Prediction of motor recovery after ischemic stroke using diffusion tensor imaging: A meta-analysis
Jing-fen Jin, Zhi-ting Guo, Yu-ping Zhang, Yuan-yuan Chen
1Nursing Department, the Second Aff i liated Hospital of Zhejiang University School of Medicine, Hangzhou 310009, China
2Neurology Department, the Second Aff i liated Hospital of Zhejiang University School of Medicine, Hangzhou 310009, China
Prediction of motor recovery after ischemic stroke using diffusion tensor imaging: A meta-analysis
Jing-fen Jin1, Zhi-ting Guo2, Yu-ping Zhang1, Yuan-yuan Chen2
1Nursing Department, the Second Aff i liated Hospital of Zhejiang University School of Medicine, Hangzhou 310009, China
2Neurology Department, the Second Aff i liated Hospital of Zhejiang University School of Medicine, Hangzhou 310009, China
BACKGROUND: This systematic review aims to investigate the prediction value of diffusion tensor imaging for motor function recovery of ischemic stroke patients.
METHODS: Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2016, Issue 9), PubMed, Embase, Clarivate Analytics, Scopus, CINAHL, Chinese Biomedical Literature Database, China National Knowledge Infrastructure and Google Scholar were searched for either motor recovery or corticospinal tract integrity by diffusion tensor imaging in different stroke phase from January 1, 1970, to October 31, 2016. The study design and participants were subjected to metrological analysis. Correlation coeff i cient (r) was used for evaluating the relationship between fractional anisotropy (FA) and motor function outcome. Correlation coeff i cient values were extracted from each study, and 95% confidence intervals (CIs) were calculated by Fisher's z transformation. Meta-analysis was conducted by STATA software.
RESULTS: Fifteen studies with a total of 414 patients were included. Meta-analysis showed that FA in the subacute phase had the signif i cant correlation with motor function outcome (ES=0.75, 95%CI 0.62–0.87), which showed moderate quality based on GRADE system. The weight correlation coeff i cient revealed that an effect size (ES) of FA in acute phase and chronic phase was 0.51 (95%CI 0.33–0.68) and 0.62 (95%CI 0.47–0.77) respectively.
CONCLUSION: This meta-analysis reveals that FA in the subacute phase after ischemic stroke is a good predictor for functional motor recovery, which shows moderate quality based on the GRADE system.
Diffusion tensor imaging; Motor function recovery; Ischemic stroke
INTRODUCTION
Stroke is a leading cause of long-term adult disability. An estimated of 50 million stroke survivors worldwide suffer from significant physical or cognitive deficits, and 25% to 74% of them require assistance or are fully dependent on caregivers for activities of daily living (ADLs).[1]In particular, considering the rising healthcare cost of stroke patients, early accurate prediction of motor function outcome after stroke is needed to set attainable rehabilitation goals, facilitate discharge planning and anticipate possible consequences such as implementing home adjustments and address the need for community support.[2]
Diffusion tensor imaging (DTI) is an advanced noninvasive magnetic resonance imaging technique used to visualize the white matter pathways and integrity of corticospinal tract (CST).[3–6]Among DTI parameters, fractional anisotropy (FA) is the most widely used sensitive index of quantif i cation of the CST lesion after stroke.[7,8]It represents the combination of properties related to diameter, density, myelination and the degree of directionality of microstructures.[9]With a range of zero (completely isotropic diffusion) to one (completely anisotropic diffusion), reduced FA might be related todisintegration of the fibers and deterioration of axonal integrity of CST.[10–12]
Over the past two decades, diffusion anisotropy parameters such as FA have been used for prediction of motor outcome in ischemic stroke patients.[13–15]However, studies reported different prediction values of FA among the three stroke phases. Two studies conf i rmed that reduced FA in subacute phase could predict poor motor outcome,[14,15]while Koyama et al[16]discovered that FA of subacute phase was not signif i cantly correlated with motor outcome (r=0.282, P=0.291). Chen and his team[17]indicated that higher FA of chronic phase was a good predictor for good motor outcome. FA was progressively decreasing from acute phase to chronic phase after ischemic stroke, and these anterograde and retrograde degenerations were accompanied by deterioration in the clinical motor function.[18]Thus, it is critical to investigate the correlation between diffusion parameters in different stroke phases and the motor functional outcome.[10]This review aims to investigate the predictive value of FA among three phases for motor function recovery in ischemic stroke patients.
METHODS
Search strategy
We searched the Cochrane Stroke Group Trails Register (last search in October 2016) and the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2016, Issue 9), PubMed, Embase, Clarivate Analytics, Scopus, CINAHL, Chinese Biomedical Literature Database, China National Knowledge Infrastructure and Google Scholar from January 1, 1970, to October 31, 2016. The search terms were "stroke", "rehabilitation" and "motor recovery" combined with "diffusion tensor imaging". Mesh terms and keywords were used in the literature search. We also hand searched the reference lists of retrieved articles. There were no language restrictions. The reference lists of all relevant articles were also screened. For example, the search strategy of Pubmed database was showed in Figure 1.
Eligible studies
Inclusion criteria: (1) full published article; (2) the study population included individuals with hemiplegia or limb function def i cit following stroke; (3) correlation study that measured FA at baseline and its relationship with motor function recovery at a future time point; (4) outcomes included motor function or functional recovery.
Exclusion criteria: (1) cerebral hemorrhage patients; (2) no extractable data (correlation coeff i cient) was available; (3) conference poster, case reports or review articles.
PRISMA flow diagram and guideline were used for literature review. Two review authors (Guo ZT and Zhang YP) read the titles and abstracts (if available) of identif i ed literature, eliminated irrelevant studies independently, and obtained full text of the remaining studies. The same two review authors examined potentially relevant studies according to the pre-determined including criteria independently and ranked these studies as relevant, irrelevant, or possibly relevant. The studies ranked initially as irrelevant were excluded, and all others were included for further assessment. Review authors resolved disagreements through discussion with other authors. If further information or data was needed to reach consensus, they contacted the study authors.
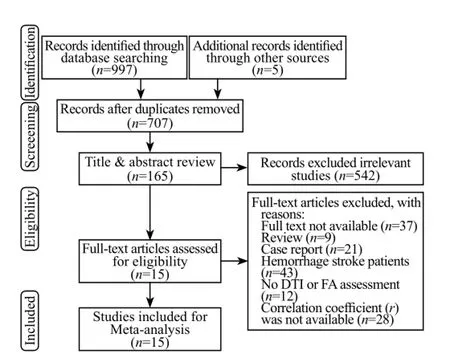
Figure 1. Flow diagram of the selection of studies and reasons for exclusion from the systematic review.
Quality assessment
Review authors assessed the methodological quality of the included studies using standardized critical appraisal instruments from the Joanna Briggs Institute in observational studies. Specifically, studies were assessed regarding the eight aspects, which were considered as essential for good reporting of observational studies except the first criterion. These included study design, participant inclusion, confounding, outcome measurement, missing data and statistical analysis. Two authors (Guo ZT and Zhang YP) conducted the quality assessment independently. All methodological quality assessment for agreement was checked between two review authors, resolving any disagreements by discussion with a third reviewer (Jin JF). Qualityassessment of level C considered as low reliability, and these studies were excluded from the analysis.
Data extraction
Two review authors (Guo ZT and Chen YY) independently extracted the information from eligible studies. They used checklists to record details of the studies contains: last name of first author, year of publication, country, title, number of participants, mean age, gender, lesion location, time from stroke onset to entry the study, clinical scale used and score, DTI parameters (FA value, rFA) and time of motor function measurement or DTI scan. All of the extracted information was checked for agreement between review authors; all discrepancies were resolved after rechecking the source papers and discussion. They contacted study authors to request more information, or missing data if necessary.
Some details of extracted data should be clarified. First, the stroke phase (acute, subacute, chronic) was allocated using Osborn criterion according to the studies data of DTI scanning time after stroke.[19]Second, Spearman correlation coefficients were extracted from all included studies, whereas the sampling distribution of Spearman correlation coefficients was problematic because the standard error (SE) depends on the value of the correlation coeff i cient. Thus, a Fisher transformation was used to convert each correlation coefficient into an approximately normal distribution.[20]Third, correlation coefficient (r) of five included studies was negative; this is related with the motor function scale. National Institutes of Health Stroke Scale (NIHSS) was used in three studies and modified Rankin Scale (mRS) in one study, these two scales were lower score with good recovery, so absolute value of r was used in the analysis. The reduction value of FA was used in another study, so the same transformation was conducted.
Statistical analysis
The results of all eligible studies were pooled to present an overall estimate of the relationship between FA and motor outcome among three stroke phases (acute, subacute, chronic). For all statistical analyses, the software STATA version14 (Stata Corporation, College Station, TX, USA) was used. The heterogeneity between studies was examined calculating the chi-square-based Q statistic (with a level of significance of P=0.05) and I2statistic. For P>0.1 or I2<50%, the included studies were identified as having acceptable heterogeneity and the Fixed-effect model was used; otherwise, the randomeffects model was used.
Overall quality of the evidence
This systematic review only had one outcome of motor function, thus the quality of evidence for outcome was evaluated according to the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group with the magnitude of effect and the influence of all plausible residual confounding taken into account.[21]Although observational studies started with a "low quality" rating,[22]the level of the evidence would be upgraded if there were large effects of the exposure according to the pooling results and potential uncontrolled confounding bias might weaken the true effect of the exposure. The following def i nitions of quality of the evidence was applied: "high quality", "moderate quality", "low quality", and "very low quality". Grades of evidence were performed using GRADE system. Any discrepancies between the 2 reviewers were solved by discussion with a third reviewer.
RESULTS
Identif i cation of relevant studies
A total of 997 published articles were identif i ed through electronic bibliographic databases. One author (Jin JF) carried out an additional search of reference lists and another 5 studies were included. Fifteen studies[5,14,15,17,20,23–32]were included for fi nal analysis. Figure 1 showed a fl ow chart of retrieved and excluded studies with their reasons for exclusion.
Characteristics of studies
Fifteen studies (Table 1) were included with a total of 414 ischemic stroke patients. The sample sizes ranged from 8 to 82 participants. The lesion location contains internal capsule (6/11), corona radiate (4/15), middle cerebral artery territory (4/15), pons (2/15). The DTI parameters reported were FA (ipsilateral and contralateral, respectively) or rFA. The studies also used a wide range of scales to measure the clinical outcome such as NIHSS (9/15), Fugl-Meyer Assessment (3/15), Barthel index scale (2/15), Motricity Index (2/15), Medical Research Council (2/15), Brunstrom Scale (2/15), Modified Rankin Scale (1/15), the Motor Assessment Scale (1/15), Nine Hole Peg Test (1/15), and Functional Ambulation Category (1/15).
Methodological quality
The quality assessment results of the included studies are shown in Table 2. All studies defined inclusion criteria clearly in the sample and measured outcomesin a reliable way. The quality levels were A (11/15) and B (4/15). The first criterion of "was studies based on a random sample?" was not applicable. One study didn't reported the confounding factors, and three studies follow up carried out over an insuff i cient time period.
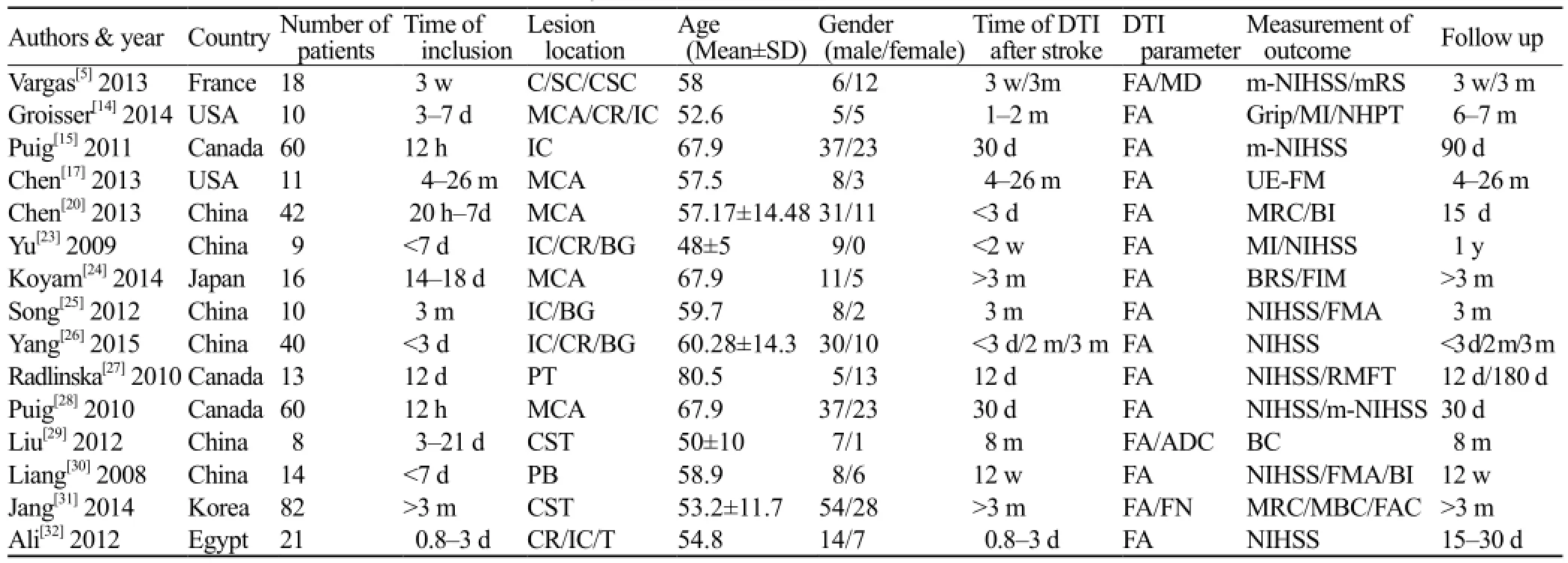
Table 1. Characteristics of the studies included in the systematic review
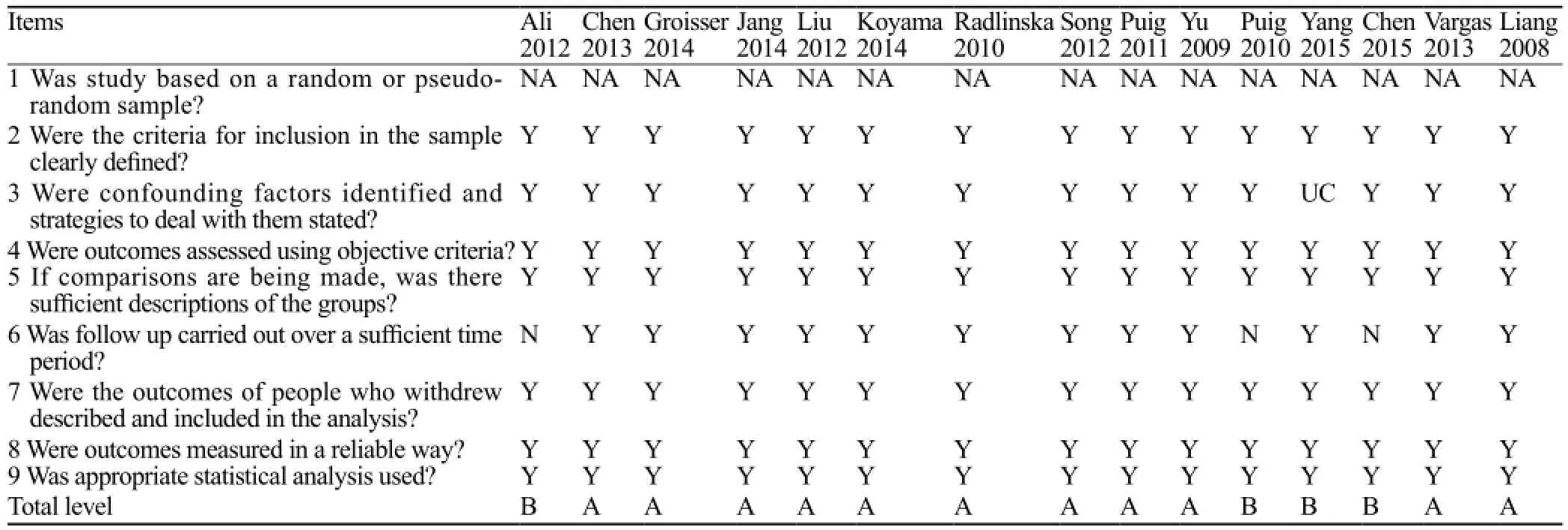
Table 2. Quality assessment of the studies included in the systematic review
The prediction value of FA in acute phase
Three studies[17,26,32]of acute phase were performed to evaluate the relationship between FA and motor function outcome. The pooled effect for all studies was 0.51 (95%CI 0.33–0.68), as shown in Figure 2. No heterogeneity was found in the analysis (P>0.1 and I2=15.4%).
The prediction value of FA in subacute phase
Totally 210 participants of seven studies[5,14,15,23,26–28]of subacute phase evaluated the relationship between FA and functional motor outcome. The overall effect size for all studies was 0.75 (95%CI 0.62–0.87) as shown in Figure 3 (P<0.1 and I2=71.7%). Considering the existing heterogeneity, we further conducted sensitivity analysis, and the results were stable after removing the largest weight article (ES 0.75; 95%CI 0.59–0.91).[15]
The prediction value of FA in chronic phase
Eight studies including 199 patients evaluated the relationship between FA and motor functional outcome.The pooled effect for all studies was 0.62 (95%CI 0.47–0.77), as shown in Figure 4 ( P<0.1 and I2=58.7%). Thus, random model was used and the heterogeneity existed in every study. We further conducted sensitivity analysis, and the results were stable after removing the largest weight article (ES 0.58; 95%CI 0.35–0.80).[31]
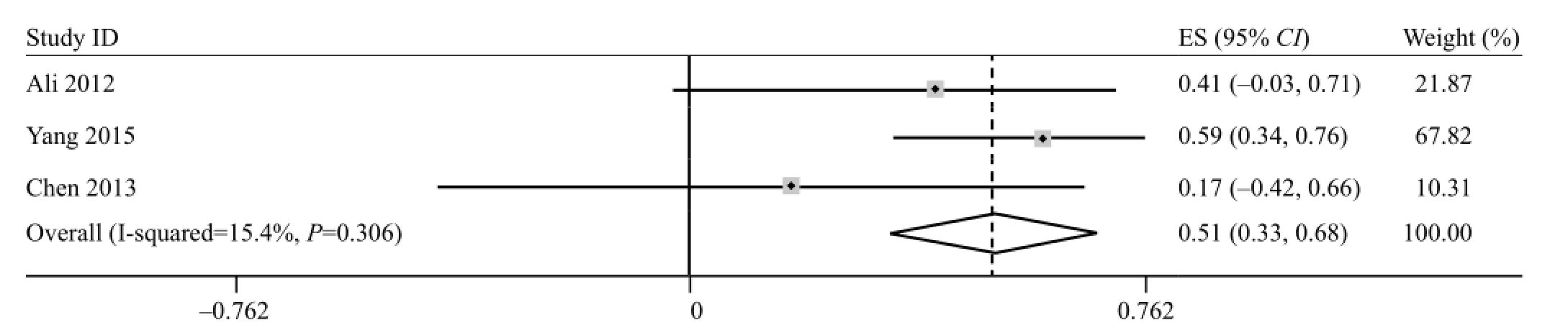
Figure 2. Forest plots of the correlation coeff i cient (r) with corresponding 95%CIs for the correlation between FA in acute phase with motor functional outcome.

Figure 3. Forest plots of the correlation coeff i cient (r) with corresponding 95%CIs for the correlation between FA in subacute phase with motor functional outcome.
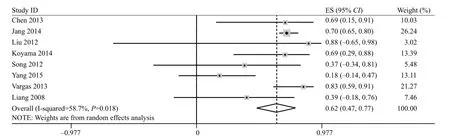
Figure 4. Forest plots of the correlation coeff i cient (r) with corresponding 95%CIs for the correlation between FA in chronic phase with motor functional outcome.
Overall quality of the evidence
The prediction value of FA in the subacute phase was judged to be of moderate quality, which means that further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. The prediction value of FA in acute or chronic phase was judged to be of very low quality and low quality. The evidence summary table based on GRADE system is shown in Table 3.
DISCUSSION
In this study, we reviewed relevant studies on therelationship between DTI parameter FA and motor recovery outcome in ischemic stroke patients, to determine which phase after stroke can predict motor recovery after stroke. This article showed a significant correlation between FA in subacute phase and motor functional outcome in IS patients (ES 0.75; 95%CI 0.62–0.87), which showed moderate quality based on GRADE system. A method to reliably predict motor recovery would help to set attainable rehabilitation goals and identify appropriate rehabilitation interventions.
The time-dependent progressive FA decrease found in the ipsi-lesional pyramidal tract provides insight on the progress of Wallerian degeneration.[32,33]The functional motor recovery predictive value of FA varied among different stroke periods. We divided stroke patients into acute phase (<3 d), subacute phase (4 d–8 w) and chronic phase (>8 w) using the Osborn criterion according to the DTI scanning time.[19]Correlation between FA and motor functional outcome was calculated respectively, so the optimal prediction phase could be decided according to the correlation coefficient (r). Actually, the pooled effect size of subacute phase was higher than chronic phase and acute phase. This finding was consistent with previous metaanalytic results of upper limb motor recovery prediction by Kumar et al.[11]It is a general agreement that FA value increases immediately after ischemic stroke onset and remains high for the next 1–2 days, then decreases significantly during the following stroke phases.[10]Our results confirmed that ES in acute phase (<3 d) was lower than other phases, which indicated that FA might not be a good predictor for motor functional outcome in acute phase after stroke. In addition, one study proposed that axial diffusivity (AD) loss in acute phase is a strong prognostic indicator of future motor function.[14]Further studies are needed to address this fi nding. FA in chronic phase showed a moderate correlation with motor recovery, one reason might be explained that reorganisation within the motor system already contributes to motor function after three months.
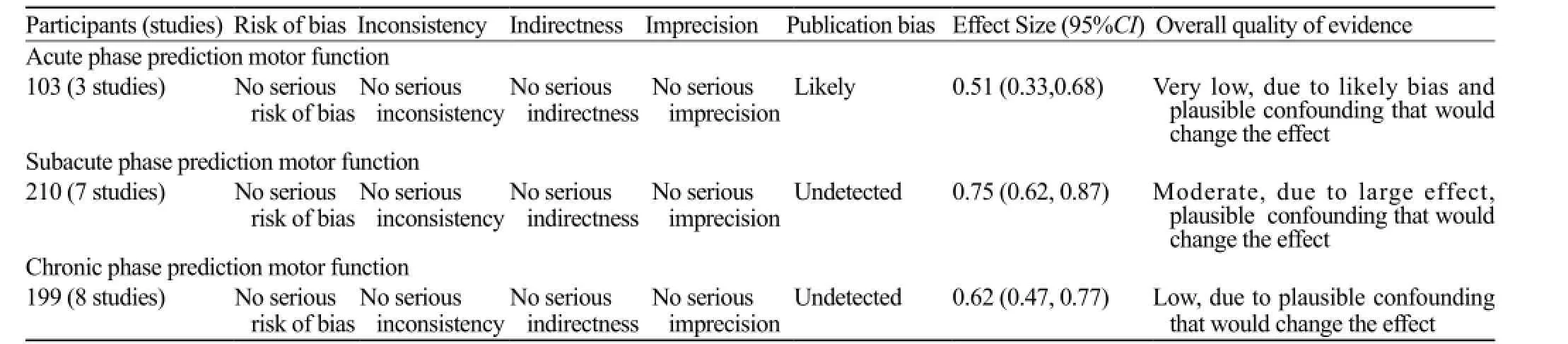
Table 3. GRADE table
Subgroup analysis of subacute and chronic phase reported heterogeneous data, which might be a limitation of our results. The motor outcome assessment scales differed among included studies. Also, the FA value obtained from analyzing the region of interest selected, which is an operator-dependent technique may have also affected the results. Only three studies were included in acute phase analysis, which is related to the feasibility of clinical trials. Our study also has some limitations. Though we listed the infraction location, no subgroup analysis was conducted, and the motor outcome measurement time point was not divided because of the literature limitation included.
CONCLUSION
Our study revealed that FA in the subacute phase after ischemic stroke was a good predictor for functional motor recovery, which showed moderate quality based on the GRADE system. Further studies might be designed prospectively to verify this finding and focus on the accurate correlation of FA in the subacute phase and motor function outcome at a certain follow-up time point.
REFERENCES
1 Miller EL, Murray L, Richards L, Zorowitz RD, Bakas T, Clark P, et al. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient: A scientif i c statement from the american heart association. Stroke. 2010;41(10):2402–48.
2 Kwakkel G, Veerbeek JM, Harmeling-van der Wel BC, van Wegen E, Kollen BJ; Early Prediction of functional Outcome after Stroke (EPOS) Investigators. Diagnostic accuracy of the Barthel Index for measuring activities of daily living outcome after ischemic hemispheric stroke: does early poststroke timing of assessment matter? Stroke. 2011;42(2):342–6.
3 Sterr A, Shen S, Szameitat AJ, Herron KA. The role of corticospinal tract damage in chronic motor recovery and neurorehabilitation: a pilot study. Neurorehabil Neural Repair. 2010;24(5):413–9.
4 Duncan PW, Goldstein LB, Matchar D, Divine GW, Feussner J. Measurement of motor recovery after stroke. Outcome assessmentand sample size requirements. Stroke. 1992;23(8):1084–9.
5 Vargas P, Gaudron M, Valabrègue R, Bertasi E, Humbert F, Lehéricy S, et al. Assessment of corticospinal tract (CST) damage in acute stroke patients: comparison of tract-specific analysis versus segmentation of a CST template. J Magn Reson Imaging. 2013;37(4):836–45.
6 Sterr A, Dean PJA, Szameitat AJ, Conforto AB, Shen S. Corticospinal tract integrity and lesion volume play different roles in chronic hemiparesis and its improvement through motor practice. Neurorehabil Neural Repair. 2014;28(4):335–43.
7 Liang Z, Zeng J, Zhang C, Liu S, Ling X, Wang F, et al. Progression of pathological changes in the middle cerebellar peduncle by diffusion tensor imaging correlates with lesser motor gains after pontine infarction. Neurorehabil Neural Repair. 2009;23(7):692–8.
8 Møller M, Frandsen J, Andersen G, Gjedde A, Vestergaard-Poulsen P, Østergaard L. Dynamic changes in corticospinal tracts after stroke detected by fi bretracking. J Neurol Neurosurg Psychiatry. 2007;78(6):587–92.
9 Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008;34(1):51–61.
10 Jang SH. Prediction of motor outcome for hemiparetic stroke patients using diffusion tensor imaging: A review. NeuroRehabilitation. 2010;27(4):367–72.
11 Kumar P, Kathuria P, Nair P, Prasad K. Prediction of upper limb motor recovery after subacute ischemic stroke using diffusion tensor imaging: A systematic review and meta-analysis. J Stroke. 2016;18(1):50–9.
12 Lindberg PG, Skejo PHB, Rounis E, Nagy Z, Schmitz C, Wernegren H, et al. Wallerian degeneration of the corticofugal tracts in chronic stroke: A pilot study relating diffusion tensor imaging, transcranial magnetic stimulation, and hand function. Neurorehabil Neural Repair. 2007;21(6):551–60.
13 Schulz R, Park CH, Boudrias MH, Gerloff C, Hummel FC, Ward NS. Assessing the integrity of corticospinal pathways from primary and secondary cortical motor areas after stroke. Stroke. 2012;43(8):2248–51.
14 Groisser BN, Copen WA, Singhal AB, Hirai KK, Schaechter JD. Corticospinal tract diffusion abnormalities early after stroke predict motor outcome. Neurorehabil Neural Repair. 2014;28(8):751–60.
15 Puig J, Pedraza S, Blasco G, Daunis-i-Estadella J, Prados F, Remollo S, et al. Acute damage to the posterior limb of the internal capsule on diffusion tensor tractography as an early imaging predictor of motor outcome after stroke. AJNR Am J Neuroradiol. 2011;32(5):857–63.
16 Koyama T, Marumoto K, Miyake H, Domen K. Relationship between diffusion tensor fractional anisotropy and long-term motor outcome in patients with hemiparesis after middle cerebral artery infarction. J Stroke Cerebrovasc Dis. 2014;23(9):2397–404.
17 Chen JL, Schlaug G. Resting state interhemispheric motor connectivity and white matter integrity correlate with motor impairment in chronic stroke. Front Neurol. 2013;4:178.
18 Gunes H, Kandis H, Saritas A, Dikici S, Buyukkaya R. The relationship between ischemic stroke and weather conditions in Duzce, Turkey. World J Emerg Med. 2015;6(3):207–11.
19 Osborn AG, Tong KA. Handbook of neuroradiology: Brain and skull. Mosby; 1996.
20 Chen L, Liu M, Bao J, Xia Y, Zhang J, Zhang L, et al. The correlation between apparent diffusion coefficient and tumor cellularity in patients: A meta-analysis. PLoS One. 2013;8(11):e79008
21 Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. Grade guidelines: 1. Introduction-grade evidence profiles and summary of fi ndings tables. J Clin Epidemiol. 2011;64(4):383–4.
22 Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
23 Yu C, Zhu C, Zhang Y, Chen H, Qin W, Wang M, et al. A longitudinal diffusion tensor imaging study on wallerian degeneration of corticospinal tract after motor pathway stroke. Neuroimage. 2009;47(2):451–458.
24 Koyama T, Marumoto K, Miyake H, Domen K. Relationship between diffusion tensor fractional anisotropy and long-term motor outcome in patients with hemiparesis after middle cerebral artery infarction. J Stroke Cerebrovasc Dis. 2014;23(9):2397–404.
25 Song F, Zhang F, Yin DZ, Hu YS, Fan MX, Ni HH, et al. Diffusion tensor imaging for predicting hand motor outcome in chronic stroke patients. J Int Med Res. 2012;40(1):126–133.
26 Yang SH Zhan SH, Lu F, Li C, Lin J. A correlation study between diffusivity of ischemic white matter fiber tract and neuro-functional recovery in patients with acute stroke by using dti technique. Chin J Magn Reson Imaging. 2015;6(10):727–33.
27 Radlinska B, Ghinani S, Leppert IR, Minuk J, Pike GB, Thiel A. Diffusion tensor imaging, permanent pyramidal tract damage, and outcome in subcortical stroke. Neurology. 2010;75(12):1048–54.
28 Puig J, Pedraza S, Blasco G, Daunis IEJ, Prats A, Prados F, et al. Wallerian degeneration in the corticospinal tract evaluated by diffusion tensor imaging correlates with motor deficit 30 days after middle cerebral artery ischemic stroke. AJNR Am J Neuroradiol. 2010;31(7):1324–30.
29 Liu X, Tian W, Li LL, Kolar B, Qiu X, Chen F, et al. Hyperintensity on diffusion weighted image along ipsilateral cortical spinal tract after cerebral ischemic stroke: A diffusion tensor analysis. Eur J Radiol. 2012;81(2):292–7.
30 Liang Z, Zeng J, Zhang C, Liu S, Ling X, Xu A, et al. Longitudinal investigations on the anterograde and retrograde degeneration in the pyramidal tract following pontine infarction with diffusion tensor imaging. Cerebrovasc Dis. 2008;25(3):209–16.
31 Jang SH, Kim K, Kim SH, Son SM, Jang WH, Kwon HG. The relation between motor function of stroke patients and diffusion tensor imaging fi ndings for the corticospinal tract. Neurosci Lett. 2014;572:1–6.
32 Ali GG, Elhameed AMA. Prediction of motor outcome in ischemic stroke involving the pyramidal tract using diffusion tensor imaging. Egyptian Journal of Radiology and Nuclear Medicine. 2012;43(1):25–31.
33 Waller A. Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fi bres. Philos Trans R Soc Lond. 1850;140:423–9.
Received December 20, 2016
Accepted after revision March 18, 2017
World J Emerg Med 2017;8(2):99–105
10.5847/wjem.j.1920–8642.2017.02.003
Zhi-ting Guo, Email: guozhitingstudy@126.com
 World journal of emergency medicine2017年2期
World journal of emergency medicine2017年2期
- World journal of emergency medicine的其它文章
- Improving hospital-based trauma care for road traff i c injuries in Malawi
- Patient tracking in earthquake emergency response in Iran: A qualitative study
- Potential impact of early physiotherapy in the emergency department for non-traumatic neck and back pain
- Fibrinogen degradation product levels on arrival for trauma patients requiring a transfusion even without head injury
- Trend of blood lactate level in acute aluminum phosphide poisoning
- The RAMA Ped Card: Does it work for actual weight estimation in child patients at the emergency department
