大黄鱼(Larimichthyscrocea)IL-10基因的克隆与表达分析
郑维兵,慕鹏飞,丁连帅,杨思司,敖敬群,艾春香,陈新华*
(1. 国家海洋局第三海洋研究所 海洋生物遗传与资源重点实验室,福建 厦门 361005;2. 厦门大学 海洋与地球学院,福建 厦门 361005)
大黄鱼(Larimichthyscrocea)IL-10基因的克隆与表达分析
郑维兵1,慕鹏飞2,丁连帅1,杨思司1,敖敬群1,艾春香2,陈新华1*
(1. 国家海洋局第三海洋研究所 海洋生物遗传与资源重点实验室,福建 厦门 361005;2. 厦门大学 海洋与地球学院,福建 厦门 361005)
白细胞介素10(Interleukin 10,IL-10)是一种抗炎细胞因子,可以抑制机体免疫反应。本研究经过分析大黄鱼基因组数据库发现了IL-10同源基因,并对其cDNA编码区序列和基因组DNA序列进行了克隆分析。大黄鱼IL-10(LycIL-10)基因由5个外显子和4个内含子构成,其序列全长1 869 bp,其开放阅读框(ORF)长555 bp,编码184个氨基酸,其N端的22个氨基酸残基为预测的信号肽,成熟肽由162个氨基酸残基组成,包含了脊椎动物IL-10标志性保守序列。LycIL-10的氨基酸序列同其他已知物种的IL-10氨基酸序列的一致性为26.49%~77.01%。Real-time PCR分析发现LycIL-10在检测的组织中为组成型表达,在脾脏和肌肉中转录水平相对较高。三联灭活细菌疫苗和聚肌苷酸胞苷酸(poly(I∶C) )刺激后,大黄鱼头肾和脾脏中LycIL-10 mRNA的转录水平会显著升高,表明LycIL-10可能参与抑制大黄鱼由细菌和病毒引起的炎症反应。
大黄鱼;白细胞介素10;分子克隆;实时定量 PCR;表达分析
1 引言
白细胞介素10(Interleukin-10, IL-10),又称细胞因子合成抑制因子,最初因其由Th2细胞分泌,并可以抑制Th1细胞合成与分泌IL-2和IFN-γ而被发现,是一种有效的抗炎因子,主要由单核/巨噬细胞和Th2细胞合成分泌[1—2]。除此之外,T细胞的其他亚群、B细胞、嗜酸性粒细胞、上皮细胞、角质细胞、间质细胞、自然杀伤性细胞(NK细胞)和肿瘤细胞等也可以分泌IL-10[3]。由于IL-10可以抑制T细胞和NK细胞活性而被广泛研究,随着研究深入,发现其主要是通过抑制活性氧(ROS)和NO产生、降低MHC II和一些巨噬细胞分泌的炎症因子(IL-1、TNF-α、IL-12和环氧酶2等)的表达从而抑制机体针对病原体入侵产生的免疫反应[4—11]。在T细胞分化的过程中IL-10也发挥重要作用,其通过抑制Th1细胞因子IFN-γ和IL-2的表达来促进T细胞向Th2分化[12—13]。
目前已经有多种哺乳动物的IL-10基因被克隆并研究[14—16],哺乳动物IL-10含6个α螺旋和2个分子内二硫键,通常以非共价键结合形成同源二聚体的形式来发挥其功能[17—18]。IL-10受体(IL-10R)含有两个亚基,IL-10R1和IL-10R2。IL-10首先与IL-10R1结合,形成IL-10/IL-10R1复合体,IL-10与IL-10R1的构象发生改变,然后与IL-10R2结合[19—22]。IL10与受体结合后通过激活接头蛋白Jak1(与IL-10R1结合)和Tyk2(与IL-10R2结合)来激活转录因子STAT3,在特定细胞中也可以激活STAT1和STAT5[23]。这些转录因子形成同源或者异源二聚体后进入细胞核促进细胞因子信号传导抑制因子3(SOCS3)的转录表达,SOCS3通过抑制促炎因子的转录表达来发挥IL-10抑制炎症反应的作用[24—26]。
有研究表明多种哺乳动物病毒也含有IL-10基因,例如Epstein-Barr病毒(EBV)、巨细胞病毒(CMV)、马疱疹病毒2型和羊口疮病毒[14,27—28]。这些病毒的IL-10可以与宿主的IL-10受体结合,并发挥宿主IL-10的生物学功能,通过此来抑制宿主的免疫反应从而达到逃避宿主免疫系统攻击的目的[29]。目前普遍认为病毒IL-10基因是其入侵宿主后从宿主获得的完全的或者不完全的IL-10转录剪切体。
Zou等运用生物信息学方法分析红鳍东方鲀(Fugurubripes)的基因组首次发现了鱼类的IL-10基因[29]。近年来,已经有多种鱼类的IL-10基因被发现,包括虹鳟(Oncorhynchusmykiss)、斑马鱼(Daniorerio)、鳕鱼(Gadusmorhua)、鲤鱼(Cyprinuscarpio)、美国黑鲈(Dicentrarchuslabrax)、白鲢(Hypophthalmichthysmolitrix)和金鱼(CarassiusauratusL.)[30—37]。在虹鳟中发现了氨基酸序列一致性高达92%的两个IL-10基因,而其他鱼类均只发现了一个IL-10基因[37]。在金鱼中,被灭活的杀鲑气单胞菌(Aeromonassalmonicida)激活的单核细胞与IL-10充分孵育后,其TNF-α1、TNF-α2、 IL-1β1、IL-10、CXCL_8/IL-8和NADPH氧化组建件p47(phox)表达量均下降,而经过IL-10预处理后,单核细胞则不会被杀鲑气单胞菌或者IFN-γ激活而产生活性氧中间产物(ROI)[36]。另外金鱼的IL-10可以诱导STAT3磷酸化并且入核,STAT3入核后迅速促进SOCS3的转录表达[36]。研究表明鲤鱼的IL-10可以抑制PMA和LPS对中性粒细胞和巨噬细胞的激活作用,并可以抑制巨噬细胞中MHC抗原呈递相关基因的转录表达[38]。一个IL-10同源基因在锦鲤孢疹病毒(Koi herpesvirus, Khv)中被发现,此病毒的IL-10基因与鲤鱼IL-10基因具有中等的相似度,并含有一个信号肽,研究发现其对病毒的复制和病毒的毒性都不是必须的[39]。锦鲤孢疹病毒IL-10(Khv IL-10)刺激斑马鱼胚胎后,发现lyz+(溶菌酶表达阳性)细胞数目增多,当IL-10R1基因表达被沉默之后,这一功能又被屏蔽,说明其可以利用斑马鱼的IL-10R1,并具有和斑马鱼IL-10相似的功能[40]。最新研究表明Khv IL-10与其宿主IL-10一样,可以通过STAT3信号通路发挥抗炎功能[41]。目前已有许多大黄鱼细胞因子被研究,例如IL-1β、IL-6、IL-8、IL-17A/F、IFN-γ和CXCL8等[42—46],但未见针对大黄鱼IL-10的相关研究。通过对大黄鱼基因组分析,我们发现了一个IL-10同源基因[47]。进一步克隆了LycIL-10基因的cDNA全长并分析了其cDNA序列和其编码蛋白的氨基酸序列特征,还对大黄鱼IL-10的组织分布和诱导表达特点进行了研究。
2 材料与方法
2.1 实验鱼
实验用大黄鱼购自福建省福州市连江县下屿村,体长为(20±1.76)cm,体质量为(100±21.5)g。采样时养殖水域温度为(20±3)℃。
2.2 疫苗制备
副溶血弧菌(Vibrioparahaemolyticus)、溶藻弧菌(Vibrioalginolyticus)、嗜水汽单胞菌(Aeromonashydrophila)均为实验室从大黄鱼病鱼中分离所得[48]。实验时将菌种从-80℃冰箱取出平板划线活化,然后挑取单菌落接种至LB培养基中,培养至OD600至0.5左右,收集菌体,用无菌PBS洗涤菌体两次后将菌体重悬制备成菌体悬液(3×109cfu/mL),最后将3种菌的悬液等体积混合。高压灭活后制成三联灭活细菌疫苗,保存于4℃冰箱备用。Poly(I∶C)粉末溶解于无菌PBS,调整浓度至1 mg/mL,保存于-20℃冰箱备用。
2.3 样品采集
健康大黄鱼组织样品采集:将健康大黄鱼置于50 mg/L的MS-222中麻醉,待大黄鱼处于麻醉状态后先抽取大黄鱼血液,后取大黄鱼脾脏、脑、肾脏、肝脏、皮肤、肌肉、心脏、鳃以及小肠等组织样品,置于液氮中速冻,运回实验室后转移至-80℃冰箱保存。
三联灭活细菌疫苗刺激组及poly(I∶C)刺激组样品采集:首先以腹腔两点注射的方法将三联灭活细菌疫苗和poly(I∶C)储存液分别注射健康的大黄鱼,每尾鱼注射200 μL,每种溶液注射35尾鱼。另用等体积PBS注射作为对照。在注射后在0 h、6 h、12 h、24 h和48 h等时间点,每个时间点取5尾大黄鱼,首先置于50 mg/L 的MS-222中麻醉,待大黄鱼处于麻醉状态后取脾脏和头肾组织样品并迅速置于液氮速冻,运回实验室后转移至-80℃冰箱保存。
2.4 RNA提取以及cDNA第一链的制备
同一组的5尾大黄鱼,每尾取10~20 mg组织混合后加入1 mL Trizol (Life Technology),用匀浆器匀浆,根据Trizol法提取RNA操作指南提取总RNA。提取后的总RNA经DNase I(TaKaRa)处理,酚/氯仿/异戊醇(25∶24∶1)(索莱宝科技有限公司)再次抽提。最后按照逆转录酶M-MLV (RNase H-)(TaKaRa)说明书将RNA逆转录为第一链cDNA后保存于-20℃冰箱备用。
2.5 大黄鱼IL-10基因克隆
根据大黄鱼基因组预测的LycIL-10 cDNA序列(GenBank登录号为XM_010738826.1)及基因组DNA序列(GenBank登录号为JPYK01015197.1),利用Primer Premier 5.0软件设计引物P1、P2(引物序列见表1)进行PCR扩增LycIL-10基因开放阅读框(ORF)和基因组DNA序列,引物(生工生物工程(上海)股份有限公司)序列如下(表1)。使用TransStart Fast Pfu Fly DNA聚合酶(全式金生物技术有限公司)进行PCR反应,反应件如下:95℃预变性3 min;然后按95℃变性30 s,58℃退火30 s,cDNA为模板时72℃延伸30 s,基因组DNA为模板时延伸90 s,进行35个循环;72℃终延伸10 min。琼脂糖(Biowest)凝胶电泳检测PCR产物大小正确后,用胶回收试剂盒(Omega Bio-Tek)回收目的条带,经平末端加A尾试剂盒(TaKaRa)加A尾后连接至pMD20-T载体(TaKaRa)中,转化EscherichiacoliDH5α感受态细胞,用氨苄青霉素(生工生物工程(上海)股份有限公司)抗性平板筛选阳性菌落,并经菌落PCR初步验证后,送至上海美吉生物医药科技有限公司进行测序验证。
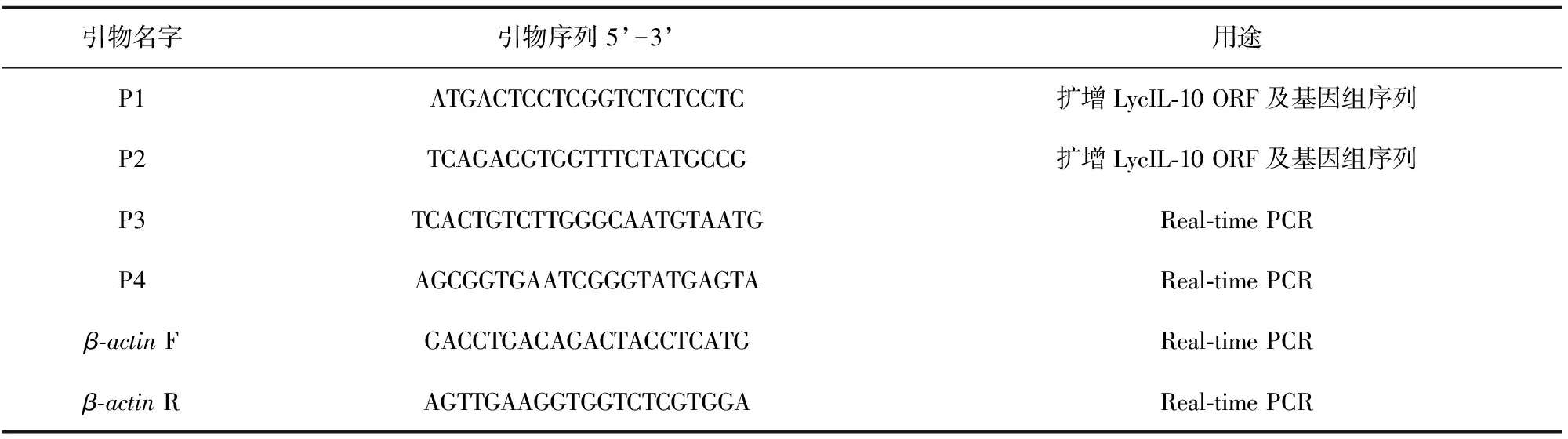
表1 引物序列表
2.6 生物信息学分析
通过SignalP 4.0和NetNGlyc 1.0在线程序(http://www.cbs.dtu.dk/services/SignalP/)和(http://www.cbs.dtu.dk/services/NetNGlyc/)分别预测LycIL10信号肽序列和天冬酰胺糖基化位点。从NCBI数据库收集其他物种的IL-10同源序列,利用DNAMAN 软件进行多序列比对分析,并用MEGA 6.0(Molecular Evolution Genetics Analysis)软件通过近邻法(Neighbor-Joining)构建系统进化树。
2.7 大黄鱼IL-10基因表达分析
为研究LycIL-10 mRNA在健康大黄鱼器官或组织中的表达,通过实时荧光定量PCR(Real-time PCR),利用引物P3和P4(序列见表1)检测LycIL-10 mRNA在健康大黄鱼脾脏、血液、脑、肾脏、肝脏、皮肤、肌肉、心脏、鳃及小肠中的转录水平。为研究大黄鱼受到免疫刺激后LycIL-10 mRNA的诱导表达特征,取经过三联灭活细菌疫苗或poly(I∶C)刺激后的大黄鱼头肾及脾脏组织(采样方法见2.3),提取总RNA制备第一链cDNA,利用表1中引物(P3/P4,β-actinF/β-actinR)进行Real-time PCR。Real-time PCR反应条件如下:95℃预变性30 s;95℃变性5 s,58℃退火15 s,72℃延伸20 s,40个循环,实验数据采用2-ΔΔCT法进行分析。
以上实验均重复3次,实验结果利用Graph Pad Prism 5进行分析及绘图,采用双尾T检验法进行统计学分析,P<0.05时视为差异显著,P<0.01即为差异极显著。
3 结果
3.1 大黄鱼IL-10基因的克隆与序列分析
通过分析大黄鱼基因组数据,我们获得了大黄鱼IL-10的cDNA编码区序列以及基因组DNA序列,GenBank登陆号分别为XM_010738826.1 和JPYK01015197.1。利用Primer Premier 5.0软件设计引物,分别以大黄鱼脾脏cDNA和肌肉基因组为模板,通过PCR扩增获得了与预测大小一致的目的片段,测序结果显示扩增得到的目的片段序列与GenBank对应序列一致。LycIL-10基因组DNA序列全长1 869 bp(图1)由5个外显子和4个内含子构成(图2)。内含子的剪切位点(5’GT-内含子-CAG-3’)如图1所示。

图1 LycIL-10的核苷酸序列及氨基酸序列分析Fig.1 Analysis of nucleotide and deduced amino acid sequences of LycIL-10起始密码子和终止密码子以灰色背景显示,方框中的DNA序列GT和CAG为内含子的剪切位点;加下划线部分为预测的信号肽序列,椭圆形中的氨基酸残基为预测的N糖基化位点Features highlighted with gray include the start and stop codon, Splice sites, GT and CAG, of the intron are shown in boxes; the putative signal peptide is underlined, a potential glycosylation site is circled with ellipse

图2 人、小鼠、虹鳟、红鳍东方鲀、斑马鱼和大黄鱼IL-10的基因组结构比较Fig.2 Comparison of the genomic organization of IL-10 genes in human, mouse, rainbow trout, fugu rubripes, zebrafish, large yellow croaker人(Homo sapiens, DQ217938.1)、小鼠(Mus musculus, M84340.1)、虹鳟(Oncorhynchus mykiss, AB118099.1)、红鳍东方鲀(Takifugu rubripes, AJ539537.1)、斑马鱼(Danio rerio, AY887900.1)和大黄鱼IL-10的基因组结构比较,其中黑色方框代表外显子黑色线条代表内含子,外显子长度标在其上方Comparison of the genomic organization of IL-10 in human (Homo sapiens, DQ217938.1), mouse (Mus musculus, M84340.1), rainbow trout (Oncorhynchus mykiss, AB118099.1), fugu rubripes (Takifugu rubripes, AJ539537.1) and zebrafish (Danio rerio, AY887900.1) and large yellow croaker. Black boxes represent exons, thin lines joining them are introns and the numerals on the black boxes depict the length of the corresponding exons

图3 LycIL-10与其他脊椎动物IL-10氨基酸多序列比对Fig.3 Alignment of LycIL-10 with IL-10 from other vertebrates通过DNAMAN软件对LycIL-10与其他脊椎动物IL-10进行氨基酸多序列比对分析。相同氨基酸用黑色背景标示(一致性等于100%),保守序列则使用红色(一致性大于等于75%)及蓝色(一致性大于等于50%)背景标示。红色方框所示为在鱼类保守的而其他高等脊椎动IL-10物所没有的两个半胱氨酸,箭头所指区域分别为信号肽和IL-10保守结构域Multiple alignment of the predicted LycIL-10 translation with other known IL-10 molecules, generated by the DNAMAN software. Black background denote conserved amino acids, whilst red (identity no less than 75%) or blue (identity no less than 50%) denote conservative substitutions. Two fish-specific cysteine residues are surrounded by a red outline. The signal peptide and IL-10 family signature residues marked by the double sided arrow

图4 鱼类及其他脊椎动物的IL-10系统进化树Fig.4 Phylogenetic tree of IL-10 from fish and other vertebrate species进化树中各物种IL-10氨基酸序列的GenBank登录号为:鲫鱼IFN-γ(Carassius auratus, ACG68885.1);人IL-10(Homo sapiens, NP_000563.1);小家鼠IL-10(Mus musculus,NP_034678.1);家犬IL-10(Canis familiaris, ABY86619.1);家猫IL-10(Felis catus, NP_001009209.1);家兔IL-10(Oryctolagus cuniculus, NP_001075514.1);野猪IL-10(Sus scrofa, NP_999206.1);原鸡IL-10(Gallus gallus, NP_001004414.2);红鳍东方鲀IL-10(Takifugu rubripes, CAD62446.1);鲈鱼IL-10(Dicentrarchus labrax, CAK29522.1);斑马鱼IL-10(Danio rerio, NP_001018621.2);青斑河豚IL-10(Tetraodon nigroviridis, AAP57415.1);热带爪蟾IL-10(Xenopus tropicalis, NP_001165400.1);虹鳟IL-10(Oncorhynchus mykiss, BAD20648.1);鲤鱼IL-10(Cyprinus carpio, BAC76885.1)Accession numbers of Genbank used for sequences of IL-10: crucian IFN-γ (Carassius auratus, ACG68885.1); human IL-10 (Homo sapiens, NP_000563.1); house mouse IL-10 (Mus musculus, NP_034678.1); dog IL-10 (Canis familiaris, ABY86619.1); domestic cat IL-10 (Felis catus, NP_001009209.1); rabbit IL-10 (Oryctolagus cuniculus, NP_001075514.1); pig IL-10 (Sus scrofa, NP_999206.1); chicken IL-10 (Gallus gallus, NP_001004414.2); fugu rubripes IL-10 (Takifugu rubripes, CAD62446.1); European seabass IL-10 (Dicentrarchus labrax, CAK29522.1); zebrafish IL-10 (Danio rerio, NP_001018621.2); spotted green pufferfish IL-10 (Tetraodon nigroviridis, AAP57415.1); tropical clawed frog IL-10 (Xenopus tropicalis, NP_001165400.1); rainbow trout IL-10 (Oncorhynchus mykiss, BAD20648.1); common carp IL-10 (Cyprinus carpio, BAC76885.1)

图5 LycIL-10 mRNA在不同组织中的相对转录水平Fig.5 Relative transcription level of LycIL-10 mRNA in different tissues
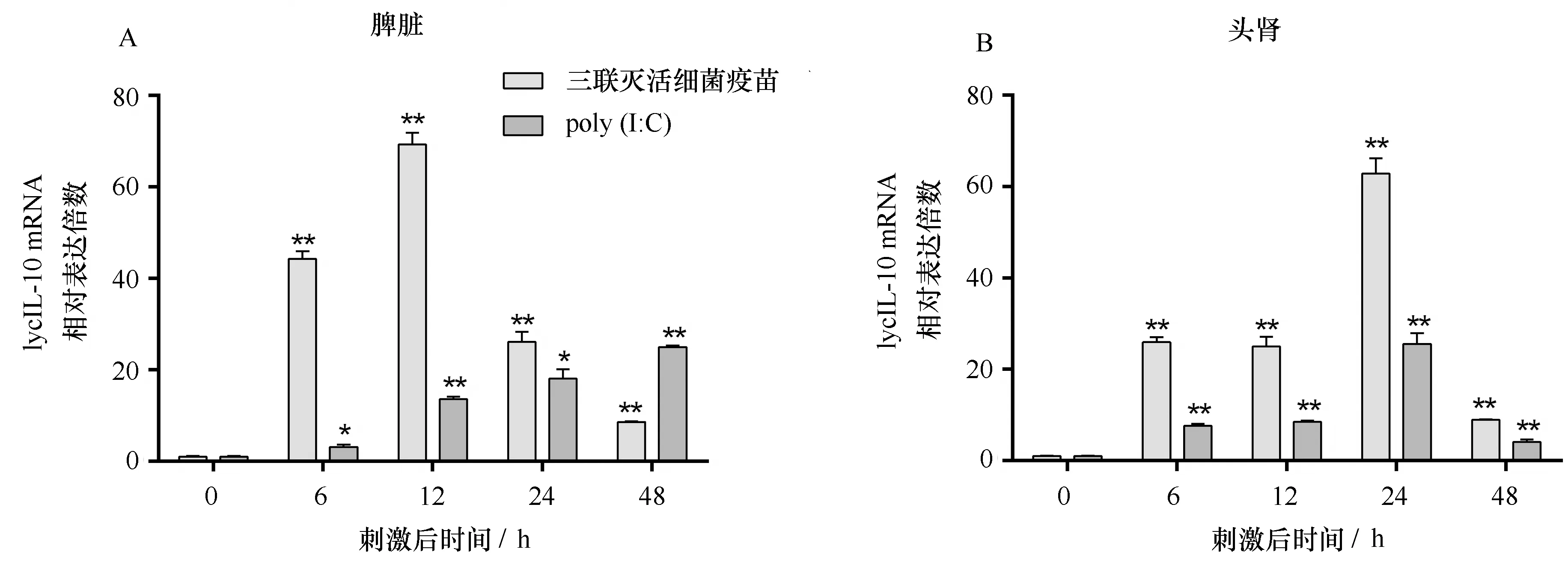
图6 三联灭活细菌疫苗和 poly(I∶C)刺激后大黄鱼的脾脏和头肾中LycIL-10 mRNA的表达分析Fig.6 Expression modulation analysis of LycIL-10 mRNA in spleen and head kidney of large yellow croaker after inactivated trivalent bacterial vaccines and poly (I∶C) stimulation
LycIL-10 ORF基因的序列全长555 bp,编码184个氨基酸,经SignalP 4.0在线预测其N端22个氨基酸残基为信号肽序列,糖基化位点在线预测显示其第146位的天冬酰胺为一个潜在N糖基化位点(图1)。成熟的LycIL-10分子包含162个氨基酸残基,预测其分子量约为18.73 kD,等电点(pI)为6.07。多序列比对发现大黄鱼IL-10与其他鱼类IL-10的氨基酸序列具有较高的一致性(43.78%~77.01%),与哺乳动物、鸟类以及两栖类IL-10的氨基酸序列一致性较低(26.49%~29.41%)。其中大黄鱼IL-10第156~172位氨基酸残基“GLYKAMGELNLLFNYIE”属于IL-10家族标志性保守序列“G-X2-KA-X2-[DE]-X-D[ILV]-[FLY]-[FILMV]-X2-[ILMV][EKQR] ”(图3)。系统进化分析发现,鱼类的IL-10聚为一支,而鸟类哺乳动物以及两栖类的IL-10聚为一支(图4)。另外,LycIL-10拥有两个在鱼类IL-10保守而在其他脊椎动物IL-10中没有的两个半胱氨酸残基序列(Cys-26,Cys-31)。
3.2 大黄鱼IL-10组织分布分析
利用Real-time PCR分析发现LycIL-10 mRNA在健康大黄鱼各个组织中均有转录,但在不同组织之间其转录水平差异较大。在脑中LycIL-10 mRNA的转录水平最低,在肌肉和脾脏中转录水平较高,分别为脑中的83.5和148.7倍,在鳃、肝脏、血液和小肠等组织中LycIL-10 mRNA转录水平分别为脑中的11.9、11.3、11.1和10.1倍,在肾脏、心脏和皮肤中分别为脑中的3.5、3.7和5.8倍(图5)。
3.3 大黄鱼IL-10基因的诱导表达分析
经过灭活三联灭活细菌疫苗和poly(I∶C)刺激后,LycIL-10 mRNA的转录水平在大黄鱼脾脏和头肾中均有显著上调(图6)。三联灭活细菌疫苗刺激后,脾脏和头肾的LycIL-10 mRNA的转录水平在刺激后6 h显著上调,脾脏在刺激后12 h达到最高,为0 h的69.3倍,头肾中则在24 h达到最高,为0 h的62.9倍。在poly(I∶C)刺激后,大黄鱼脾脏和头肾的LycIL-10 mRNA转录水平也在刺激后6 h显著上调,脾脏中LycIL-10 mRNA的转录水平在刺激后48 h达到最高,头肾在刺激后24 h达到最高,分别为0 h的25.0和25.5倍。
4 讨论
IL-10作为一种重要的抗炎因子,在高等哺乳动物、鸟类、两栖类和鱼类已经被广泛研究,而有关大黄鱼IL-10基因的研究目前尚未见报道。本文根据大黄鱼基因组数据得到了LycIL-10基因的cDNA序列及基因组DNA序列,经PCR扩增后测序结果与基因组数据中结果一致。经分析发现LycIL-10基因由5个外显子和4个内含子构成,这与哺乳动物以及已知鱼类如红鳍东方鲀、虹鳟、斑马鱼、鲤鱼以及大西洋鳕鱼的基因组结构一致,并且各个外显子长度比较保守,而各个内含子长度差异比较大,一般而言小鼠和人等哺乳动物的IL-10基因内含子比较长,鱼类的IL-10基因内含子较哺乳动物短,而在鱼类中虹鳟IL-10基因的内含子长度与哺乳动物比较相似[29—31,33,35]。LycIL-10基因ORF序列全长555 bp,推测其编码184个氨基酸,经SignalP在线预测其N端22个氨基酸残基为信号肽序列,糖基化位点在线预测显示其146位的天冬酰胺是一个潜在N糖基化位点。小鼠和虹鳟的IL-10均含一个预测的糖基化位点,红鳍东方鲀IL-10则有2个预测的糖基化位点,研究表明原核大肠杆菌表达的重组小鼠IL-10具备所有小鼠IL-10的生物学功能,这说明糖基化位点可能对IL-10发挥其生物学功能不是必需的[1,5]。多序列比对发现LycIL-10包含了在脊椎动物中保守的4个半胱氨酸(Cys-30,Cys-80,Cys-130,Cys-136),和两个鱼类中保守的半胱氨酸(Cys-26,Cys-31),目前还没有研究表明这两个半胱氨酸对鱼类IL-10结构和功能方面的作用。LycIL-10具有IL-10家族的特征序列“G-X2-KA-X2-[D,E]-X-D[ILV]-[FLY]-[FILMV]-X2-[ILMV][EKQR]”,这一段序列在所有脊椎动物的IL-10中都是保守的。LycIL-10基因编码的氨基酸序列与人IL-10的氨基酸序列的一致性为27.57%,与其他已知的哺乳动物和鸟类以及两栖类IL-10的氨基酸序列的一致性为26.49%~29.41%,与已知的其他鱼类的IL-10氨基酸序列有较高的一致性,为43.78%~77.01%,系统进化树结果与此结果一致。
Real-time PCR检测结果显示,LycIL-10 mRNA在脾脏中转录水平最高,在肌肉中次之,在鳃、小肠、肝脏和血液中的表达为中等,在皮肤、心脏、肾脏和脑中的转录水平均较低。在金鱼中,IL-10 mRNA的转录水平也是在脾脏中最高,这可能是因为在鱼类中脾脏是一个主要的免疫器官,富含T细胞较多,而IL-10对于T细胞分化有重要作用[36]。鳃和小肠中IL-10 mRNA的转录水平中等,可能是由于它们都是鱼类重要的粘膜免疫器官,其组织微环境波动较大,IL-10可能对于维持这两个组织的免疫稳态有重要作用[49]。在斑马鱼和虹鳟中,IL-10 mRNA则是在肾脏中的转录水平最高在肌肉中转录水平较低,而在大黄鱼中LycIL-10 mRNA在肾脏中的相对转录水平较低而在肌肉中转录水平较高,鱼类的肾脏在鱼类免疫方面发挥重要作用,但是LycIL-10 mRNA在肾脏的转录水平并不高,这一结果可能与鱼自身的一些生理特征和所处环境有关[31,33]。
IL-10是一个有效的抗炎因子,可以有效抑制炎症反应,避免由于获得性免疫过强而引起的组织损伤,主要在炎症反应中后期发挥作用[50]。副溶血弧菌、溶藻弧菌、嗜水汽单胞菌均为大黄鱼致病菌,这3种细菌经过灭活后制备的三联灭活细菌疫苗可以诱导大黄鱼产生类似细菌引起的炎症反应[48]。Poly(I∶C)为双链RNA类似物,与双链RNA病毒相似,可以诱导大黄鱼产生类似病毒引起的炎症反应[51]。脾脏和头肾均为大黄鱼重要的免疫器官,在先天性免疫和获得性免疫方面均发挥重要作用。大黄鱼脾脏和头肾中LycIL-10 mRNA的转录水平在受到三联灭活疫苗刺激后分别在12 h和24 h达到最高,在受到poly(I∶C)刺激后分别在48 h和24 h达到最高。大黄鱼在受到细菌或者poly(I∶C)刺激后在6 h、12 h一些炎症早期的细胞因子如IL-1β和IL-17的转录水平即达到最高[43,45]。而LycIL-10 mRNA转录水平则在受到免疫刺激后24 h、48 h达到最大,表明其可能在免疫刺激晚期发挥抑制炎症反应的作用,这与IL-10在人和哺乳动物里的研究结果一致[50]。在斑马鱼和虹鳟中,经LPS刺激后其IL-10 mRNA的转录水平在头肾中亦均上升,大西洋鳕鱼经过poly(I∶C)刺激后,其头肾中IL-10 mRNA转录水平显著升高,这些结果与我们的实验结果一致,说明鱼类IL-10可能在抑制细菌和病毒引起的炎症反应中发挥一定作用[35,37,40]。另外分析发现三联灭活细菌疫苗刺激后大黄鱼脾脏和头肾的LycIL-10 mRNA的转录水平上调倍数均高于poly(I∶C)刺激后LycIL-10 mRNA的上调倍数。说明LycIL-10可能在抗细菌引起的炎症反应方面发挥更重要的作用。
5 结论
本文首次报道了LycIL-10基因,分析了LycIL-10基因的序列特征,并通过多序列比对以及进化分析阐明了LycIL-10氨基酸序列的基本结构特点。本文研究了LycIL-10 mRNA在各个健康组织中的转录水平。首次阐明了大黄鱼受免疫刺激后LycIL-10 mRNA的表达特征,结果表明LycIL-10可能在在抗细菌引起的炎症反应中起着重要作用。本研究为进一步了解LycIL-10的特征及功能奠定了基础。
[1] Moore K W, de Waal Malefyt R, Coffman R L, et al. Interleukin-10 and the interleukin-10 receptor[J]. Annual Review of Immunology, 2001, 19(1): 683-765.
[2] Fiorentino D F, Bond M W, Mosmann T R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones[J]. The Journal of Experimental Medicine, 1989, 170(6): 2081-2095.
[3] Thomson A W, Lotze M T. The Cytokine Handbook, Two-volume Set[M]. 4th ed. London: Gulf Professional Publishing, 2003.
[4] Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10[J]. The Journal of Experimental Medicine, 1991, 174(6): 1549-1555.
[5] Ding L, Shevach E M. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function[J]. The Journal of Immunology, 1992, 148(10): 3133-3139.
[6] Fiorentino D F, Zlotnik A, Mosmann T R, et al. IL-10 inhibits cytokine production by activated macrophages[J]. The Journal of Immunology, 1991, 147(11): 3815-3822.
[7] Fiorentino D F, Zlotnik A, Vieira P, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells[J]. The Journal of Immunology, 1991, 146(10): 3444-3451.
[8] Aste-Amezaga M, Ma Xiaojing, Sartori A, et al. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10[J]. The Journal of Immunology, 1998, 160(12): 5936-5944.
[9] Gazzinelli R T, Oswald I P, James S L, et al. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages[J]. The Journal of Immunology, 1992, 148(6): 1792-1796.
[10] Mertz P M, DeWitt D L, Stetler-Stevenson W G, et al. Interleukin 10 suppression of monocyte prostaglandin H synthase-2. Mechanism of inhibition of prostaglandin-dependent matrix metalloproteinase production[J]. The Journal of Biological Chemistry, 1994, 269(33): 21322-21329.
[11] Niiro H, Otsuka T, Kuga S, et al. IL-10 inhibits prostaglandin E2production by lipopolysaccharide-stimulated monocytes[J]. International Immunology, 1994, 6(4): 661-664.
[12] de Waal Malefyt R, Yssel H, de Vries J E. Direct effects of IL-10 on subsets of human CD4+T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation[J]. The Journal of Immunology, 1993, 150(11): 4754-4765.
[13] Groux H, Bigler M, de Vries J E, et al. Inhibitory and stimulatory effects of IL-10 on human CD8+T cells[J]. The Journal of Immunology, 1998, 160(7): 3188-3193.
[14] Vieira P, de Waal-Malefyt R, Dang M N, et al. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI[J]. Proceedings of the National Academy of Sciences of the United States of America, 1991, 88(4): 1172-1176.
[15] Villinger F, Brar S S, Mayne A, et al. Comparative sequence analysis of cytokine genes from human and nonhuman primates[J]. The Journal of Immunology, 1995, 155(8): 3946-3954.
[16] Hash S M, Brown W C, Rice-Ficht A C. Characterization of a cDNA encoding bovine interleukin 10: kinetics of expression in bovine lymphocytes[J]. Gene, 1994, 139(2): 257-261.
[17] Syto R, Murgolo N J, Braswell E H, et al. Structural and biological stability of the human interleukin 10 homodimer[J]. Biochemistry, 1998, 37(48): 16943-16951.
[18] Walter M R, Nagabhushan T L. Crystal structure of interleukin 10 reveals an interferon gamma-like fold[J]. Biochemistry, 1995, 34(38): 12118-12125.
[19] Yoon S I, Logsdon N J, Sheikh F, et al. Conformational changes mediate interleukin-10 receptor 2 (IL-10R2) binding to IL-10 and assembly of the signaling complex[J]. The Journal of Biological Chemistry, 2006, 281(46): 35088-35096.
[20] Liu Y, Wei S H, Ho A S, et al. Expression cloning and characterization of a human IL-10 receptor[J]. The Journal of Immunology, 1994, 152(4): 1821-1829.
[21] Tan J C, Braun S, Rong Hong, et al. Characterization of recombinant extracellular domain of human interleukin-10 receptor[J]. The Journal of Biological Chemistry, 1995, 270(21): 12906-12911.
[22] Tan J C, Indelicato S R, Narula S K, et al. Characterization of interleukin-10 receptors on human and mouse cells[J]. The Journal of Biological Chemistry, 1993, 268(28): 21053-21059.
[23] Wehinger J, Gouilleux F, Groner B, et al. IL-10 induces DNA binding activity of three STAT proteins (Stat1, Stat3, and Stat5) and their distinct combinatorial assembly in the promoters of selected genes[J]. FEBS Letters, 1996, 394(3): 365-370.
[24] Auernhammer C J, Bousquet C, Melmed S. Autoregulation of pituitary corticotroph SOCS-3 expression: characterization of the murine SOCS-3 promoter[J]. Proceedings of the National Academy of Sciences of the United States of America, 1999, 96(12): 6964-6969.
[25] Berlato C, Cassatella M A, Kinjyo I, et al. Involvement of suppressor of cytokine signaling-3 as a mediator of the inhibitory effects of IL-10 on lipopolysaccharide-induced macrophage activation[J]. The Journal of Immunology, 2002, 168(12): 6404-6411.
[26] Qin Hongwei, Wilson C A, Roberts K L, et al. IL-10 inhibits lipopolysaccharide-induced CD40 gene expression through induction of suppressor of cytokine signaling-3[J]. The Journal of Immunology, 2006, 177(11): 7761-7771.
[27] Hsu D H, de Waal Malefyt R, Fiorentino D F, et al. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1[J]. Science, 1990, 250(4982): 830-832.
[28] Kotenko S V, Saccani S, Izotova L S, et al. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10)[J]. Proceedings of the National Academy of Sciences of the United States of America, 2000, 97(4): 1695-1700.
[29] Zou J, Clark M S, Secombes C J. Characterisation, expression and promoter analysis of an interleukin 10 homologue in the puffer fish,Fugurubripes[J]. Immunogenetics, 2003, 55(5): 325-335.
[30] Savan R, Igawa D, Sakai M. Cloning, characterization and expression analysis of interleukin-10 from the common carp,CyprinuscarpioL.[J]. European Journal of Biochemistry, 2003, 270(23): 4647-4654.
[31] Inoue Y, Kamota S, Ito K, et al. Molecular cloning and expression analysis of rainbow trout (Oncorhynchusmykiss) interleukin-10 cDNAs[J]. Fish & Shellfish Immunology, 2005, 18(4): 335-344.
[32] 肖凡书, 昌鸣先, 孙军, 等. 鲢(Hypophthalmichthysmolitrix)IL-10基因的克隆及表达分析[J]. 自然科学进展, 2006, 16(2): 183-189.
Xiao Fanshu, Chang Mingxian, Sun Jun, et al. Cloning and expression analysis of interleukin-10 from the silver carp,Hypophthalmichthysmolitrix[J]. Progress in Natural Science, 2006, 16(2): 183-189.
[33] Zhang Dianchang, Shao Yanqing, Huang Yanqin, et al. Cloning, characterization and expression analysis of interleukin-10 from the zebrafish (Daniorerion)[J]. BMB Reports, 2005, 38(5): 571-576.
[34] Pinto R D, Nascimento D S, Reis M I R, et al. Molecular characterization, 3D modelling and expression analysis of sea bass (DicentrarchuslabraxL.) interleukin-10[J]. Molecular Immunology, 2007, 44(8): 2056-2065.
[35] Seppola M, Larsen A N, Steiro K, et al. Characterisation and expression analysis of the interleukin genes, IL-1β, IL-8 and IL-10, in Atlantic cod (GadusmorhuaL.)[J]. Molecular Immunology, 2008, 45(4): 887-897.
[36] Grayfer L, Hodgkinson J W, Hitchen S J, et al. Characterization and functional analysis of goldfish (CarassiusauratusL.) interleukin-10[J]. Molecular Immunology, 2011, 48(4): 563-571.
[37] Harun N O, Costa M M, Secombes C J, et al. Sequencing of a second interleukin-10 gene in rainbow troutOncorhynchusmykissand comparative investigation of the expression and modulation of the paraloguesinvitroandinvivo[J]. Fish & Shellfish Immunology, 2011, 31(1): 107-117.
[38] Piazzon M C, Savelkoul H F J, Pietretti D, et al. Carp IL10 has anti-inflammatory activities on phagocytes, promotes proliferation of memory T cells, and regulates B cell differentiation and antibody secretion[J]. The Journal of Immunology, 2015, 194(1): 187-199.
[39] van Beurden S J, Forlenza M, Westphal A H, et al. The alloherpesviral counterparts of interleukin 10 in European eel and common carp[J]. Fish & Shellfish Immunology, 2011, 31(6): 1211-1217.
[40] Sunarto A, Liongue C, McColl K A, et al. Koi herpesvirus encodes and expresses a functional interleukin-10[J]. Journal of Virology, 2012, 86(21): 11512-11520.
[41] Piazzon M C, Wentzel A S, Tijhaar E J, et al. Cyprinid herpesvirus 3 Il10 inhibits inflammatory activities of carp macrophages and promotes proliferation of IgM+B cells and memory T cells in a manner similar to carp Il10[J]. The Journal of Immunology, 2015, 195(8): 3694-3704.
[42] Chen Ruanni, Su Yongquan, Wang Jun, et al. Molecular characterization and expression analysis of interferon-gamma in the large yellow croakerLarimichthyscrocea[J]. Fish & Shellfish Immunology, 2015, 46(2): 596-602.
[43] Wu Jun, Shi Yuhong, Zhang Xueheng, et al. Molecular characterization of anIL-1βgene from the large yellow croaker (Larimichthyscrocea) and its effect on fish defense againstVibrioalginolyticusinfection[J]. Zoological Research, 2015, 36(3): 133-141.
[44] Mu Yinnan, Wang Kunru, Ao Jingqun, et al. Molecular characterization and biological effects of a CXCL8 homologue in large yellow croaker (Larimichthyscrocea)[J]. Fish & Shellfish Immunology, 2015, 44(2): 462-470.
[45] Ding Yang, Ao Jingqun, Ai Chunxiang, et al. Molecular and functional identification of three interleukin-17A/F (IL-17A/F) homologues in large yellow croaker (Larimichthyscrocea)[J]. Developmental & Comparative Immunology, 2016, 55: 221-232.
[46] Zhu Qian, Li Chan, Yu Zhenxing, et al. Molecular and immune response characterizations of IL-6 in large yellow croaker (Larimichthyscrocea)[J]. Fish & Shellfish Immunology, 2016, 50: 263-273.
[47] Ao Jingqun, Mu Yinnan, Xiang Lixin, et al. Genome sequencing of the perciform fishLarimichthyscroceaprovides insights into molecular and genetic mechanisms of stress adaptation[J]. PLoS Genetics, 2015, 11(4): e1005118.
[48] Zheng Wenbiao, Tian Chen, Chen Xinhua. Molecular characterization of goose-type lysozyme homologue of large yellow croaker and its involvement in immune response induced by trivalent bacterial vaccine as an acute-phase protein[J]. Immunology Letters, 2007, 113(2): 107-116.
[49] Salinas I, Zhang Yong’an, Sunyer J O. Mucosal immunoglobulins and B cells of teleost fish[J]. Developmental & Comparative Immunology, 2011, 35(12): 1346-1365.
[50] Ouyang Wenjun, Rutz S, Crellin N K, et al. Regulation and functions of the IL-10 family of cytokines in inflammation and disease[J]. Annual Review of Immunology, 2011, 29(1): 71-109.
[51] Mu Yinnan, Wan Xiang, Lin Kebing, et al. Liver proteomic analysis of the large yellow croaker (Pseudosciaenacrocea) following polyriboinosinic: polyribocytidylic acid induction[J]. Fish Physiology and Biochemistry, 2013, 39(5): 1267-1276.
Cloning and expression analysis of large yellow croaker (Larimichthysrocea) IL-10 gene
Zheng Weibing1, Mu Pengfei2, Ding Lianshuai1, Yang Sisi1, Ao Jingqun1,Ai Chunxiang2, Chen Xinhua1
(1.KeyLaboratoryofMarineBiogeneticResources,ThirdInstituteofOceanography,StateOceanicAdministration,Xiamen361005,China; 2.CollegeofOceanandEarthSciences,XiamenUniversity,Xiamen361005,China)
Interleukin-10 (IL-10) is a central anti-inflammatory cytokine that demonstrates immunosuppressive function. In this research, an interleukin-10 (IL-10) homologue has been identified by analyzed the genomic data of large yellow croaker (Larimichthyscrocea). Subsequently, the open reading frame (ORF) of cDNA and the genomic DNA sequences have been cloned and analyzed. The genomic DNA of large yellow croaker IL-10(LycIL-10) consisted of 1 869 bp that contains five exons and four introns, sharing the same organization with mammalian IL-10 genes. The open reading frame of LycIL-10 consisted 555 bp that give a predicted 184 amino acid IL-10 molecule. It contains a predicted signal peptide of 22 amino acids in the N-terminal and a mature peptide of 162 amino acids, which contained the vertebrate IL-10 family signature. The LycIL-10 exhibits a conserved IL-10 motif signature and shares 26.49%-77.01% amino acid sequence identity with other known IL-10. Real-time PCR analysis showed that LycIL-10 was constitutively expressed in all tissues tested, especially in spleen and muscle. The LycIL-10 mRNA transcription level could significantly increase in the spleen and head kidney of large yellow croaker after trivalent inactivated vaccines or poly(I∶C) stimulated, indicating that LycIL-10 may be involved in suppressing the inflammatory induced by bacterium or virus in the large yellow croaker.
large yellow croaker; interleukin-10; molecule cloning; real-time PCR; expression analysis
10.3969/j.issn.0253-4193.2017.04.005
2016-07-01;
2016-07-31。
国家自然科学基金资助项目(31001131 ,31372556);厦门南方海洋研究中心资助项目(13GZP002NF08,14CZP049SF02)。
郑维兵(1976—),男,福建省宁德市人,高级技术员,研究方向为水产养殖。E-mail:wbzhen@tio.ory.cn
*通信作者:陈新华(1968—),男,湖北省武汉市人,研究员,研究方向为鱼类分子免疫学。E-mail:chenxinhua@tio.org.cn
S917.4
A
0253-4193(2017)04-0050-11
郑维兵,慕鹏飞,丁连帅,等. 大黄鱼(Larimichthyscrocea)IL-10基因的克隆与表达分析[J].海洋学报,2017,39(4):50—60,
Zheng Weibing, Mu Pengfei, Ding Lianshuai, et al. Cloning and expression analysis of large yellow croaker (Larimichthysrocea) IL-10 gene[J]. Haiyang Xuebao,2017,39(4):50—60, doi:10.3969/j.issn.0253-4193.2017.04.005

