硫粉为硫源,多元醇辅助合成硫化钼纳米片
王 辉 邹德春,2,*
(1中国科学院北京纳米能源与系统研究所,国家纳米科学中心,北京100083;2北京大学化学与分子工程学院,北京100871)
硫粉为硫源,多元醇辅助合成硫化钼纳米片
王 辉1邹德春1,2,*
(1中国科学院北京纳米能源与系统研究所,国家纳米科学中心,北京100083;2北京大学化学与分子工程学院,北京100871)
本文以普通硫粉和钼酸钠为原料,通过两相法在170-200°C下经8 h制备得到二硫化钼纳米片,同时基于聚集-聚并模型提出其三步法生长机理。其中,硫粉的再组装保证了其向H2S的转化,使钼酸钠得以还原,因此在本方法中起到关键作用。本法制备得到的硫化钼纳米片富含可能由于各种位错引起的未饱和硫原子,有利于其在催化加氢等领域的应用。此外,由于二维过渡金属硫族化合物结构相似,所以本方法及其机理可能同样适用于其他二维过渡金属硫族化合物。本方法为绿色、便捷制备二硫化钼纳米片提供了一个选择。
二硫化钼纳米片;硫粉;聚集和聚并模型;乙二醇;两相法
1 Introduction
Molybdenum disulfide(MoS2)nanosheets have received increasing attention as a promising materialfor Hydrogen evolution reaction(HER)catalysis,energy storage,and semiconductor device because of its excellent chemical,physical,optical and electrical properties and low cost during the last decades1-4. However,the HER activity of MoS2nanosheets,as has been reported by both experimentaland theoretical studies,are limited to the density per unit surface area and turnover frequency of catalytically active edge sites5-13.Therefore,many efforts have beendevoted to prepare MoS2nanosheets with abundantexposed edge sites13-25.However,the effective scale-up of MoS2nanosheets still remains a major challenge.
Currently,four main approaches have been employed to obtain MoS2nanosheets in laboratories14-25,including micromechanical cleavage,chemical vapor deposition,Li ion intercalation,and liquid-phase exfoliation.The micromechanicalcleavage method is reported to have low yield and time-consuming14.Chemical vapor deposition method,in spite of good crystallinity of the resulting MoS2nanosheets also has the limitation of its high cost, poor transferability and complex manipulation15.The latter two methods can be achieved by taking advantage of the sandwiched S-Mo-S layer structure of MoS2thatare held togetherby weak van der Waals interaction.The interaction and hydration of lithium cations combined with the strong affinity of organic solvents or surfactants give rise to the exfoliation of MoS2in organic solvents16-20,whereas it is still challenging for effective scale-up because of expensive intercalating agents used,long reaction time (atleastseveraldays),strictstorage,high sensitivity to environmentconditions,and possible structure deformations.The liquidphase exfoliation of MoS2powder is achievable in appropriate organic solution or aqueous surfactants solution by means of sonication and centrifugation21-25.Nevertheless,both organic solvents and aqueous surfactant exfoliated MoS2usually show multilayer stacks with size as large as 200-400 nm,which results in the reducing number of active sites.Moreover,the dispersed concentrations of resulting MoS2nanosheets are generally too low to require large quantities of exfoliated MoS2nanosheets.Due to the aforementioned limitations of approaches to obtain MoS2nanosheets,a green,convenient,low-cost,and large-scale approach for preparing MoS2has been pursued for a long time.
Herein,we reportthe preparation of MoS2nanosheets of with abundantactive sites by a revised one-pot,polyol-mediated method for the first time and propose a three-step growth mechanism of the MoS2nanosheets based on the aggregation and coalescence model.Compared with the aforementioned methods,this method is proved to be able to prepare scale-up MoS2nanosheets more easily without any hazardous chemicals in 8 h,in which only sulfur powder,polyol(ethylene glycol,diethylene glycol),and Na2MoO4were used.Meanwhile,the size of nanosheets could be adjusted by changing the polyol-to-water ratio.More importantly, the growth mechanism may be suitable for other transition metal dichalogenides(TMDCs).
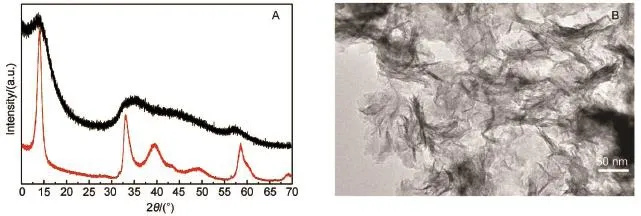
Fig.1(A)XRD patterns ofobtained MoS2nanosheets at170°C for 8 h(red line:after calcination in N2at 400°C for 4 h, black line:before calcination);(B)TEMimage of obtained MoS2nanosheets at 170°C for 8 h(color online)
2 Experimental
2.1 Chemicals and materials
Ethylene glycol(EG,≥95%),diethylene glycol(DEG,≥99%),absolute ethyl alcohol(≥99.7%),sublimed sulfur(≥98%),Na2MoO4(≥99%)were purchased from Beijing Reagent Company.All chemicals were used as received,exceptfor sublimed sulfur slightly ground before use.Deionized(DI)water with a resistivity of 18.2 MΩ·cm was used as well,and MoS2syntheses were conducted in 50 mL PTFE-lined stainless autoclaves.
2.2 Synthesis of MoS2nanosheets
In a standard synthesis,0.14 g ground sublimed sulfur and 0.36 g Na2MoO4were added into a 50 mL PTFE-lined autoclave with 30 mL mixture of H2O and polyol.Then the autoclave was sealed and heated at170°C in an electric drying oven for 24 h.The final black products were precipitated with DI water and absolute ethyl alcohol by centrifugation at4000 rpm for 30 min thrice to wash out polyoland possible sulfur particles.The average yield is about 92%.
2.3 Characterization
X-ray diffraction(XRD)patterns of the samples were recorded with Cu Kαradiation(Bruker/AXS D8 Advance,Netherlands). Scanning electron microscopy(SEM)and transmission electron microscopy(TEM)images were obtained by Hitachi SU8020 (Japan)and FEI Tecnai G2 F20 S-TWIN TMP(U.S.A.),respectively.
3 Results and discussion
3.1 Synthesis of MoS2nanosheets
Fig.1A shows that all the diffraction peaks of the MoS2nanosheets agree well with the hexagonal MoS2phase(JCPDS CARDNo.37-1492).These peaks are atapproximately 2θ=14.3°, 32.8°,39.5°,49.6°,58.3°,69.0°,which correspond to the(002),(100),(103),(105),(110),(201)crystalplanes,respectively.The high intensity of(002)peak implies the good stack of MoS2nanosheets.The broadened peaks(as black line shown)suggest the poor crystallinity of as-prepared sample.Fig.2B shows thatthe average length of as-prepared MoS2sheets are ca 150 nm with differentwidth,when reactat170°C for 8 h.The nanosheets are rich in unsaturated sulfur atoms due to the dislocations,which presentas tiny rings11,26,resulted from the random overlap of small nanoflakes composing nanosheets according to the aggregation and coalescence model27-35.Similar structure had been proved to be beneficial for the HER catalysis11,12,and Zhou et al.26has also provided a detailed classification for the dislocations.
3.2 Effects of reaction temperature and holding time on the formation of MoS2nanosheets
In our scheme,the reaction temperature plays a major role on the transformation of sulfur powder and the growth of MoS2nanosheets,and the holding time also matters much as it affects the density of unsaturated sulfur atoms.Generally,sublimed sulfur tends to aggregate into insoluble sulfur above 159°C in open or close systems and totally precipitate owing to their large size(ca 20μm).To avoid such case,the sublimed sulfur was ground for ca.10 min were used in our protocol.The transformation of sulfur powder(composed of S8)into low reactive liquid sulfur beads with appropriate holding temperatures in airtightautoclaves makes the preparation of MoS2nanosheets successful(detailed in Fig.S1 (Supporting Information(SI))).The liquid sulfur beads contains abundantlong free radicalchains(S8)nwhose reactivity decreased with enhancing reaction temperature,which can reach to a maximum atca.190°C36.The liquids beads spread evenly in the whole polyol at last and subsequently reacted with polyol gradually,generating H2S for reducing Na2MoO4.Therefore,a length nucleation occurred mainly due to the slow reaction between the low-activity(S8)nand polyol.For EG,the reaction begins at160°C and can be expressed as37:

Fig.2 TEMimages of MoS2nanosheets prepared with differentholding time at 170°C for(A)8 h,(B)12 h,(C)16 h,(D)20 h The inseted images are selected area electron diffraction(SAED)patterns of the matching samples.
Fig.2(A-D)shows the improved crystallinity of the as-prepared MoS2nanosheets with increasing holding time.When the holding time was less than 7 h,most of time no MoS2nanosheet was obtained,butgrey solutions.After 8 h,smaller black nanosheets with poor crystallinity appeared atthe bottom of the autoclave in a grey solution as shown in Fig.2A.Fig.2B shows thatmany ordered rings emerged after 12 h to reduce surface energy without obvious improvement in crystallinity.Fig.2C shows the crystal planes realign to reduce total system energy and crystal defects during the 12-16 h,which has been detailed by Wang38.At16-20 h,some regions with good crystallinity appeared as shown in Fig.2D,which can improve the electrical conductivity of MoS2nanosheets.
3.3 Influence of polyoland volume ratio of polyolto water
EG and DEG have been widely used in poyloy process as solvents,ruducing and stabilizer agents39,40to prepare various NPs such as metals,alloys,oxides,and Metal chalcogenides41-48due to their exellcent physical and chemical properties.Fig.3(A-F) shows thatthe MoS2nanosheets prepared with EG and DEG differ significantly in dispersity and length atthe same contiditon,which can be attribute to their different reducing powers and viscosities. As shown in Fig.3(A,C,F)the volume ratio of EG to water affects slightly on the size of as-prepared MoS2nanosheets,and the average allare ca 70-100 nm in length.The case can be attribuated to the dramatical decrease of EG viscosity when the temperature increased to 170-200°C.Moreover,every EG molecule only possess two hydrogen bond acceptors,therefore,the affinity of EG molecule on the MoS2nanosheets(or nanoflakes)is poor,giving rise to the free movementof MoS2nanosheets(or nanoflakes)at high temperature.In other words,the thermal energy of MoS2nanosheets is comparable to the aggregation barrier of the systems according Jolte′s stability hypothesis35,thus all the MoS2nanosheets have a similarly finalaverage size.However,as shown in Fig.3(B,D,F),the average size of the MoS2nanosheets prepared with DEG are roughly proportionalto the increasing volume ratio of DEGto water,which increased from ca.70 to 150 nm.The higher viscosity and more hydrogen bond accpetors(three)may accountfor the difference between the MoS2nanosheets prepared with EG and DEG.However,when the volume ratio of EG or DEG to water decreased to 1:4-1:5,high uniform MoS2nanospheres were obtained as Fig.3G(prepared with EG)and Fig.3H(prepared with DEG)show.
3.4 Growth mechanism
The three-step formation proccess of MoS2nanosheets can be expressed as follows:the reassembly of ground sublimed sulfur, the reduction of Na2MoO4to MoS3nuclei,the formation of MoS2nanoflakes,and the formation of MoS2nanosheets(Fig.4).To takeEG as an example for further details.Before heating,mostof the ground sulfur powder floated on the interface of the mixture of water and EG.Atapproximate 100°C,the sulfur powder adsorbed on foams stemming from the boiling water was sprayed on the inner wallof autoclave because of the burstof foams.When the temperature reached to 120°C,the water and EGseparated with Na2MoO4in water,and the sulfur powder began to meltinto liquid beads.Then with the rising temperature,the high viscous liquids beads,which are insoluble in water,slipped into EG by gravity spread evenly in EG finally.At the critical polymerization temperature 159°C,the S8composing of liquid sulfur beads began to transfer into low active insoluble sulfur(S8)n.Meanwhile,EG surmounted the onsetpotentialof oxidation(160°C)40,and began to reactwith S8and(S8)n,followed by the generation of H2S.Increasing H2S then migrated to the water-EG interface gradually, which result in the reduction of Na2MoO4into MoO42-ions gradually(detailed in SI 2).By the aid of sub-criticalwater,the MoO42-
ions transferred into MoS3nuclei49,50,which would be aggregated or coalesced due to van der Wall forces and violent collisions caused by Brownian movement because of the high density of MoS3nearby the water-EG interface33-35.The MoS3then was trapped in EG and decomposed into MoS2soon for their poor thermostability.As a result,the MoS2and NPs grew into MoS2nanofakes with poor crystallinity according to Kotov et al.28just as displayed in Fig.2A.Then,the nanofalkes grew larger gradually untilto the the maximalsize determined by the aggregation barrier ofthe system35.In the process,the crystallinity of MoS2nanosheets improved by the rearrange of atoms and specialcrystalplanes to reduce defects and the totalsystem energy,as elaborated in a latest research of Wang38.However,the crystallinity improvement of MoS2was limited by low reaction temperature,indacated by the discolations shown in Fig.2(B-D)and the smallwhite rings on the nanosheets shown in Fig.4.We speculated that the growth mechanism may be applicable to other TMDCs because of their similar structure and properties.
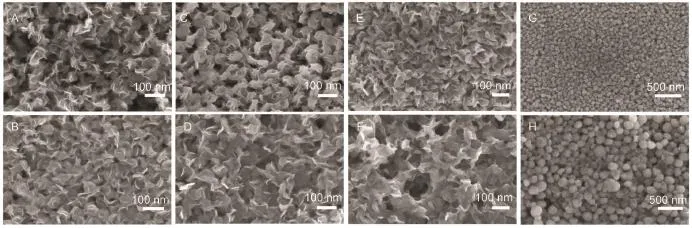
Fig.3 MoS2nanosheets prepared with EG(A,C,E)and DEG(B,D,F)with different volume ratio of EG/DEG to water at 170°C heating for 8 h (A,B)1:1,(C,D)1:2,(E,F)1:3,(G,H)1:5

Fig.4 Scheme of the three-step growth mechanism of MoS2nanosheets
4 Conclusions
MoS2nanosheets were successfully prepared with a novlonepot,two-phase method with sulfur powder and Na2MoO4at170-200°C for 8 h without transfer agents.The as-prepared MoS2nanosheets are rich in unsaturated S atoms thatare crucialto the HER ctatlysis.Moreover,a possible three-step growth mechansim was proposed,which may be employed for other TMDCs for their similar structures.We hope this facile,green,low-cost method may be an effective method to perpare scale-up TMDCs in the near future.
Supporting Information:available free of charge via the internetathttp://www.whxb.pku.edu.cn.
(1)Radisavljevic,B.;Radenovic,A.;Brivio,J.;Giacometti,V.;Kis, A.Nat.Nanotechnol.2011,6,147.doi:10.1038/nnano.2010.279
(2)Mark,K.F.;He,K.;Shan,J.;Heinz,T.F.Nat.Nanotechnol. 2012,7,494.doi:10.1038/nnano.2012.96
(3)Splendiani,A.;Sun,L.;Zhang,Y.;Li,T.;Kim,J.;Chim,C.Y.; Galli,G.;Wang,F.Nano Lett.2010,10,1271.doi:10.1021/ nl903868w
(4)Li,Y.;Wang,H.;Xie,L.;Liang,Y.;Hong,G.;Dai,H.J.Am.Chem.Soc.2011,133,7296.doi:10.1021/ja201269b
(5)Dagge,M.;Chianelli,R.R.J.Catal.1994,149,414. doi:10.1006/jcat.1994.1308
(6)Jaramillo,T.F.;Jorgensen,K.P.;Bonde,J.;Nielsen,J.H.; Horch,S.;Chorkendorff,I.Science 2007,317,100. doi:10.1126/science.1141483
(7)Karunadasa,H.I.;Montalvo,E.;Sun,Y.;Majda,M.;Long,J. R.;Chang,C.J.Science 2002,335,698.doi:10.1126/ science.1215868
(8)Benck,J.D.;Chen,Z.;Kuritzky,A.L.;Froman,A.J.; Jaramillo,T.F.ACS Catal.2012,2,1916.doi:10.1021/ cs300451q
(9)Benchk,J.D.;Hellstern,T.R.;Kibsgaard,J.;Chakthranont,P.; Jaramillo,T.F.ACS Catal.2014,2,3957.doi:10.1021/ cs500923c
(10)Kibsgarrd,J.;Chen,Z.;Beinecke,B.N.;Jaramillo,T.F.Nat. Mater.2012,11,963.doi:10.1038/nmat3439
(11)Xie,J.;Zhang,J.;Li,S.;Grote,F.;Zhang,X.;Zhang,H.;Wang, R.;Lei,Y.;Pan,B.;Xie,Y.J.Am.Chem.Soc.2013,135,17881. doi:10.1021/ja408329q
(12)Xie,J.;Zhang,H.;Li,S.;Wang,R.;Sun,X.;Zhou,M.;Zhou, J.;Lou,X.W.;Xie,Y.Angew.Chem.Int.Ed.2013,25,5807. doi:10.1039/C4SC02019G
(13)Lee,Y.;Zhang,X.Q.;Zhang,W.;Chang,M.T.;Lin,C.T.; Chang,K.D.;Yu,Y.C.;Wang,J.T.;Chang,C.S.;Li,L.J.;Lin, T.W.Adv.Mat.2012,24,2320.doi:10.1002/adma.201104798
(14)Novoselov,K.S.;Jiang,D.;Schedin,F.;Booth,T.J.; Khotkevich,V.V.;Morozov,S.V.;Geim,A.K.Proc.Natl. Acad.Sci.U.S.A.2005,102,10451.doi:10.1073/ pnas.0502848102
(15)Zhan,Y.;Liu,Z.;Najmaei,S.;Ajayan,P.M.;Lou,J.Small 2012,10,966.doi:10.1002/smll.201102654
(16)Bissessur,R.;Heising,J.;Hirpo,W.;Kanatzidis,M.Chem. Mater.1996,8,318.doi:10.1021/cm950378+
(17)Danot,M.;Mansot,J.L.;Golub,A.S.;Protzenko,G.A.; Fabritchnyi,P.B.;Novikov,Y.N.;Rouxel,J.Mater.Res.Bull. 1994,29,833.doi:10.1016/0025-5408(94)90003-5
(18)Golub,A.S.;Zubavichus,Y.V.;Slovokhotov,Y.L.;Novikov,Y. N.;Danot,M.Solid State Ionics 2000,128,151.doi:10.1016/ S0167-2738(99)00347-1
(19)Tachibana,H.;Yamanaka,Y.;Sakai,H.;Abe,M.;Matsumoto, M.Chem.Mater.2000,12,854.doi:10.1021/cm990664b
(20)Benavente,E.;Santa Ana,M.A.;Mendizabal,F.;Gonzalez,G. Coord.Chem.Rev.2002,224,87.doi:10.1016/S0010-8545(01) 00392-7
(21)Coleman,J.;Lotya,M.;OʹNeill,A.;Bergin,S.D.;King,P.J.; Khan,U.;Young,K.;Gaucher,A.;De,S.;Smith,R.J.;Shvets, I.V.;Arora,S.;Stanton,G.;Kim,H.Y.;Lee,K.;Kim,G.T.; Duesberg,G.S.;Hallam,T.;Boland,J.J.;Wang,J.J.;Donegan, J.F.;Grunlan,J.;Moriarty,G.;Shmeliov,A.;Nicholls,R.J.; Perkins,J.M.;Grieveson,E.M.;Theuwissen,K.;McComb,D. W.;Nellist,P.D.;Nicolosi,V.Science 2011,331,568. doi:10.1126/science.1194975
(22)Zhou,K.G.;Mao,N.N.;Wang,H.X.;Peng,Y.;Zhang,H.L. Angew.Chem.Int.Ed.2011,50,10839.doi:10.1002/ ange.201105364
(23)O′Neill,A.;Khan,Umar;Coleman,J.N.Chem.Mater.2012, 24,2414.doi:10.1021/cm301515z
(24)Voiry,D.;Salehi,M.;Silva,R.;Fujita,T.;Chen,M.;Asefa,T.; Shenoy,V.B.;Eda,G.;Chhowalla,M.Nano Lett.2013,13, 6222.doi:10.1021/nl403661s
(25)Jawaid,A.;Nepal,D.;Park,K.;Jespersen,M.;Qualley,A.; Mirau,P.;Drummy,L.F.;Vaia,R.A.Chem.Mater.2016,28, 337.doi:10.1021/acs.chemmater.5b04224
(26)Zhou,W.;Zou,X.L.;Najmaei,S.;Zheng,L.;Shi,Y.M.;Kong, J.;Lou,J.;Ajayan,P.M.;Yakobson,B.I.;Idrobo,J.Nano Lett. 2013,13,2615.doi:10.1021/nl4007479
(27)Kalsin,A.M.;Fialkowski,M.;Paszewski,M.;Smoukov,S.K.; Bishop,K.J.M.;Grzybowski,B.A.Science 2006,321,420. doi:10.1126/science.1125124
(28)Tang,Z.Y.;Zhang,Z.L.;Wang,Y.;Glotzer,S.C.;Kotov,N.A. Science 2006,314,274.doi:10.1126/science.1128045
(29)Glotzer,S.C.;Solomon,M.J.Nat.Mater.2007,6,557. doi:10.1038/nmat1949
(30)Min,Y.J.;Akbulut,M.;Kristiansen,K.;Golan,Y.;Israelachvili, J.Nat.Mater.2008,7,527.doi:10.1038/nmat2206
(31)Schliehe,C.;Juarez,B.H.;Pelletier,M.;Jander,S.;Greshnykh, D.;Nagel,M.;Meyer,A.;Foerster,S.;Kornowski,A.;Klinke, C.;Weller,H.Science 2010,329,550.doi:10.1126/ science.1188035
(32)Xia,Y.S.;Nguyen,T.D.;Yang,M.;Lee,B.;Santos,A.; Podsiadlo,P.;Tang,Z.Y.;Glotzer,S.C.;Kotov,N.A.Nat. Nanotechnol.2011,6,580.doi:10.1038/nnano.2011.121
(33)Plote,J.;Erler,R.;Thunemann,A.F.;Emmerling,F.;Kraehnert, R.Chem.Commun.2010,46,9209.doi:10.1039/C0CC03238G
(34)Polte,J.;Ahner,T.T.;Delissen,F.;Sokolov,S.;Emmerling,F.; Thunemann,A.F.;Kraehnert,R.J.Am.Chem.Soc.2010,132, 1296.doi:10.1021/ja906506j
(36)Fairbrother,F.;Gee,G.;Merrall,G.T.J.Polym.Sci.1955,16, 459.doi:10.1002/pol.1955.120168231
(37)Zheng,Y.;Cheng,Y.;Wang,Y.;Zhou,L.;Bao,F.;Jia,C. J.Phys.Chem.B 2006,110(16),8284.doi:10.1021/jp060351l
(38)Fei,L.F.;Lei,S.J.;Zhang,W.B.;Lu,W.;Lin,Z.Y.;Lam,C. H.;Chai,Y.;Wang,Y.Nat.Commun.2016,7,1.doi:10.1038/ ncomms12206
(39)Yue,H.R.;Zhao,Y.J.;Gong,J.L.Chem.Soc.Rev.2012,41, 4218.doi:10.1039/C2CS15359A
(40)Biacchi,A.J.;Schaak,R.E.ACS Nano 2011,5,8089. doi:10.1021/nn2026758
(41)Fifvet,F.;Lagier,J.P.;Blin,B.Solid State Ionics 1989,32,198.doi:10.1016/0167-2738(89)90222-1
(42)Silvert,P.V.;Tekaia-Elhsissen,K.Solid State Ionics 1995,82, 53.doi:10.1016/0167-2738(95)00198-F
(43)Bonet,F.;Delmas,V.;Grugeon,S.;Urina,H.R.;Silvert,P.Y.; Tekaia-Elhsissen,K.Nano Structured Material1999,11,1277. doi:10.1016/S0965-9773(99)00419-5
(44)Schmitt,P.;Brem,N.;Schunk,S.;Feldmann,C.Adv.Funct. Mater.2011,21,3037.doi:10.1002/adfm.201100655
(45)Feldmann,C.Adv.Funct.Mater.2003,13,101.doi:10.1002/ adfm.200390014
(46)Sun,Y.G.;Xia,Y.N.Science 2002,298,2176.doi:10.1126/ science.1077229
(47)Chen,J.Y.;Herricks,T.;Geissler,M.;Xia,Y.N.J.Am.Chem. Soc.2004,126,10854.doi:10.1021/ja0468224
(48)Rusitskiy,A.;Xia,Y.N.J.Am.Chem.Soc.2016,138,3161. doi:10.1021/jacs.5b13163
(49)Akiya,N.;Savage,P.E.Chem.Rev.2002,102,2725. doi:10.1021/cr000668w
(50)Erickson,B.E.;Helz,G.R.Geochim.Cosmochim.Ac.2000, 64,1149.doi:10.1016/S0016-7037(99)00423-8
Polyol-Mediated Synthesis of MoS2Nanosheets Using Sulfur Powder as the Sulfur Source
WANGHui1ZOU De-Chun1,2,*
(1Beijing Institute of Nanoenergy and Nanosystems,Chinese Academy of Sciences,National Center for Nanoscience and Technology, Beijing 100083,P.R.China;2College of Chemistry and Molecular Engineering,Peking University,Beijing 100871,P.R.China)
MoS2nanosheets are prepared with sulfur powder and Na2MoO4by a one-pottwo-phase method at170-200°C for 8 h.In addition,a three-step growth mechanism based on the aggregation and coalescence modelis proposed.The reassembly ofsulfur powder ensures the transformation from sulfur powder to H2S to reduce Na2MoO4and plays a key role in the successfulpreparation of MoS2nanosheets.The as-prepared MoS2nanosheets are rich in unsaturated sulfur atoms,probably resulting from the dislocation cores of the MoS2nanosheets,which have been found to be beneficialfor hydrogen evolution reaction catalysis.The method and growth mechanism adopted in this study may be applied to other transition metaldichalogenides for similar structures.The facile and green method provides an alternative for the preparation of MoS2nanosheets.
MoS2nanosheet;Sulfur powder;Aggregation and coalescence model;Ethylene glycol; Two-phase method
O643;O611.4
Polte,J.CrystEngComm 2015,17,6809.
10.1039/ c5ce01014d
doi:10.3866/PKU.WHXB201702081
Received:November23,2016;Revised:February 6,2017;Published online:February 8,2017.
*Corresponding author.Email:dczou@pku.edu.cn;Tel:+86-10-62759799.
The projectwas supported by the“Thousand Talents”Program of China for Pioneering Researchers and Innovative Teams,National Natural Science Foundation of China(51573004,91333107),and Natural Science Foundation of Beijing,China(Z160002).
国家顶尖千人计划,国家自然科学基金(51573004,91333107)及北京市自然科学基金(Z160002)资助项目©Editorialoffice of Acta Physico-Chimica Sinica

