 707.
 Cardiovascular Innovations and Applications2017年1期
Cardiovascular Innovations and Applications2017年1期
- Cardiovascular Innovations and Applications的其它文章
- Hospitalization for Congenital Heart Disease in Beijing: Patient Characteristics and Temporal Trends
- The Effect of Home-Based Cardiac Rehabilitation on Functional Capacity,Behavior, and Risk Factors in Patients with Acute Coronary Syndrome in China
- Catheter Ablation of Atrial Fibrillation:Where Are We?
- Role of Second-Generation Drug-Eluting Stents and Bypass Grafting in Coronary Artery Disease: A Systematic Review and Meta-analysis
- Inherited Cardiomyopathies: Genetics and Clinical Genetic Testing
- Depression, Anxiety, and Cardiovascular Disease in Chinese: A Review for a Bigger Picture
