Trend of blood lactate level in acute aluminum phosphide poisoning
Peyman Erfantalab, Kambiz Soltaninejad, Shahin Shadnia, Nasim Zamani, Hossein Hassanian-Moghaddam, Arezou Mahdavinejad, Behrooz Hashemi Damaneh
1Toxicological Research Center, Excellent Center of Clinical Toxicology, Department of Clinical Toxicology, Loghman Hakim Hospital, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2Department of Forensic Toxicology, Legal Medicine Research Center, Legal Medicine Organization, Tehran, Iran
Trend of blood lactate level in acute aluminum phosphide poisoning
Peyman Erfantalab1, Kambiz Soltaninejad2, Shahin Shadnia1, Nasim Zamani1, Hossein Hassanian-Moghaddam1, Arezou Mahdavinejad1, Behrooz Hashemi Damaneh1
1Toxicological Research Center, Excellent Center of Clinical Toxicology, Department of Clinical Toxicology, Loghman Hakim Hospital, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2Department of Forensic Toxicology, Legal Medicine Research Center, Legal Medicine Organization, Tehran, Iran
BACKGROUND: Aluminum phosphide (AlP) poisoning is common in the developing countries. There is no specif i c antidote for the treatment of acute AlP poisoning. Early diagnosis of poisoning and outcome predictors may facilitate treatment decisions. The objective of this study was to determine the trend of blood lactate level in acute AlP poisoning to evaluate its role as a prognostic factor.
METHODS: This was a prospective study on acute AlP intoxicated patients during one year. Demographic data, clinical and laboratory data on admission, and outcome were recorded in a selfmade questionnaire. Blood lactate levels were analyzed every two hours for 24 hours.
RESULTS: Thirty-nine (27 male, 12 female) patients were included in the study. The mortality rate was 38.5%. The mean blood pressure, pulse rate, blood pH and serum bicarbonate level were significantly different between the survivors and non-survivors groups. Blood lactate level was signif i cantly higher in the non-survivors group during 8 to 16 hours post ingestion.
CONCLUSION: Blood lactate level could be used as an index of severity of acute AlP poisoning.
Aluminum phosphide; Poisoning; Blood lactate; Prognostic factor
INTRODUCTION
Aluminum phosphide (AlP) is a fumigant that has been used to protect stored grains.[1]It is a common cause of intentional poisoning with high mortality in developing countries.[2,3]AlP is known as "rice tablet" in Iran and marketed in 3 g tablets under brand name of Phostoxin®which contain 56%.[2]
Severe intoxication cases may present with various degrees of metabolic acidosis with signs and symptoms like tachypnea, hyperpnea, tachycardia, hypotension, decreased mental status, and multisystem organ failure.[1]
Studies using Glasgow Coma Scale (GCS), electrocardiogram (ECG), blood glucose level and scoring systems like Acute Physiology and Chronic Health Evaluation (APACHE) and Simplified Acute Physiology Score (SAPS) have attempted to predict mortality in acute AlP poisoning. The data regarding their utility are inconsistent.[4–7]
Blood lactate levels have been used as a prognostic factor in critically ill patients.[8,9]Lactate has also been studied for prognostication in patients with poisoning from metformin, acetaminophen, beta blockers, carbon monoxide, cyanide and paraquat.[10–16]
The aim of this study is to determine the prognostic utility of blood lactate in acute poisoned patients admitted to the Medical Toxicology Intensive Care Unit (MTICU).
METHODS
This is a prospective study of patients with acute AlP poisoning admitted to the MTICU between March 2014 and March 2015. The diagnosis was based on the historyof exposure and positive silver nitrate test on stomach content and exhale breath samples.[17]
Subjects with a history of diabetes mellitus, cardiovascular, respiratory, renal, hepatic failure, or coingestions were excluded from data analysis as were those who received medical management for AlP poisoning prior to admission.
In all cases, gastric decontamination was performed with sodium bicarbonate (44 mEq, orally), permanganate potassium (1:10 000 orally), and activated charcoal (1 g/kg, orally) in the first 6 hours. The patients were also treated with magnesium sulfate (4–6 g/IV infusion for 24 hours), calcium gluconate (4–6 g/IV infusion for 24 hours), fluid therapy, vitamin E (400 mg/q12 hours for 24 hours, IM), and N-acetylcysteine (100 mg/kg/q8 hours for 24 hours, IV infusion). Blood lactate levels were analyzed every two hours for 24 hours using a portable lactometer (StatStrip®Lactate Xpress™ Meter, Nova Biomedical, USA).
Data collection
Data collected on each subject included demographic information, the amount of AlP ingested, time between exposure and admission to the hospital, GCS, vital signs, laboratory data on admission, and clinical outcome. Individual data of the patients were kept confidential in all stages of the study. This study was approved by the ethical committee of Shahid Beheshti University of Medical Sciences (Grant No. 5980).
Statistical analysis
All data were analyzed by social package for statistical analysis (SPSS) software version 16. The data were expressed as mean±SD for continuous or discrete variables and as a frequency and percentage for categorical variables. Chi-square test was used for statistical analysis of qualitative variables. The normal distribution of quantitative variables was tested by Kolmogorov-Smirnov test. The statistical comparison was done with Mann-Whitney U-test for nonparametric variables and independent student t-test for parametric variables. Optimal threshold for lactate levels were determined by receiver operating characteristics (ROC) analysis. P values of 0.05 or less were considered to be statistically signif i cant.
RESULTS
A total of 39 patients (27 male, 12 female) were included in the study. In all of the cases, the reason for admission was suicide. The mortality rate was 38.5% (15/39). Table 1 shows the comparison between survivors and non-survivors groups based on demographic, clinical, and laboratory data.
Plots of the distribution of blood lactate levels according to survival status and time after onset of poisoning are presented in Figures 1 and 2. Blood lactate levels were signif i cantly higher in the non-survivors group between 8 and 16 hours post ingestion (Table 2). Figure 3 indicatesblood lactate levels in survivors and non-survivors groups during the fi rst 24 hours post ingestion.

Table 1. Comparison of survived and non-survived AlP intoxicated cases based on demographic data, clinical, and paraclinical parameters on admission time
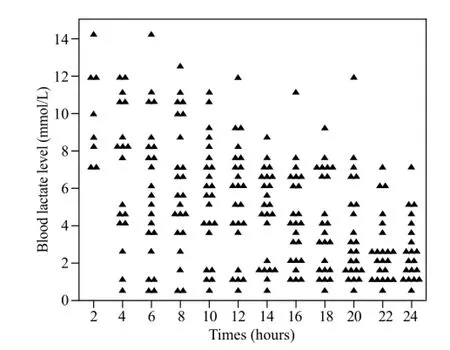
Figure 1. Distribution of the patients according to blood lactate levels in the fi rst 24 hours post ingestion in the survivors group.
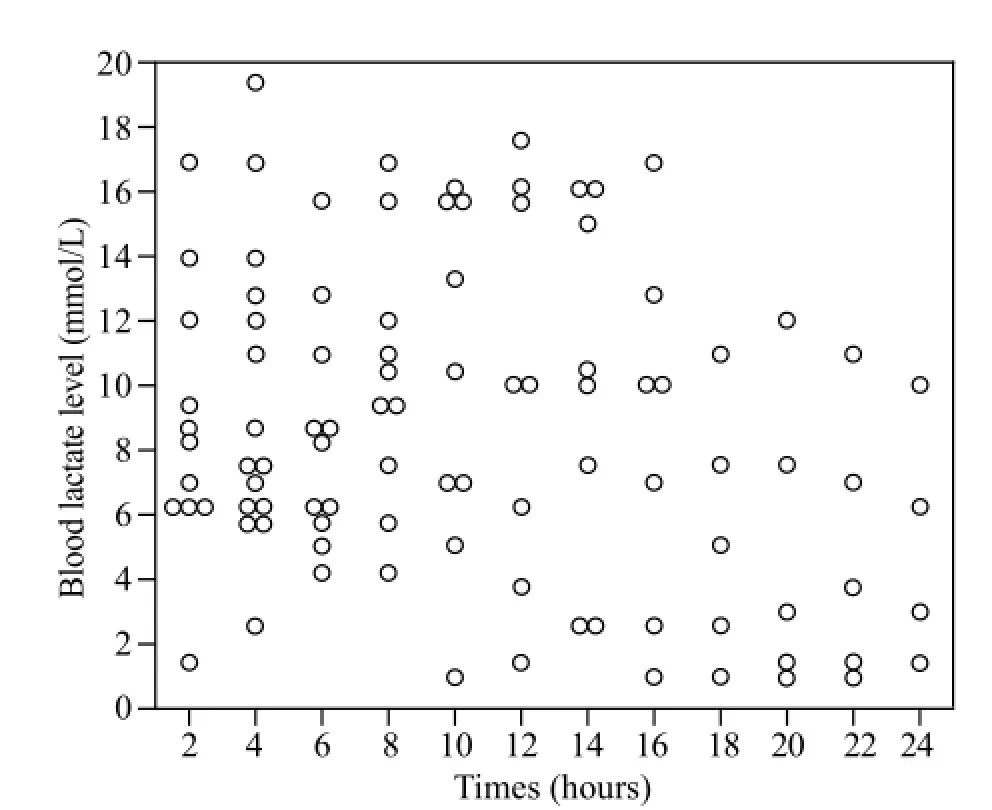
Figure 2. Distribution of the patients according to blood lactate levels in the fi rst 24 hours post ingestion in the non-survivors group.
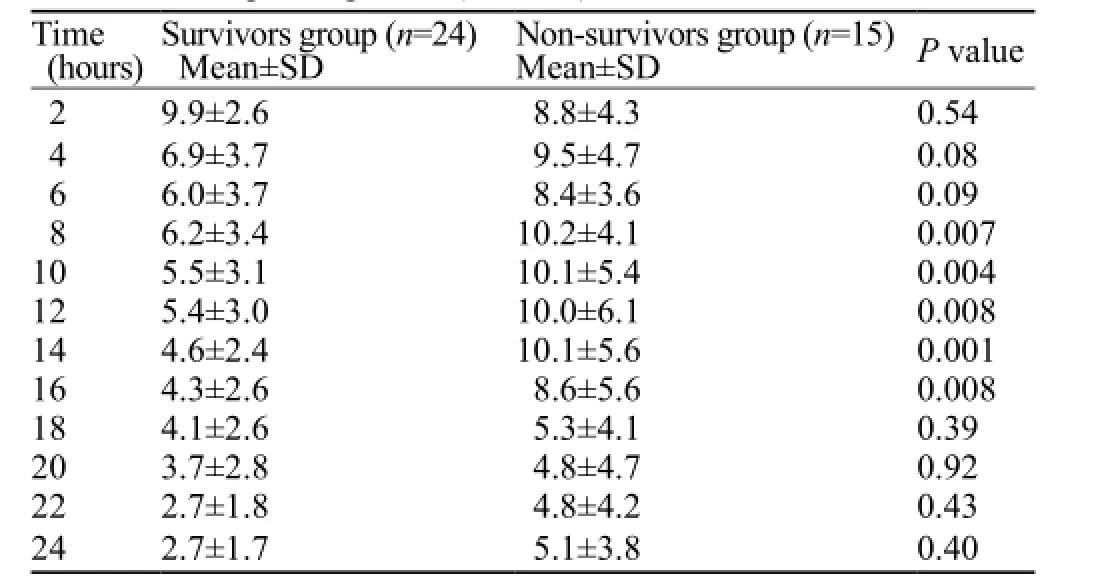
Table 2. Blood lactate level in survivors and non-survivors groups in different times post ingestion (mmol/L)
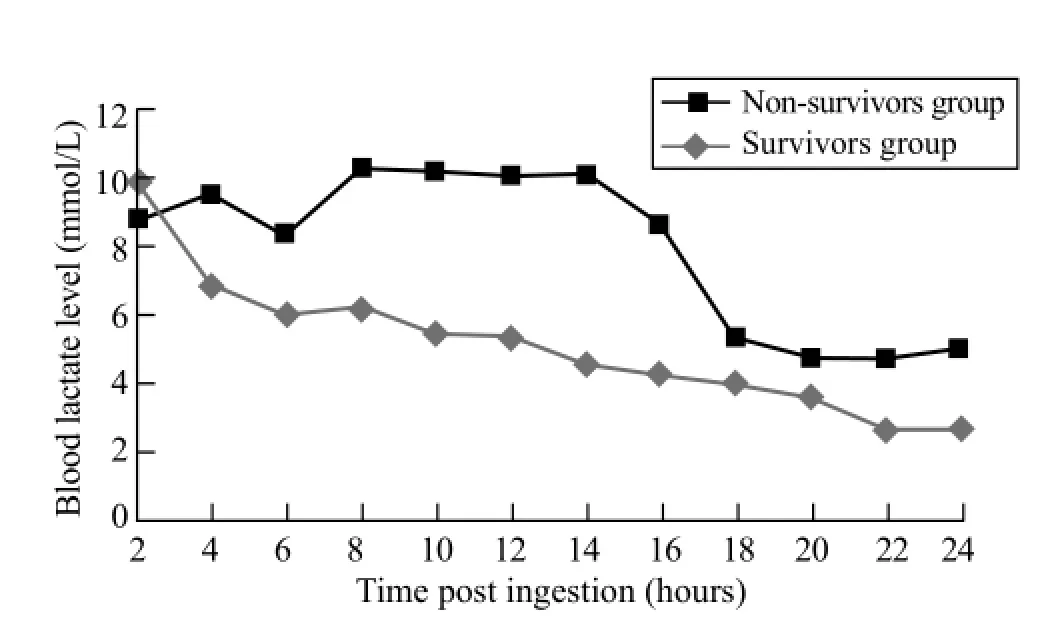
Figure 3. Blood lactate level trend in survivors and non-survivors groups in the fi rst 24 hours post ingestion.
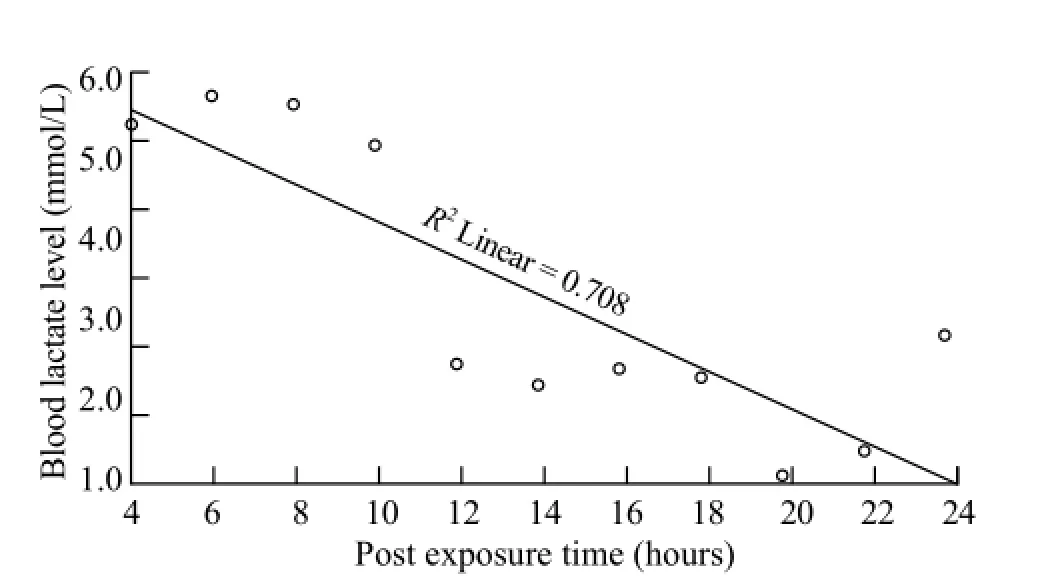
Figure 4. Blood lactate levels versus time post ingestion based on receiver operating characteristics analysis data.

Table 3. Statistical analysis of receiver operating characteristics and relative risk of death according to blood lactate levels in different times post ingestion
Table 3 shows the results of ROC analysis and relative risk for death with regard to blood lactate levels at 4 to 24 hours post ingestion. An area under the ROCcurve for the first 2-hour post ingestion was 0.364.The curve in Figure 4 was constructed as blood lactate levels versus time post ingestion based on ROC analysis data, and a line was drawn to separate the patients who are at risk of death from those who are not. The curve plots the line of best fi t connecting the lactate level of 5.4 mmol/L at 4 hours and 2.8 mmol/L at 24 hours post ingestion. The levels above the line are used to predict the likelihood of death.
The data shows significant correlation between blood lactate levels and serum pH at 6 to 22 hours postingestion: 6 hours (r =–0.36, P=0.036), 10 hours (r =–0.38, P=0.03), 14 hours (r =–0.54, P=0.002), 18 hours (r =–0.28, P=0.01), 22 hours (r =–0.51, P=0.007). Also the results showed signif i cant correlation between blood lactate levels and time of death in the non-survivors group (r =–0.52, P=0.04).
DISCUSSION
AlP is a fumigant with a high mortality rate in acute poisoning.[2]The most common clinical manifestations are due to cardiovascular and respiratory toxicity which are the most common causes of mortality.[1]
There is no specific antidote for the treatment of acute AlP poisoning. Early diagnosis and reliable predictors of mortality may guide care and appropriate resource utilization for these patients.
Previously studied prognostic variables including age, lack of vomiting, dose ingested, GCS, blood pressure, severe acidosis, hyperglycemia, and electrocardiographic abnormality have yielded inconsistent results.[4–7]
Our study demonstrated that blood pressure, pulse rate, blood pH, and serum bicarbonate level were significantly different between survivors and non-survivors groups, which is in concordance with the results of our previous studies.[4–6]
Hyperlactatemia is defined as the serum lactate level of ≥2 mmol/L.[18]In general, the mechanisms of hyperlactatemia including hypoperfusion lead to cellular hypoxia, increased activity of Na+/K+–ATPase in normoxia, increased pyruvate and lactate due to increased anaerobic glycolysis, decreased lactate clearance, muscle hyperactivity due to seizures, and impaired electron transfer and oxidative phosphorylation.[18–21]
Evaluation of the blood lactate level is generally used in diagnosis and management of the patients with signs and symptoms of sepsis or shock and is a sign of tissue hypoperfusion.[22]Blood lactate level has been reported to be a poor prognostic factor predicting death in hospital and ICU-admitted patients.[23,24]The role of blood lactate level has been studied as a prognostic factor in drug and chemical poisoning too.[10–16,25]
Hyperlactatemia in AlP toxicity is a result of both energy insuff i ciency and oxidative stress, with a possible interaction with mitochondrial electron transport chain and the inhibition of cytochrome c, which may lead to metabolic acidosis and increased lactate production.[26–29]
Within our study population, AlP poisoned patients generally have higher levels of blood lactate within the first hours post ingestion. Blood lactate level could be successfully used as a prognostic factor in acute AlP poisoning within the 8 to 16 hours post ingestion. Also our results show significant correlation between blood lactate levels and pH at 6 to 22 hours post ingestion.
The limitation of this study is the small number of cases, so it is suggested that the same study be done as a multicenter with a large number of cases.
CONCLUSION
Evaluation of blood lactate level could be used as an index of severity of poisoning and for decision making about treatment provided in acute AlP poisoning.
ACKNOWLEDGMENT
The authors wish to convey their full appreciation to dear Rama. B Rao M.D. FACMT, assistant attending physician in New York-Presbyterian hospital and assistant professor of medicine in Weill Cornell Medical College, Cornell University, for her sophisticated editing of this paper. Also the authors wish to thank the nurses of Loghman Hakim Hospital Poison Center; especially Mrs. S. Bana-Jafari, the head nurse of MTICU and Mrs. M. Rezvani the staff of MTICU.
Funding: This study was supported by a grant from Toxicological Research Center of Shahid Beheshti University of Medical Sciences.
Ethical approval: This study was approved by the ethical committee of Shahid Beheshti University of Medical Sciences (Grant No. 5980).
Conflicts of interest: The authors have no conflict of interests. The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding source.
Contributors: All authors read and approved the fi nal version of the manuscript.
REFERENCES
1 Proudfoot AT. Aluminum and zinc phosphide poisoning. Clin Toxicol (Phila). 2009;47(2):89–100.
2 Soltaninejad K, Nelson LS, Bahreini SA, Shadnia S. Fatal aluminum phosphide poisoning in Tehran-Iran from 2007 to 2010. Indian J Med Sci. 2012;66(3–4):66–70.
3 Singh SP, Aggarwal AD, Oberoi SS, Aggarwal KK, Thind AS, Bhullar DS, et al. Study of poisoning trends in north India-a perspective in relation to world statistics. J Forensic Leg Med. 2013;20(1):14–8.
4 Shadnia S, Sasanian G, Allami P, Hosseini A, Ranjbar A, Amini-Shirazi N, et al. A retrospective 7-years study of aluminum phosphide poisoning in Tehran: opportunities for prevention. Hum Exp Toxicol. 2009;28(4):209–13.
5 Shadnia S, Mehrpour O, Soltaninejada K. Simplified acute physiology score in the prediction of acute aluminum phosphide poisoning outcome. Indian J Med Sci. 2010;64(12):532–9.
6 Soltaninejad K, Beyranvand MR, Momenzadeh SA, Shadnia S. Electrocardiographic findings and cardiac manifestations in acute aluminum phosphide poisoning. J Forensic Leg Med. 2012;19(5):291–3.
7 Mehrpour O, Alfred S, Shadnia S, Keyler DE, Soltaninejad K, Chalaki N, et al. Hyperglycemia in acute aluminum phosphide poisoning as a potential prognostic factor. Hum Exp Toxicol. 2008;27(7):591–5.
8 Fall PJ, Szerlip HM. Lactic acidosis: from sour milk to septic shock. J Intensive Care Med. 2005;20(5):255–71.
9 Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45(5):524–8.
10 Dell'aglio DM, Perino LJ, Kazzi Z, Abramson J, Schwartz MD, Morgan BW. Acute metformin overdose: examining serum pH, lactate level, and metformin concentrations in survivors versus nonsurvivors: a systematic review of the literature. Ann Emerg Med. 2009;54(6):818–23.
11 Seidowsky A, Nseir S, Houdret N, Fourrier F. Metforminassociated lactic acidosis: a prognostic and therapeutic study. Crit Care Med. 2009;37(7):2191–6.
12 Bernal W, Donaldson N, Wyncoll D, Wendon J. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: a cohort study. Lancet. 2002;359(9306):558–63.
13 Mégarbane B, Deye N, Malissin I, Baud FJ. Usefulness of the serum lactate concentration for predicting mortality in acute beta-blocker poisoning. Clin Toxicol (Phila). 2010;48(10):974–78.
14 Inoue S, Saito T, Tsuji T, Tamura K, Ohama S, Morita S. Lactate as a prognostic factor in carbon monoxide poisoning: a case report. Hum Exp Toxicol. 2011;30(8):836–43.
15 Baud FJ, Borron SW, Mégarbane B, Trout H, Lapostolle F, Vicaut E, et al. Value of lactic acidosis in the assessment of the severity of acute cyanide poisoning. Crit Care Med. 2002;30(9): 2044–50.
16 Lee Y, Lee JH, Seong AJ, Hong CK, Lee HJ, Shin DH, et al. Arterial lactate as a predictor of mortality in emergency department patients with paraquat intoxication. Clin Toxicol (Phila). 2012;50(1):52–6.
17 Braithwaite R. Metals and Anions. In: Moffat AC, et al. (Eds) Clarke's Analysis of Drugs and Poisons, Vol. 1, 3rd ed., London, UK: Pharmaceutical Press; 2004;273.
18 Gunnerson KJ. Clinical review: The meaning of acid–base abnormalities in the intensive care unit – epidemiology. Crit Care. 2005;9(5):508–16.
19 Hulme J, Sherwood N. Severe lactic acidosis following alcohol related generalized seizures. Anaesthesia. 2004;59(12):1228–30.
20 Jorens PG, Demey HE, Schepens PJ, Coucke V, Verpooten GA, Couttenye MM, et al. Unusual D-lactic acid acidosis from propylene glycol metabolism in overdose. J Toxicol Clin Toxicol. 2004;42(2):163–9.
21 Benaissa ML, Mégarbane B, Borron SW, Baud FJ. Is elevated plasma lactate a useful marker in the evaluation of pure carbon monoxide poisoning? Intensive Care Med. 2003;29(8):1372–5.
22 Kompanje EJ, Jansen TC, van der Hoven B, Bakker J. The fi rst demonstration of lactic acid in human blood in shock by Johann Joseph Scherer (1814–1869) in January 1843. Intensive Care Med. 2007;33(11):1967–71.
23 Rishu AH, Khan R, Al-Dorzi HM, Tamim HM, Al-Qahtani S, Al-Ghamdi G, et al. Even mild hyperlactatemia is associated with increased mortality in critically ill patients. Crit Care. 2013;17(5):R197.
24 Nichol AD, Egi M, Pettila V, Bellomo R, French C, Hart G, et al. Relative hyperlactatemia and hospital mortality in critically ill patients: a retrospective multi-centre study. Crit Care. 2010;14(1):R25.
25 Manini AF, Kumar A, Olsen D, Vlahov D, Hoffman RS. Utility of serum lactate to predict drug-overdose fatality. Clin Toxicol. 2010;48(7):730–6.
26 Anand R, Kumari P, Kaushal A, Bal A, Wani WY, Sunkaria A, et al. Effect of acute aluminum phosphide exposure on rats: a biochemical and histological correlation. Toxicol Lett. 2012;215(1):62–9.
27 Dua R, Sunkaria A, Kumar V, Gill KD. Impaired mitochondrial energy metabolism and kinetic properties of cytochrome oxidase following acute aluminium phosphide exposure in rat liver. Food Chem Toxicol. 2010;48(1):53–60.
28 Louriz M, Dendane T, Abidi K, Madani N, Abouqal R, Zeggwagh AA. Prognostic factors of acute aluminum phosphide poisoning. Indian J Med Sci. 2009;63(6):227–34.
29 Anand R, Sharma DR, Verma D, Bhalla A, Gill KD, Singh S. Mitochondrial electron transport chain complexes, catalase and markers of oxidative stress in platelets of patients with severe aluminum phosphide poisoning. Hum Exp Toxicol. 2013;32(8):807–16.
Received May 8, 2016
Accepted after revision January 9, 2017
World J Emerg Med 2017;8(2):116–120
10.5847/wjem.j.1920–8642.2017.02.006
Shahin Shadnia, Email: shahin1380@yahoo.com
 World journal of emergency medicine2017年2期
World journal of emergency medicine2017年2期
- World journal of emergency medicine的其它文章
- Improving hospital-based trauma care for road traff i c injuries in Malawi
- Patient tracking in earthquake emergency response in Iran: A qualitative study
- Prediction of motor recovery after ischemic stroke using diffusion tensor imaging: A meta-analysis
- Potential impact of early physiotherapy in the emergency department for non-traumatic neck and back pain
- Fibrinogen degradation product levels on arrival for trauma patients requiring a transfusion even without head injury
- The RAMA Ped Card: Does it work for actual weight estimation in child patients at the emergency department
