原花青素对多囊卵巢综合征合并动脉粥样硬化模型大鼠的作用
金铉顺,李梦京,金 石,张国杰,元奎昌
·论著·
原花青素对多囊卵巢综合征合并动脉粥样硬化模型大鼠的作用
金铉顺,李梦京,金 石,张国杰,元奎昌*
目的 探究原花青素对多囊卵巢综合征(PCOS)合并动脉粥样硬化模型大鼠的作用及其机制。方法 2015年1—10月,采用随机数字表法从20只健康雌性清洁级SD大鼠中选取15只为建模组,在其颈背部皮下埋置双氢睾酮(DHT)缓释片,并且定期更换DHT缓释片的同时,给予高脂饲料配方,给予充分的颗粒饲料和水,建立PCOS合并动脉粥样硬化模型大鼠;其余5只大鼠为对照组,常规饲养。16周后,分别检测两组大鼠体质量、雄激素、瘦素、胰岛素水平,光学显微镜下观察其主动脉内膜病理情况,以验证PCOS合并动脉粥样硬化模型大鼠成功与否。将建模成功的15只大鼠采用随机数字表法分为模型组、辛伐他汀组、原花青素组,各5只。对照组与模型组大鼠给予0.9%氯化钠溶液,辛伐他汀组大鼠给予辛伐他汀片,原花青素组大鼠给予原花青素。治疗4周后,检测4组大鼠脂代谢指标〔总胆固醇(TC)、三酰甘油(TG)、低密度脂蛋白(LDL)、高密度脂蛋白(HDL)〕水平、脂代谢关键基因〔过氧化物酶体增殖物激活受体γ(PPAR-γ)、3-羟基-3-甲基戊二酰辅酶A还原酶(HMG-CoA还原酶)、ATP结合盒转运子A1(ABCA1)、肝脏X受体α(LXR-α)、法尼醇受体(FXR)〕mRNA表达水平。结果 建模组体质量、雄激素、瘦素、胰岛素水平均低于对照组(P<0.05)。光学显微镜下观察大鼠主动脉内膜病理情况,结果显示,与对照组相比,建模组大鼠主动脉内膜出现大量粥样斑块及胆固醇结晶。模型组TC、TG、LDL水平高于对照组(P<0.05);辛伐他汀组TC、TG、LDL水平低于模型组,HDL水平高于对照组、模型组(P<0.05);原花青素组TC、TG、LDL水平低于模型组,TC、LDL水平低于辛伐他汀组,HDL水平高于对照组、模型组(P<0.05)。模型组PPAR-γ、HMG-CoA还原酶、FXR mRNA表达水平低于对照组(P<0.05);辛伐他汀组PPAR-γ、HMG-CoA还原酶、ABCA1、LXR-α、FXR mRNA表达水平高于模型组,HMG-CoA还原酶、ABCA1、LXR-α、FXR mRNA表达水平高于对照组(P<0.05);原花青素组PPAR-γ、HMG-CoA还原酶、ABCA1、LXR-α、FXR mRNA表达水平高于对照组、模型组,HMG-CoA还原酶mRNA表达水平高于辛伐他汀组(P<0.05)。4组大鼠主动脉内膜病理情况:与对照组相比,模型组大鼠主动脉内膜出现大量粥样斑块及胆固醇结晶;与模型组相比,辛伐他汀组大鼠主动脉内膜粥样斑块缩小,同时胆固醇结晶也减少;与辛伐他汀组相比,原花青素组大鼠主动脉内膜粥样斑块缩小更多,同时胆固醇结晶也减少更多。结论 原花青素可改善PCOS合并动脉粥样硬化模型大鼠脂代谢水平并缩减动脉粥样斑块。
多囊卵巢综合征;动脉粥样硬化;原花青素类;大鼠;辛伐他汀
金铉顺,李梦京,金石,等.原花青素对多囊卵巢综合征合并动脉粥样硬化模型大鼠的作用[J].中国全科医学,2017,20(12):1463-1468.[www.chinagp.net]
JIN X S,LI M J,JIN S,et al.Effect of procyanidine on rat models of polycystic ovary syndrome complicated with atherosclerosis[J].Chinese General Practice,2017,20(12):1463-1468.
多囊卵巢综合征(polycystic ovary syndrome,PCOS)是女性常见的一类内分泌疾病[1],以高雄激素血症及长期不排卵为主要特征,同时可伴有痤疮、月经量少、肥胖、代谢紊乱及心血管系统功能紊乱等临床表现[2-3]。研究统计,PCOS影响着全世界约7%女性的健康[3]。内分泌紊乱是引起机体代谢失调的前提。多项研究表明,大多数PCOS患者存在代谢综合征,其表现如高血压、脂代谢异常、肥胖、胰岛素抵抗[4]。同时,PCOS患者心血管疾病患病率明显增加,成为影响患者预后的主要因素[4-6]。但PCOS与代谢紊乱是否与心血管疾病相关,尚未见明确报道。研究显示,80%以上的PCOS患者患有心血管疾病,特别是动脉粥样硬化[7]。原花青素是存在于多种植物,如葡萄、蓝莓、紫薯、樱桃等中的一类含多酚羟基的黄酮类化合物[8-9]。研究显示,合理摄入原花青素可起到抗氧化、抑制肿瘤及抗感染等多种作用[10-11]。在动脉粥样硬化的发生与发展过程中,体内氧化应激增强所致脂质过氧化、泡沫细胞的激活以及机体脂质代谢异常,是引起动脉粥样斑块形成的重要因素[12-15]。ZHANG等[16]通过对比动脉粥样硬化患者服用原花青素前后动脉粥样斑块大小、动脉内膜厚度等指标发现,原花青素可明显减小动脉粥样斑块,提示原花青素具有抗动脉粥样硬化的疗效。因此,结合原花青素抗氧化的特性以及脂质代谢异常在动脉粥样硬化中扮演的重要角色,本研究观察原花青素对PCOS合并动脉粥样硬化模型大鼠的作用及其机制,以期为探究PCOS病因及其多项并发症的病因提供理论指导。
1 材料与方法
1.1 实验动物 2015年1—10月,选取健康雌性清洁级SD大鼠20只,体质量(170±20)g,4周龄,由延边大学实验动物中心提供(合格证号为医动字第220010016号)。饲养环境:相对湿度50%~70%,温度20~25 ℃,饲料由延边大学实验动物中心提供(执行标准GB149241994),动物实验房的合格号为医动字第220010015号。
1.2 药品与仪器 原花青素购于默沙东(中国)有限公司;辛伐他汀片购于杭州康泰药业有限公司;雄激素、瘦素、胰岛素、总胆固醇(TC)、三酰甘油(TG)、低密度脂蛋白(LDL)、高密度脂蛋白(HDL)检测试剂盒购于上海碧云天生物技术有限公司;DM2500型光学显微镜、DFA450摄像头购于上海日立电器有限公司。
1.3 研究方法
1.3.1 PCOS合并动脉粥样硬化模型大鼠的建立 采用随机数字表法选取15只大鼠为建模组,在其颈背部皮下埋置双氢睾酮(DHT)缓释片(5 mg/90 d,美国Innovative Research公司,美国密西西比州Reckelhoff JF生理实验室赠予),并且在定期更换DHT缓释片的同时,给予高脂饲料配方(4%胆固醇+6%花生油+90%基础饲料,由沈阳市于洪区前民动物实验饲料厂加工成颗粒饲料),给予充分的颗粒饲料和水。其余5只大鼠为对照组,常规饲养。16周后,分别检测两组大鼠体质量,按照检测试剂盒说明书检测雄激素、瘦素、胰岛素水平,DM2500型光学显微镜下观察其主动脉内膜病理情况,以验证PCOS合并动脉粥样硬化模型大鼠成功与否。
1.3.2 治疗方法 将建模成功的15只大鼠采用随机数字表法分为模型组、辛伐他汀组、原花青素组,各5只。对照组与模型组大鼠灌胃给予10 ml/kg 0.9%氯化钠溶液,1次/d;辛伐他汀组大鼠灌胃给予4 mg/kg辛伐他汀片,1次/d;原花青素组大鼠灌胃给予50 mg/kg原花青素,1次/d。
1.3.3 检测脂代谢指标(TC、TG、LDL、HDL)水平 治疗4周后,抽取4组大鼠尾静脉血,分离血浆,采用试剂盒检测血浆TC、TG、LDL、HDL水平。
1.3.4 检测脂代谢关键基因〔过氧化物酶体增殖物激活受体γ(PPAR-γ)、3-羟基-3-甲基戊二酰辅酶A还原酶(HMG-CoA还原酶)、ATP结合盒转运子A1(ABCA1)、肝脏X受体α(LXR-α)、法尼醇受体(FXR)〕mRNA表达水平 治疗4周后,断颈处死各组大鼠,收集颈动脉血后分离血浆,采用实时荧光定量PCR法检测脂代谢关键基因(PPAR-γ、HMG-CoA还原酶、ABCA1、LXR-α、FXR)mRNA表达水平。PCR反应条件:95 ℃预变性10 min;95 ℃变性15 s,60 ℃退火30 s,72 ℃延伸30 s,共40个循环;72 ℃末次延伸5 min。以GAPDH为内参,采用2-ΔΔCT法计算目的基因mRNA表达水平。
1.3.5 观察大鼠主动脉内膜病理情况 采用DM2500型光学显微镜、DFA450摄像头观察大鼠主动脉内膜病理情况。

2 结果
2.1 PCOS合并动脉粥样硬化大鼠模型的鉴定 建模组体质量、雄激素、瘦素、胰岛素水平均低于对照组,差异有统计学意义(P<0.05,见表1)。光学显微镜下观察大鼠主动脉内膜病理情况,结果显示,与对照组相比,建模组大鼠主动脉内膜出现大量粥样斑块及胆固醇结晶(见图1)。

Table 1 Comparison of general data between control group and modeling group

组别只数体质量(g)雄激素(ng/ml)瘦素(ng/ml)胰岛素(mU/L)对照组5196±173.7±0.43.98±0.0129.4±0.9建模组15176±211.8±0.62.14±0.4618.2±0.3t值1.9196.55015.48127.325P值0.071<0.001<0.001<0.001

图1 光学显微镜下观察对照组、建模组主动脉内膜病理情况(HE染色,×400)
Figure 1 Pathological conditions of the aortic tunica intima in control group and modeling group observed by optical microscopy
2.2 对照组、模型组、辛伐他汀组、原花青素组脂代谢指标水平比较 对照组、模型组、辛伐他汀组、原花青素组TC、TG、LDL、HDL水平比较,差异有统计学意义(P<0.05)。其中模型组TC、TG、LDL水平高于对照组,差异有统计学意义(P<0.05);辛伐他汀组TC、TG、LDL水平低于模型组,HDL水平高于对照组、模型组,差异有统计学意义(P<0.05);原花青素组TC、TG、LDL水平低于模型组,TC、LDL水平低于辛伐他汀组,HDL水平高于对照组、模型组,差异有统计学意义(P<0.05,见表2)。
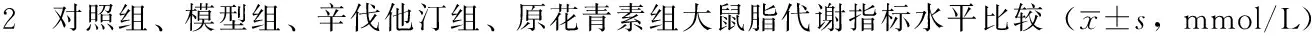
Table 2 Comparison of levels of indicators of lipid metabolism in control group,model subgroup,simvastatin subgroup and procyanidine subgroup

组别只数TCTGLDLHDL对照组51.52±0.341.24±0.081.16±0.131.23±0.18模型组53.44±0.14a2.98±0.07a2.96±0.32a1.01±0.25辛伐他汀组51.81±0.33b1.62±0.72b1.31±0.32b1.98±0.43ab原花青素组51.13±0.42bc1.02±0.62b0.92±0.23bc2.23±0.51abF值48.52516.68263.48912.824P值<0.001<0.001<0.001<0.001
注:TC=总胆固醇,TG=三酰甘油, LDL=低密度脂蛋白,HDL=高密度脂蛋白;与对照组比较,aP<0.05;与模型组比较,bP<0.05;与辛伐他汀组比较,cP<0.05
2.3 对照组、模型组、辛伐他汀组、原花青素组脂代谢关键基因mRNA表达水平比较 对照组、模型组、辛伐他汀组、原花青素组PPAR-γ、HMG-CoA还原酶、ABCA1、LXR-α、FXR mRNA表达水平比较,差异有统计学意义(P<0.05)。其中模型组PPAR-γ、HMG-CoA还原酶、FXR mRNA表达水平低于对照组,差异有统计学意义(P<0.05);辛伐他汀组PPAR-γ、HMG-CoA还原酶、ABCA1、LXR-α、FXR mRNA表达水平高于模型组,HMG-CoA还原酶、ABCA1、LXR-α、FXR mRNA表达水平高于对照组,差异有统计学意义(P<0.05);原花青素组PPAR-γ、HMG-CoA还原酶、ABCA1、LXR-α、FXR mRNA表达水平高于对照组、模型组,HMG-CoA还原酶mRNA表达水平高于辛伐他汀组,差异有统计学意义(P<0.05,见表3)。
2.4 对照组、模型组、辛伐他汀组、原花青素组大鼠主动脉内膜病理情况 与对照组相比,模型组大鼠主动脉内膜出现大量粥样斑块及胆固醇结晶;与模型组相比,辛伐他汀组大鼠主动脉内膜粥样斑块缩小,同时胆固醇结晶也减少;与辛伐他汀组相比,原花青素组大鼠主动脉内膜粥样斑块缩小更多,同时胆固醇结晶也减少更多(见图2)。

Table 3 Comparison of mRNA expressions of lipid metabolism-associated genes in control group,model subgroup,simvastatin subgroup and procyanidine subgroup

组别只数PPAR-γmRNAHMG-CoA还原酶mRNAABCA1mRNALXR-αmRNAFXRmRNA对照组512.3±3.38.5±1.34.5±1.29.1±2.28.2±1.4模型组55.0±0.8a3.6±0.8a2.5±0.55.5±1.14.2±0.8a辛伐他汀组516.3±4.3b18.5±3.3ab13.9±3.2ab18.3±3.7ab15.3±3.6ab原花青素组518.5±5.3ab27.5±6.1abc17.6±4.4ab22.2±4.9ab17.4±4.2abF值12.04244.75033.61627.68523.098P值<0.001<0.001<0.001<0.001<0.001
注:PPAR-γ=过氧化物酶体增殖物激活受体γ,HMG-CoA还原酶=3-羟基-3-甲基戊二酰辅酶A还原酶,ABCA1=ATP结合盒转运子A1,LXR-α=肝脏X受体α,FXR=法尼醇受体;与对照组比较,aP<0.05;与模型组比较,bP<0.05;与辛伐他汀组比较,cP<0.05

图2 光学显微镜下观察对照组、模型组、辛伐他汀组、原花青素组主动脉内膜病理情况(HE染色,×400)
Figure 2 Pathological conditions of the aortic tunica intima in control group,model subgroup,simvastatin subgroup and procyanidine subgroup observed by optical microscopy
3 讨论
PCOS是起源于青春期的生殖内分泌紊乱疾病,也是育龄期妇女最常见的内分泌疾病,患病率达5%~10%,在无排卵性不育的妇女中为50%~60%[17]。PCOS临床表现多样,以高雄激素血症和长期无排卵为主要特征,多表现为肥胖、不孕和卵巢囊性增大,且PCOS易引发多种并发症,尤其是心血管疾病,80%以上的PCOS患者患有心血管疾病,特别是动脉粥样硬化[18]。PCOS患者伴有动脉粥样硬化的可能机制如下[19]:(1)游离脂肪酸的抑制作用减弱,导致LDL和TG合成增加,同时脂蛋白酯酶和肝脂酶的活性降低,肝脏对LDL和TG的清除率降低。(2)脂质沉积代谢异常,导致脂蛋白酯酶活性受到抑制,从而增加了TG水平,降低了HDL水平,PCOS伴肥胖使血中雄激素、瘦素、胰岛素抵抗(IR)水平增加,在其协同作用下进一步加重代谢紊乱。
原花青素是存在于多种植物内的一类含多酚羟基的黄酮类化合物。研究显示,原花青素具有抗动脉粥样硬化的作用[20]。因此,结合原花青素抗氧化的特性以及脂质代谢异常在动脉粥样硬化中扮演的重要角色,本研究观察原花青素对PCOS合并动脉粥样硬化模型大鼠的作用及其机制,结果显示,建模组体质量、雄激素、瘦素、胰岛素水平均低于对照组;光学显微镜下观察大鼠主动脉内膜病理情况,结果显示,与对照组相比,建模组大鼠主动脉内膜出现大量粥样斑块及胆固醇结晶。说明PCOS合并动脉粥样硬化模型大鼠建模成功。TC、TG、LDL和HDL是评价动脉粥样硬化的常见指标,TC、TG、LDL水平越高,HDL水平越低,表明动脉粥样硬化程度越严重[18]。模型组TC、TG、LDL水平高于对照组;辛伐他汀组TC、TG、LDL水平低于模型组,HDL水平高于对照组、模型组;原花青素组TC、TG、LDL水平低于模型组,TC、LDL水平低于辛伐他汀组,HDL水平高于对照组、模型组。PPAR-γ、HMG-CoA还原酶、ABCA1、LXR-α、FXR为大鼠脂代谢关键基因,其mRNA能够启动脂质代谢有关酶的基因转录,从而促进脂肪氧化分解;其mRNA表达水平越高,越有利于脂肪氧化分解,改善动脉粥样硬化症状[21]。本研究结果显示,模型组PPAR-γ、HMG-CoA还原酶、FXR mRNA表达水平低于对照组;辛伐他汀组PPAR-γ、HMG-CoA还原酶、ABCA1、LXR-α、FXR mRNA表达水平高于模型组,HMG-CoA还原酶、ABCA1、LXR-α、FXR mRNA表达水平高于对照组;原花青素组PPAR-γ、HMG-CoA还原酶、ABCA1、LXR-α、FXR mRNA表达水平高于对照组、模型组,HMG-CoA还原酶mRNA表达水平高于辛伐他汀组。上述结果证明,原花青素治疗PCOS合并动脉粥样硬化效果显著,甚至强于临床常用药辛伐他汀。
由于本研究样本量小,临床代表性差,进一步的治疗效果还需要临床试验研究;且PCOS合并动脉粥样硬化的发病机制需进一步明确,因而本研究未能确定原花青素的具体治疗机制,有待进一步的研究探讨。
综上所述,原花青素可改善PCOS合并动脉粥样硬化模型大鼠脂代谢水平,并缩减动脉粥样斑块。
作者贡献:金铉顺、李梦京、金石、张国杰、元奎昌进行文章的构思与设计、研究的实施与可行性分析、数据收集、数据整理、统计学处理、结果的分析与解释、撰写论文、论文的修订、英文的修订,金铉顺负责文章的质量控制及审校、对文章整体负责、监督管理。
本文无利益冲突。
[1]GURSOY CALAN O,CALAN M,YESIL SENSES P,et al.Increased adipsin is associated with carotid intima media thickness and metabolic disturbances in polycystic ovary syndrome[J].Clin Endocrinol(Oxf),2016,85(6):910-917.
[2]QUINN M M,KAO C N,AHMAD A,et al.Raising threshold for diagnosis of polycystic ovary syndrome excludes population of patients with metabolic risk[J].Fertil Steril,2016,106(5):1244-1251.
[3]MISHRA S,DAS A K,DAS S.Hypovitaminosis D and associated cardiometabolic risk in women with PCOS[J].J Clin Diagn Res,2016,10(5):BC01-4.
[4]ABBOTT D H,LEVINE J E,DUMESIC D A.Translational insight into polycystic ovary syndrome(PCOS) from female monkeys with PCOS-like traits[J].Curr Pharm Des,2016,22(36):5625-5633.
[5]SAYDAM B O,YILDIZ B O.Gut-brain axis and metabolism in polycystic ovary syndrome[J].Curr Pharm Des,2016,22(36):5572-5587.
[6]DING N,CHANG J,JIAN Q,et al.Luteal phase clomiphene citrate for ovulation induction in women with polycystic ovary syndrome:a systematic review and meta-analysis[J].Gynecol Endocrinol,2016,32(11):866-871.
[7]FRØSSING S,NYLANDER M,AZIZ M,et al.Atrial natriuretic peptide,copeptin and adrenomedullin levels in polycystic ovary syndrome:a case-control study[J].Gynecol Endocrinol,2016:1-4.DOI:10.1080/09513590.2016.1202915.
[8]MAO J T,XUE B,SMOAKE J,et al.MicroRNA-19a/b mediates grape seed procyanidin extract-induced anti-neoplastic effects against lung cancer[J].J Nutr Biochem,2016,34:118-125.DOI:10.1016/j.jnutbio.2016.05.003.
[9]SUN P,WANG T,CHEN L,et al.Trimer procyanidin oligomers contribute to the protective effects of cinnamon extracts on pancreatic β-cells in vitro[J].Acta Pharmacol Sin,2016,37(8):1083-1090.
[10]KIM H W,JEONG J Y,SEOL K H,et al.Effects of edible films containing procyanidin on the preservation of pork meat during chilled storage[J].Korean J Food Sci Anim Resour,2016,36(2):230-236.
[11]CHEN L,CHEN L,WANG T,et al.Preparation of methylated products of a-type procyanidin trimers in cinnamon bark and their protective effects on pancreatic β-cell[J].J Food Sci,2016,81(5):C1062-1069.
[12]LIU Y,LIAO W J,ZHU Z,et al.Effect of procyanidine on VEGFR-2 expression and transduction pathway in rat endothelial progenitor cells under high glucose conditions[J].Genet Mol Res,2016,15(1):gmr.15016925.DOI:10.4238/gmr.15016925.
[13]KILARI E K,PUTTA S.Biological and phytopharmacological descriptions of litchi chinensis[J].Pharmacogn Rev,2016,10(19):60-65.
[14]DECEAN H,FISCHER-FODOR E,TATOMIR C,et al.Vitis vinifera seeds extract for the modulation of cytosolic factors BAX-α and NF-κB involved in UVB-induced oxidative stress and apoptosis of human skin cells[J].Clujul Med,2016,89(1):72-81.
[15]KYRALEOU M,KOTSERIDIS Y,KOUNDOURAS S,et al.Effect of irrigation regime on perceived astringency and proanthocyanidin composition of skins and seeds of Vitis vinifera L.cv.Syrah grapes under semiarid conditions[J].Food Chem,2016,203:292-300.DOI:10.1016/j.foodchem.2016.02.052.
[16]ZHANG J Q,GAO B W,WANG J,et al.Critical role of FoxO1 in granulosa cell apoptosis caused by oxidative stress and protective effects of grape seed procyanidin B2[J].Oxid Med Cell Longev,2016,2016:6147345.DOI:10.1155/2016/6147345.
[17]崔馨月,侯丽辉,郝松莉,等.多囊卵巢综合征脂代谢、氧化应激与动脉粥样硬化的关系[J].医学研究杂志,2014,43(5):12-14,25. CUI X Y,HOU L H,HAO S L,et al.Relationship between lipid metabolism,oxidative stress and atherosclerosis in polycystic ovary syndrome[J].Journal of Medical Research,2014,43(5):12-14,25.
[18]梁小红.56例多囊卵巢综合症血脂水平测定及意义研究[J].大家健康,2016,10(20):73. LINAG X H.Measurement and significance of serum lipid levels in 56 patients with polycystic ovary syndrome[J].For All Health,2016,10(20):73.
[19]代晶芳,李晓林,孙宝治.多囊卵巢综合征易感基因多态性的研究进展[J].山东医药,2013,53(34):98-101.DOI:10.3969/j.issn.1002-266X.2013.34.043. DAI J F,LI X L,SUN B Z.Research progress of susceptibility gene polymorphism in polycystic ovary syndrome[J].Shandong Medical Journal,2013,53(34):98-101.DOI:10.3969/j.issn.1002-266X.2013.34.043.
[20]洪岭,童晓文.多囊卵巢综合征与炎症因子[J].中华妇产科杂志,2007,42(10):713-715.DOI:10.3760/j.issn:0529-567x.2007.10.019. HONG L,TONG X W.Polycystic ovary syndrome and inflammatory factors[J].Chinese Journal of Obstetrics and Gynecology,2007,42(10):713-715.
[21]陈爱群,李建梅,张丽燕,等.对46例 PCOS患者内分泌激素及脂代谢的分析[J].中国妇幼健康研究,2014,25(6):1088-1090.DOI:10.3969/j.issn.1673-5293.2014.06.063. CHEN A Q,LI J M,ZHANG L Y,et al.Analysis of endocrine hormone and lipid metabolism of 46 cases of polycystic ovary syndrome[J].Chinese Journal of Woman and Child Health Research,2014,25(6):1088-1090.DOI:10.3969/j.issn.1673-5293.2014.06.063.
(本文编辑:崔丽红)
Effect of Procyanidine on Rat Models of Polycystic Ovary Syndrome Complicated with Atherosclerosis
JINXuan-shun,LIMeng-jing,JINShi,ZHANGGuo-jie,YUANKui-chang*
DepartmentofCardiology,YanbianUniversityHospital,Yanji133000,China
*Correspondingauthor:YUANKui-chang,Associatechiefphysician;E-mail:7250599@qq.com
Objective To explore the effect and mechanism of procyanidine on rat models of polycystic ovary syndrome(PCOS) complicated with atherosclerosis.Methods This study was conducted between January and October 2015.For modeling the rats of PCOS complicated with atherosclerosis,we randomly assigned 20 female healthy and clean SD rats to control group(n=5,conventional feeding) and modeling group〔n=15,embedding and regular replacement of dihydrotestosterone(DHT) sustained-release tablets in the subcutaneous tissue of the neck and back and giving enough high-fat food,pellet and water〕.Body weight,androgen,leptin and insulin levels were measured in both groups after 16 weeks of intervention.Aortic tunica intima specimen was taken from both groups and detected by optical microscopy,and the results suggested that the rat models of PCOS complicated with arteriosclerosis were successfully built only in modeling group.Then we randomly divided modeling group into model subgroup,simvastatin subgroup and procyanidine subgroup with 5 rats in each.Both control group and model subgroup were fed with 10 ml/kg 0.9% sodium chloride solution,simvastatin subgroup and procyanidine subgroup were fed with 4 mg/kg simvastatin tablets,50 mg/kg procyanidine,respectively,once a day,for 4 weeks.At the end of this round of intervention,the rat tail vein blood was taken for measuring the levels of lipid metabolism indicators 〔total cholesterol(TC),triacylglycerol(TG),low-density lipoprotein(LDL) and high-density lipoprotein(HDL)〕.Then,all the rats were sacrificed,the carotid artery blood was collected to detect the mRNA expressions of lipid metabolism-associated key genes〔peroxisome proliferator-activated receptor gamma(PPAR-γ),3-hydroxy-3-methyl-glutaryl-CoA(HMG-CoA) reductase,ATP-binding cassette transporter A1(ABCA1),liver X receptor alpha(LXR-α) and farnesoid X receptor(FXR)〕.Results Modeling group had lower body weight,androgen,leptin and insulin levels than control group(P<0.05).There were a lot of atherosclerotic plaques and cholesterol crystals in the aortic tunica intima of modeling group but not in control group found by optical microscopy.TC,TG and LDL levels were higher in model subgroup than in control group(P<0.05);simvastatin subgroup had lower TC,TG and LDL levels but higher HDL level than model subgroup(P<0.05);higher HDL level was noted in simvastatin subgroup than in control group(P<0.05);procyanidine subgroup had lower TC,TG,LDL levels but higher HDL level than model subgroup,lower TC and LDL levels than simvastatin subgroup,and higher HDL level than control group(P<0.05).Compared with control group,model subgroup had lower PPAR-γ,HMG-CoA reductase and FXR mRNA expressions(P<0.05);the mRNA expressions of all the 5 genes were higher in simvastatin subgroup than in model subgroup(P<0.05);HMG-CoA reductase,ABCA1,LXR-α and FXR mRNA expressions were higher in simvastatin subgroup than in control group(P<0.05);procyanidine subgroup had higher PPAR-γ,HMG-CoA reductase,ABCA1,LXR-α,FXR mRNA expressions than both control group and model subgroup,and it had higher HMG-CoA reductase mRNA expression than simvastatin subgroup(P<0.05).Optical microscopy displayed that,much more atherosclerotic plaques and cholesterol crystals appeared in the aortic tunica intima in model subgroup than in control group;atherosclerotic plaques and cholesterol crystals were less in simvastatin subgroup than in model subgroup;procyanidine group showed even less atherosclerotic plaques and cholesterol crystals than simvastatin subgroup.Conclusion Procyanidine could be used to improve the lipid metabolism and reduce the atherosclerotic plaques in rats with PCOS complicated with atherosclerosis.
Polycystic ovary syndrome;Atherosclerosis;Proanthocyanidins;Rats;Simvastatin
国家自然科学基金资助项目(81460229);吉林省教育厅“十二五”科学技术研究项目(吉教科合字[2012]第18号);延边大学科技发展计划项目〔延大科合字(2011)第13号〕
R 711.75 R 543.5
A
10.3969/j.issn.1007-9572.2017.12.011
2016-11-23;
2017-02-21)
133000 吉林省延吉市,延边大学附属医院心内科
*通信作者:元奎昌,副主任医师;E-mail:7250599@qq.com

