Neuroprotective ef f ect of the Chinese medicine Tiantai No. 1 and its molecular mechanism in the senescence-accelerated mouse prone 8
Ying-hong Li, Xu-sheng Wang, Xiao-lin Chen, Yu Jin Hong-bo Chen, Xiu-qin Jia Yong-feng Zhang Zheng-zhi Wu
1 First Af filiated Hospital of Shenzhen University (Shenzhen Second People’s Hospital), Shenzhen, Guangdong Province, China
2 School of Life Sciences, Tsinghua University, Beijing, China
3 Guangzhou Medical University, Guangzhou, Guangdong Province, China
4 Shenzhen Key Laboratory of Health Sciences and Technology, Graduate School at Shenzhen, Tsinghua University, Beijing, China
Nathanael J. Yates1, Marcus K. Giacci1, Ryan L. O’Hare Doig1,2, Wissam Chiha1,2, Bethany E. Ashworth1, Jade Kenna1, Carole A. Bartlett1, Melinda Fitzgerald1,*
1 Department of Experimental and Regenerative Neurosciences, School of Animal Biology, The University of Western Australia, Crawley, Western Australia, Australia
2 Department of Experimental and Regenerative Neurosciences, School of Anatomy, Physiology and Human Biology, The University of Western Australia, Crawley, Western Australia, Australia
Neuroprotective ef f ect of the Chinese medicine Tiantai No. 1 and its molecular mechanism in the senescence-accelerated mouse prone 8
Ying-hong Li1,*,#, Xu-sheng Wang2,#, Xiao-lin Chen3, Yu Jin1, Hong-bo Chen4, Xiu-qin Jia1, Yong-feng Zhang1, Zheng-zhi Wu1,*
1 First Af filiated Hospital of Shenzhen University (Shenzhen Second People’s Hospital), Shenzhen, Guangdong Province, China
2 School of Life Sciences, Tsinghua University, Beijing, China
3 Guangzhou Medical University, Guangzhou, Guangdong Province, China
4 Shenzhen Key Laboratory of Health Sciences and Technology, Graduate School at Shenzhen, Tsinghua University, Beijing, China
Tiantai No. 1, a Chinese medicine predominantly composed of powdered Rhizoma Gastrodiae, Radix Ginseng, and Ginkgo leaf at a ratio of 2:1:2 and dissolved in pure water, is neuroprotective in animal models of various cognitive disorders, but its molecular mechanism remains unclear. We administered Tiantai No. 1 intragastrically to senescence-accelerated mouse prone 8 (SAMP8) mice (a model of Alzheimer’s disease) at doses of 50, 100 or 150 mg/kg per day for 8 weeks and evaluated their behavior in the Morris water maze and expression of Alzheimer’s disease-related proteins in the brain. Tiantai No. 1 shortened the escape latency in the water maze training trials, and increased swimming time in the target quadrant during the spatial probe test, indicating that Tiantai No. 1 improved learning and memory in SAMP8 mice. Immunohistochemistry revealed that Tiantai No. 1 restored the proliferation potential of Ki67-positive cells in the hippocampus. In addition, mice that had received Tiantai No. 1 had fewer astrocytes, and less accumulation of amyloid-beta and phosphorylated tau. These results suggest that Tiantai No. 1 is neuroprotective in the SAMP8 mouse model of Alzheimer’s disease and acts by restoring neuronal number and proliferation potential in the hippocampus, decreasing astrocyte inf i ltration, and reducing the accumulation of amyloid-beta and phosphorylated tau.
nerve regeneration; neuroprotective ef f ects; Alzheimer’s disease; Tiantai No. 1; SAMP8; amyloid-beta; autophagy-lysosome pathway; ubiquitin proteasome pathway; tau phosphorylation; neuronal apoptosis; astrocytosis; neural regeneration
Graphical Abstract

Introduction
There are approximately 35.6 million people with dementia worldwide and this number is predicted to increase to more than 115 million by 2050 (Morley et al., 2012). The loss of neurons in the central nervous system in Alzheimer’s disease (AD) results in profound pathological changes causing many behavioral and psychological symptoms (Zhang et al., 2013). The pathological hallmarks of AD include extracellular senile plaques, which are mainly composed of 40- to 42-amino-acid amyloid-beta (AB) peptides, and intracellular neurofibrillary tangles (Gold and El Khoury, 2015; Gouras et al., 2015). These hallmarks occur mostly in the cortex and the hippocampus of the brain (Selkoe and Hardy, 2016). Moreover, AB acts by triggering a series of reactions known as the amyloid cascade, which eventually leads to dementia (Hardy and Selkoe, 2002; Huang and Mucke, 2012; Galimberti and Scarpini, 2016). However, the detailed etiology and pathogenesis of AD remain largely unknown.
Autophagic dysfunction in neurodegenerative diseases such as AD is associated with protein misprocessing and over-accumulation (Klionsky and Emr, 2000). Under normal conditions, the autophagic/lysosomal pathway makes efficient use of recycled organelles and substrate proteins (Bernard and Klionsky, 2013), whereas attenuation of autophagy under pathological conditions leads to the accumulation of proteins and lysosomal particles (Platt et al., 2012). These particles contain toxic lysosomal hydrolases, which enhance the formation of AB (Komatsu et al., 2006; Jamasbi et al., 2016). Thus, autophagy is strongly associated with AB accumulation and the development of AD.
The Chinese medicine Tiantai No. 1 has been used for the prevention and treatment of AD for many years (Wu et al., 2010). Previous pharmacodynamics studies have indicated that Tiantai No. 1 enhances learning and memory in various models of agnosia, improves neuronal ultrastructure and synaptic plasticity in models of AD, and increases the regeneration of cholinergic fi bers (Wu et al., 2008, 2010a). Moreover, Tiantai No. 1 induces the dif f erentiation of neuron-like cells from mesenchymal stem cells (Wu et al., 2010b), and has an antagonistic effect on tunicamycin-induced endoplasmic reticulum stress in mesenchymal stem cells. In addition, Tiantai No. 1 protects neurons against AB toxicity and regulates the expression of nuclear factor kappa B and cyclic adenosine monophosphate response element binding protein (Li et al., 2015). Few studies have investigated the mechanism underlying the neuroprotective ef f ect of Tiantai No. 1 in AD.
To this end, we explored the neuroprotective effects and possible molecular mechanism of Tiantai No. 1 in the senescence-accelerated mouse prone 8 (SAMP8) model of AD. The results suggest that Tiantai No. 1 acts by restoring the number and proliferation potential of neurons in the hippocampus, decreasing infiltration of astrocytes, and reducing accumulation of AB and phosphorylated (p-) tau.
Materials and Methods
Animals
We used male specific-pathogen-free, 9- and 15-week-old SAMP8 mice, weighing 20—24 g, as an animal model of AD. SAMP8 mice are used in dementia research because of their characteristic learning and memory def i cits in old age (Flood and Morley, 1998; Qiu et al., 2010). Age-matched senescence-accelerated-resistant mice (SAMR1) were used as controls. The mice were obtained from the First Af fi liated Hospital of Tianjin University of Traditional Chinese Medicine, China, and housed at 24 ± 1°C, 50 ± 5% humidity, and under a 12-hour light/dark cycle, with free access to food and water. All experiments were carried out in accordance with the Chinese guidelines for animal care and use and were approved by the Animal Ethics Committee of the Shenzhen Institute of Integrated Traditional and Western Medicine, China.
Drugs
Tiantai No. 1 is predominantly composed of Rhizoma Gastrodiae, Radix Ginseng, and Ginkgo leaf. These herbs were purchased individually from Beijing Tongrentang (Group) Co., Ltd., China, and tested and approved by the Shenzhen Municipal Drug Inspection. The three herbs were mixed at a ratio of 2:1:2 (China Pharmacopoeia Committee, 2010). An extract was obtained by water extraction and alcohol precipitation, freeze-dried at –40°C and refrigerated. Pure water was used to dissolve the extracts to the required concentration, and the prepared medicine was stored in a refrigerator until use.
Group assignment
All mice were acclimatized for one week before the experiment. SAMP8 mice were then randomly allocated to six groups at 10 and 16 weeks of age, to receive the following treatments (n = 6/group): 0.5 mL/mouse/d of distilled water, intragastrically (AD model control; SAMP8); 50, 100, or 150 mg/kg/d Tiantai No. 1, intragastrically (SAMP8-L, SAMP8-M, and SAMP8-H, respectively); 1 g/kg/d 4-phenylbutyric acid, orally (SAMP8-PBA); 1 mg/kg/d rapamycin, orally (SAMP8-RAPA). SAMP8 mice that received 4-PBA or RAPA were positive controls (Wu et al., 2008). Age-matched SAMR1 mice served as normally aging reference mice and were given an equivalent amount (0.5 mL/mouse/d) of distilled water intragastrically. In all groups, treatments were given orally every day for 8 weeks (Figure 1A).
Morris water maze test
We performed the test after 8 weeks of treatment. The procedure was similar to that described by Morris (1984), with minor modifications. A circular pool (diameter 100 cm, height 40 cm), half-f i lled with opaque water at 25—27°C, was placed in the center of the room. All external visual cues were kept constant for the spatial orientation of the mice. A black platform (diameter 6 cm, height 25 cm) was placed in the center of one of the arbitrarily designated left, trained, right, and opposite quadrants and provided the only escape from the water. The task consisted of visible-platform acquisition training, hidden-platform training, and a probe trial. For the fi rst two days, the platform was clearly marked with a fl ag located 1 cm above the surface of the water in the southwest quadrant of the pool (visible-platform training).
The Morris water maze was performed as described previously (Wang et al., 2013).
Immunof l uorescence and immunohistochemistry
Mouse brain tissue was fixed with 4% formaldehyde and cut into 8—10 μm-thick frozen sections. The sections were incubated with rabbit anti-light chain-3B (LC3B), anti-AB, and anti-p-tau (Ser396) polyclonal antibodies (1:150; Jiake Life Science, Shenzhen, China). Next, the sections were incubated with CY3/fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit antibody (1:200; Beyotime Institute of Biotechnology, Jiangsu, China) at room temperature for 1 hour in the dark, followed by incubation with 4′,6-diamidino-2-phenylindole (DAPI) (1:1,000; Biosharp Biotech, Hefei, China) for 5 minutes. They were then washed three times with phosphate-buf f ered saline (PBS) to remove excess staining solution. For immunohistochemistry, the same primary antibodies were used, with a horseradish peroxidase-conjugated secondary antibody (1:200; Beyotime Institute of Biotechnology). Subsequently, the proteins were visualized with 3,3′-diaminobenzidine (0.2 mL; Beyotime Institute of Biotechnology). The sections were viewed under a laser scanning confocal microscope (FV1000S-SIM/IX81; Olympus, Tokyo, Japan). Eight to 12 sections from each of the six mice per group were examined, and representative images from all analyzed tissue sections were presented. For the Ki67 tissue section staining assay, 8 microscope views per section were randomly selected and the cell positive for Ki67 was counted, and 8—10 sections per sample were analyzed, and representative image was shown. All analyses were performed manually.
Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) assay
Brain sections were rinsed twice with PBS-Tween 20, for 2 minutes each time. Sections were incubated with 3% H2O2in PBS for 10 minutes to block endogenous peroxidase activity, rinsed in PBS-Tween 20 for 3 × 2 minutes, and incubated in TdT Reaction Buf f er (Roche, Basle, Switzerland) for 10 minutes. afterwards, sections were incubated with TdT Reaction Mixture (Roche) for 1—2 hours at 37—40°C in a humidified chamber, and rinsed in Stop/Wash Buffer (Roche) for 10 minutes followed by PBS-Tween 20 for 3 × 2 minutes. They were then incubated with streptavidin-horseradish peroxidase (Roche) in PBS for 20 minutes at room temperature, rinsed in PBS-Tween 20 for 3 × 2 minutes, incubated with 3,3′-diaminobenzidine (Roche) for 1—2 minutes, rinsed in tap water, counterstained with Gill’s hematoxylin for 30 seconds, and rinsed under running tap water for 5 minutes. All sections were dehydrated through 95% ethanol for 5 minutes, 100% ethanol for 3 × 2 minutes, permeabilized in xylene for 2 × 5 minutes, and mounted with xylene-based mounting medium.
Statistical analysis
Data are expressed as the mean ± SEM. Statistical analysis was performed in GraphPad Prism 5.01 soThware (GraphPad SoTh-ware, Inc., CA, USA) using one-way analysis of variance followed by Dunnett’s t test. P < 0.05 was considered statistically signif i cant.
Results
Tiantai No. 1 attenuated memory def i cit in SAMP8 miceIn the Morris water maze, there was no signif i cant dif f erence in the mean escape latency between 10-week-old SAMR1 and SAMP8 mice (Figure 1B). However, the escape latency was significantly greater in 24-week-old SAMP8 mice than in SAMR1 mice at the same age (P < 0.01). SAMP8-PBA and SAMP8-RAPA mice had shorter escape latencies than SAMP8 mice (P < 0.05). Importantly, SAMP8 mice that received Tiantai No. 1 also had signif i cantly shorter escape latencies during the visible-platform training trial (P < 0.05; Figure 1C). A probe test was carried out to further evaluate the ef f ect of Tiantai No. 1 on the cognitive impairment of SAMP8 mice. This showed that SAMR1 mice and SAMP8-PBA mice searched preferentially in the target quadrant, where the platform had been during the training trials (n = 6, P < 0.01), whereas untreated SAMP8 mice showed no signif i cant preference for that quadrant. SAMP8 mice that received Tiantai No. 1 also preferentially searched in the target quadrant (n = 6, P < 0.05; Figure 1D). These results indicate that Tiantai No. 1 attenuated the cognitive impairment observed in the AD mouse models.
Tiantai No. 1 reduced AB accumulation and restored the proliferation of cells in the hippocampus
Amyloid plaques were rarely detected in SAMR1 mice, with significantly more observed in SAMP8 control mice. SAMP8-PBA and SAMP8-RAPA mice showed markedly less amyloid plaque accumulation than SAMP8 controls. A dose-dependent decrease in the number of amyloid plaques was observed in SAMP8 mice that had received Tiantai No. 1 (P < 0.05) (Figure 2A, C).
There was a signif i cant correlation between the hippocampal levels of Ki67 and working memory errors. Ki67 protein expression was detected in the hippocampus of 24-weekold aged SAMR1 and the dif f erent groups of SAMP8 mice.The hippocampal levels of Ki67 were signif i cantly attenuated in SAMP8 mice, but appeared restored in SAMP8-PBA and SAMP8-RAPA mice (P < 0.01). Tiantai No. 1-treated mice showed a dose-dependent increase in Ki67 expression (P <0.05 vs. SAMP8; Figure 2B, D).
Together, these data suggest that Tiantai No. 1 attenuated amyloid accumulation and restored Ki67 expression in the hippocampus in AD mouse models.
Tiantai No. 1 attenuated neuronal death, tau phosphorylation, and astrocytosis in the brain in SAMP8 mice
TUNEL assay signals were lower in SAMP8-PBA and SAMP8-RAPA mice, and in Tiantai No. 1-treated SAMP8 mice, than in SAMP8 controls (Figure 3A). We examined p-tau-immunopositive neurons in the hippocampus of the brain; they were markedly fewer in SAMR1, SAMP8-PBA and SAMP8-RAPA mice than in SAMP8 control mice. In the Tiantai No. 1-treated SAMP8 groups, there were also signif i cantly fewer p-tau-immunopositive cells in the hippocampus than in the SAMP8 control mice, and the reduction in number was dose-dependent (Figure 3B).
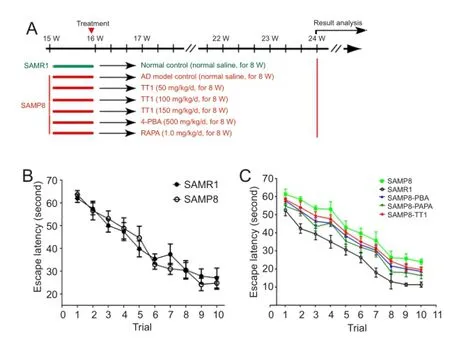
Figure 1 Tiantai No. 1 attenuated memory def i cit in SAMP8 mice.
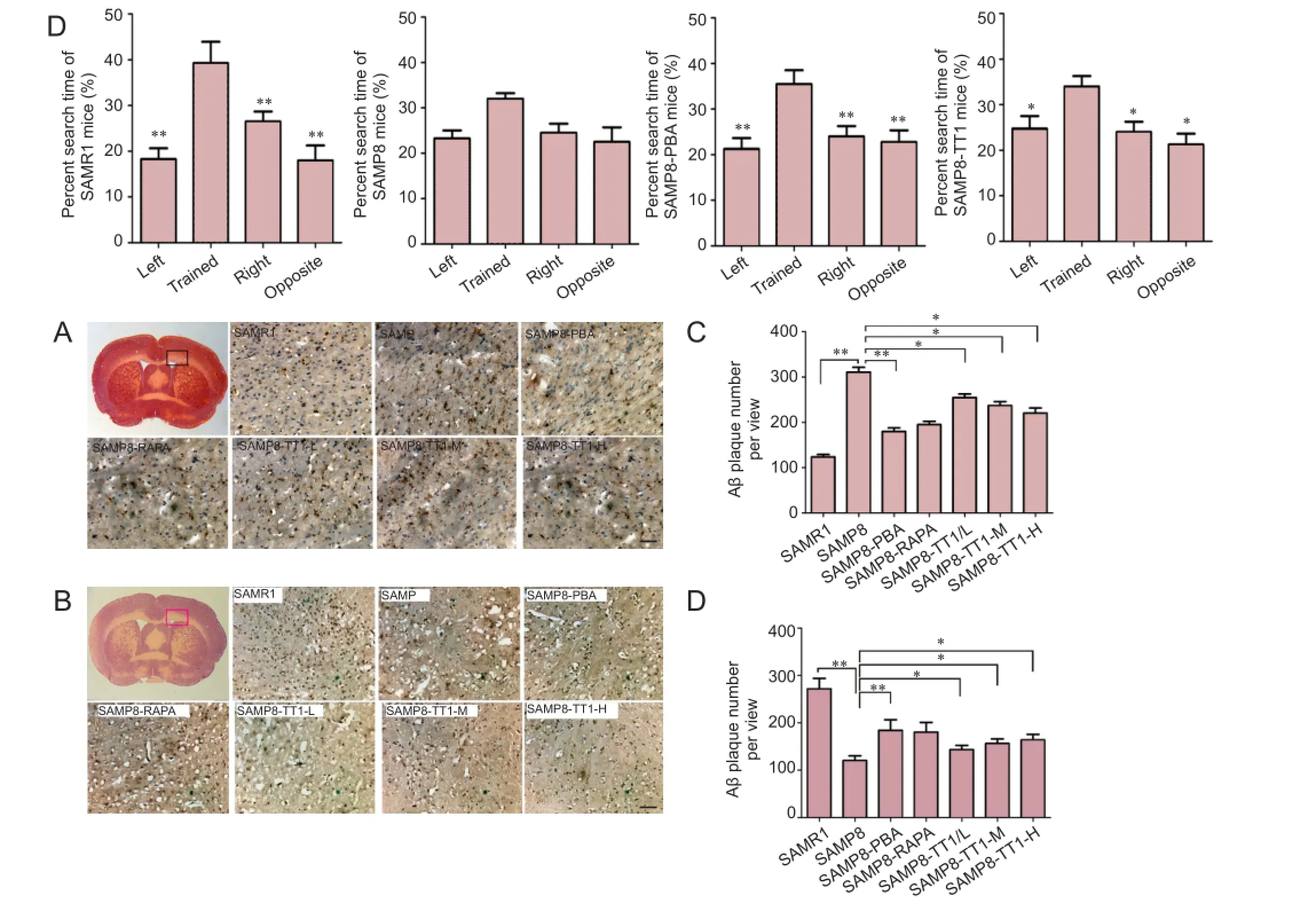
Figure 2 Tiantai No. 1 reduces amyloid-beta accumulation and restores proliferation of cells in the hippocampus (immunohistochemistry).
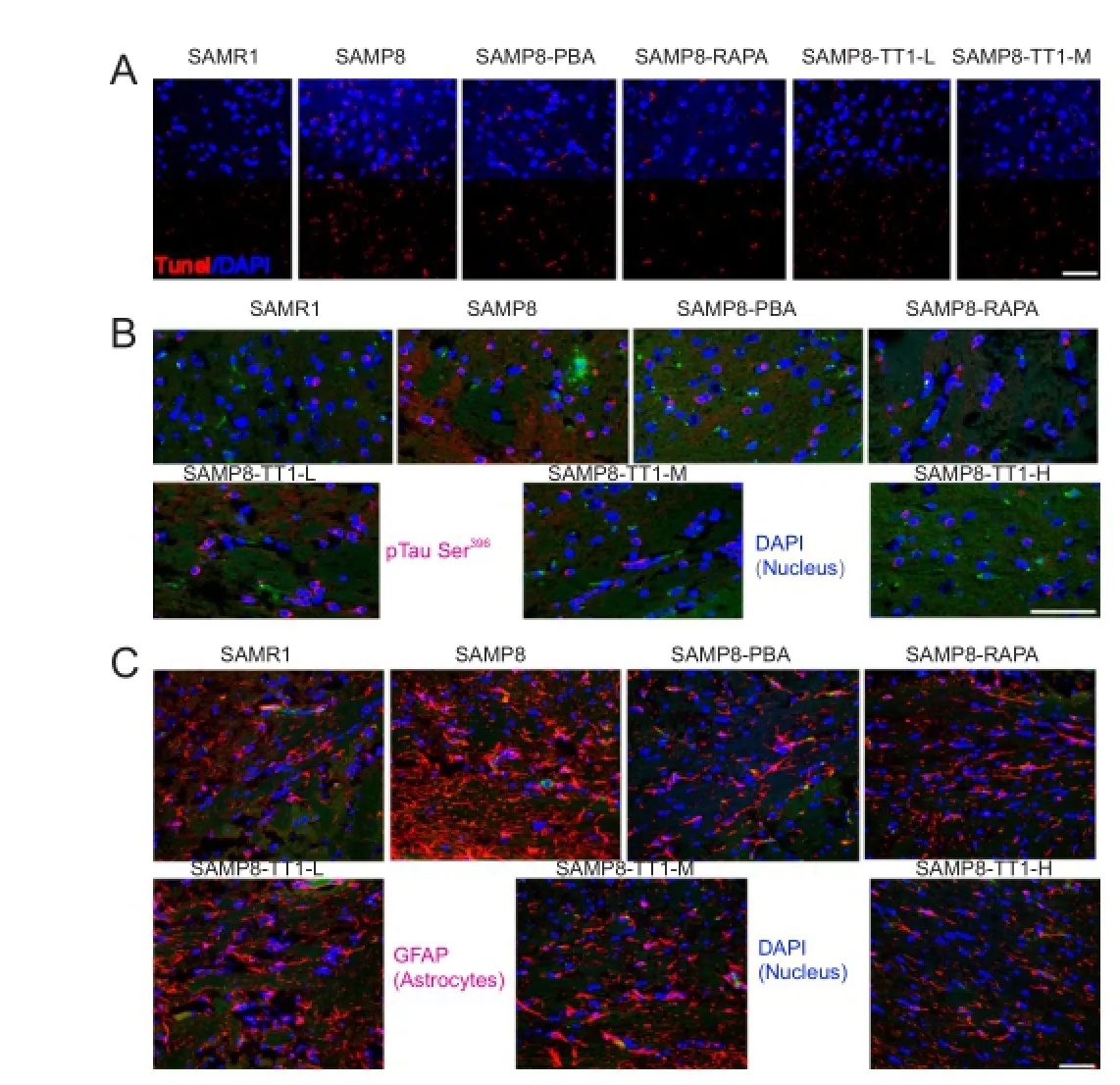
Figure 3 Tiantai No. 1 attenuates cell apoptosis, tau phosphorylation and astrocytosis in the brain of SAMP8 mice (immunof l uorescence).
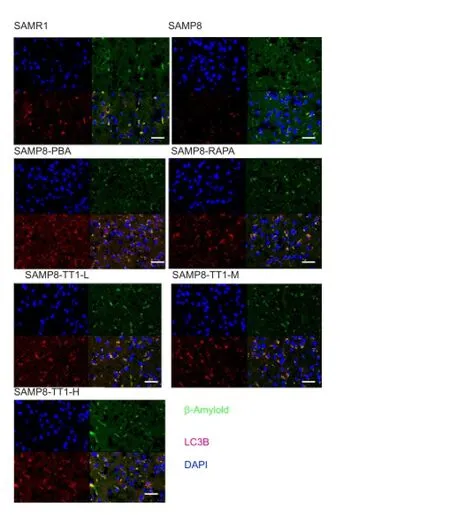
Figure 4 Tiantai No. 1 af f ects the lysosomal pathway in SAMP8 mice (immunof l uorescence).
Brain inf l ammation, microgliosis and astrocytosis are the secondary hallmarks of AD pathology (Belbin et al., 2008; Lee and Landreth, 2010). In the present study, the area of GFAP staining (a marker of astrocytosis) in the hippocampus was significantly smaller in SAMR1, SAMP8-PBA and SAMP8-RAPA mice than in SAMP8 mice. Mice that received Tiantai No. 1 showed a dose-dependent decrease in GFAP-positive cells in the hippocampus (Figure 3C).
Tiantai No. 1 increased autophagy in the hippocampus in SAMP8 mice
To determine whether Tiantai No. 1 enhances the autophagic process in SAMP8 mice, double-staining for LC3B and AB was performed. Confocal microscopy revealed that LC3B and AB were largely co-localized. Further immunostaining analysis showed that Tiantai No. 1, like 4-PBA and RAPA, increased the number of LC3B-positive autophagosomes, and reduced the accumulation of AB (Figure 4). This result suggests that Tiantai No. 1 decreases AB accumulation by enhancing autophagy.
Discussion
Interest in the use of traditional Chinese medicines for the treatment of AD is increasing, because no currently available therapies (cholinesterase inhibitors or N-methyl-D-aspartate receptor antagonists) successfully halt the progression of the disease (Wu et al., 2011; Luo et al., 2016). Recently, researchers have isolated many active compounds from herbs, which have the potential to alleviate dementia and neurodegenerative syndrome, and have fewer side ef f ects than conventional drugs (Wang et al., 2013, 2016). This makes traditional Chinese medicines promising candidates in the treatment of AD, but there have been few investigations to date of their cellular and molecular mechanisms. Here, we investigated the neuroprotective ef f ects of Tiantai No. 1 and explored its possible mechanisms in SAMP8 mouse models of AD.
The memory def i cit observed in SAMP8 mice in the Morris water maze, demonstrated by a longer escape latency in SAMP8 mice than in SAMR1 mice, was attenuated after 8 weeks of treatment with Tiantai No. 1. However, Tiantai No. 1 also decreased the escape latency in SAMP8 mice during the training trial, similarly to that in positive control mice (SAMP8-PBA and SAMP8 RAPA). These results indicate that Tiantai No. 1 attenuated cognitive impairment in AD mice, consistent with previous pharmacodynamics and molecular studies (Wu et al., 2008). This demonstrates that Tiantai No. 1 markedly improves cognitive impairment in SAMP8 mice, and provides further evidence that Tiantai No. 1 is a strong candidate for the treatment of AD.
There were a large number of amyloid plaques in the hippocampus of SAMP8 mice, compared with very few inSAMR1 mice. Tiantai No. 1 treatment appeared to decrease amyloid plaque accumulation in a dose-dependent manner; this is an interesting and important result, as amyloid plaque formation is one of the most characteristic features of AD, indicating that Tiantai No. 1 could reduce or prevent AD progression. Consistent with this result, LC3B staining also showed that Tiantai No. 1 enhanced the autophagic process. Indeed, this may underlie the ef f ect of Tiantai No. 1 on AB plaques, because amyloid degradation may depend on autophagy (Cavieres et al., 2015); this hypothesis needs further investigation, for example by using PC12 cells to explore the neuroprotection imparted by Tiantai No. 1.
Tiantai No. 1 increased cell proliferation in hippocampal tissue, shown by a dose-dependent increase in Ki76 expression levels in Tiantai No. 1-treated mice. This is also an interesting result, as it indicates another possible neuroprotective mechanism of Tiantai No. 1, in which it promotes nerve cell proliferation. Neuronal degeneration is the major cause of AD symptoms, so it would be of great clinical interest to further evaluate the role of Tiantai No. 1 in the promotion of neuronal proliferation both in vivo and in vitro.
In vivo and in vitro methods must be used to identify the pathways and mechanisms of cell death during AD.
Acknowledgments:We are grateful for the technical support by School of Life Sciences, Tsinghua University (Beijing, China), the Shenzhen Key Laboratory of Health Sciences and Technology, Graduate School at Shenzhen, Tsinghua University (Beijing, China) and Guangzhou Medical University (Guangzhou, Guangdong Province, China).
Author contributions:YHL was responsible for study design, fundraising for the study and data collection. XSW was responsible for the research work, data collection, statistical analysis and paper writing. YJ and XLC were in charge of data collection and statistical analysis. HBC was in charge of study design. XQJ and YFZ supported the references and collected data. ZZW provided the critical revision of the paper for intellectual content, study conception and administrative support. All authors approved the fi nal version of the paper.
Conf l icts of interest:None declared.
Plagiarism check:This paper was screened twice using CrossCheck to verify originality before publication.
Peer review:This paper was double-blinded and stringently reviewed by international expert reviewers.
Behl C (2000) Apoptosis and Alzheimer’s disease. J Neural Transm 107:1325-1344.
Belbin O, Dunn JL, Chappell S, Ritchie AE, Ling Y, Morgan L, Pritchard A, Warden DR, Lendon CL, Lehmann DJ, Mann DM, Smith AD, Kalsheker N, Morgan K (2008) A SNP in the ACT gene associated with astrocytosis and rapid cognitive decline in AD. Neurobiol Aging 29:1167-1176.
Bernard A, Klionsky DJ (2013) Autophagosome formation: tracing the source. Develop Cell 25:116-117.
Cavieres VA, González A, Muñoz VC, Yef i CP, Bustamante HA, Barraza RR, Tapia-Rojas C, Otth C, Barrera MJ, González C, Mardones GA, Inestrosa NC, Burgos PV (2015) Tetrahydrohyperforin inhibits the proteolytic processing of amyloid precursor protein and enhances its degradation by atg5-dependent autophagy. PLoS One 10:e0136313.
China Pharmacopoeia Committee (2010) Pharmacopoeia of the People’s Republic of China. Beijing: Chinese Medical Science and Technology Press.
Flood JF, Morley JE (1998) Learning and memory in the SAMP8 mouse. Neurosci Biobehav Rev 22:1-20.
Galimberti D, Scarpini E (2016) Emerging amyloid disease-modifying drugs for Alzheimer’s disease. Expert Opin Emerg Dr 21:5-7.
Gold M, El Khoury J (2015) beta-amyloid, microglia, and the inf l ammasome in Alzheimer’s disease. Semin Immunopathol 37:607-611.
Gouras GK, Olsson TT, Hansson O (2015) Beta-amyloid peptides and amyloid plaques in Alzheimer’s disease. Neurotherapeutics 12:3-1i.
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353-356.
Huang Y, Mucke L (2012) Alzheimer mechanisms and therapeutic strategies. Cell 148:1204-1222.
Jamasbi E, Wade JD, Separovic F, Hossain MA (2016) Amyloid beta (A beta) peptide and factors that play important roles in Alzheimer’s disease. Curr Med Chem 23:884-892.
Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290:1717-1721.
Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K (2006) Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441:880-884.
Lee CY, Landreth GE (2010) The role of microglia in amyloid clearance from the AD brain. J Neural Transm (Vienna) 117:949-960.
Li YH, Wu ZZ, Cao MQ, Li M, Sun KH, Yang M, Chen MY, Huang AC (2015) Ef f ect of Tiantai No.1 on gene expression prof i les in hippocampus of Alzheimer’s disease rats by bioinformatic analysis. Chin J Integr Med 21:123-131.
Luo HB, Li Y, Liu ZJ, Cao L, Zhang ZQ, Wang Y, Zhang XY, Liu Z, Shi XQ (2016) Protective effect of tetrahydroxy stilbene glucoside on learning and memory by regulating synaptic plasticity. Neural Regen Res 11:1480-1486.
Morley JE, Armbrecht HJ, Farr SA, Kumar VB (2012) The senescence accelerated mouse (SAMP8) as a model for oxidative stress and Alzheimer’s disease. Biochim Biophys Acta 1822:650-656.
Platt FM, Boland B, van der Spoel AC (2012) The cell biology of disease: lysosomal storage disorders: the cellular impact of lysosomal dysfunction. J Cell Biol 199:723-734.
Qiu L, Zheng L, Zhang Y (2010) Effect and mechanism of action of qingkailing on learning and memory capacity of SAMP8 mouse. Zhongguo Zhongxiyi Jiehe Zazhi 30:738-742.
Roos WP, Kaina B (2006) DNA damage-induced cell death by apoptosis. Trends Mol Med 12:440-450.
Roos WP, Kaina B (2013) DNA damage-induced cell death: from specif i c DNA lesions to the DNA damage response and apoptosis. Cancer Lett 332:237-248.
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. Embo Mol Med 8:595-608.
Stadelmann C, Bruck W, Bancher C, Jellinger K, Lassmann H (1998) Alzheimer disease: DNA fragmentation indicates increased neuronal vulnerability, but not apoptosis. J Neuropathol Exp Neurol 57:456-464. Wang HM, Wang LW, Liu XM, Li CL, Xu SP, Farooq AD (2013) Neuroprotective ef f ects of forsythiaside on learning and memory def i cits in senescence-accelerated mouse prone (SAMP8) mice. Pharmacol Biochem Behav 105:134-141.
Wang Y, Cai B, Shao J, Wang TT, Cai RZ, Ma CJ, Han T, Du J (2016) Genistein suppresses the mitochondrial apoptotic pathway in hippocampal neurons in rats with Alzheimer’s disease. Neural Regen Res 11:1153-1158.
Wu ZZ, Zhong Q, Sun SP (2010a) A prospective multicenter randomized double-blinded controlled clinical trial on ef f ects of tiantai no.1 in treating mild cognitive impairment. Zhongguo Zhongxiyi Jiehe Zazhi 30:255-258.
Wu ZZ, Huang AC, de Vellis J, Li YH (2008) Ef f ect of Tiantai No.1 on beta-amyloid-induced neurotoxicity and NF-kappa B and cAMP responsive element-binding protein. Chin J Integr Med 14:286-292.
Wu ZZ, Li YH, Huang AC, Li M, Zhang XL, Wang JG, Yang M, Chen MY (2010b) Endoplasmic reticulum stress induced by tunicamycin and antagonistic effect of Tiantai No.1 (1) on mesenchymal stem cells. Chin J Integr Med 16:41-49.
Zhang L, Ma Q, Yang W, Qi X, Yao Z, Liu Y, Liang L, Wang X, Ma C, Huang L, Xu Y, Zhu H, Deng W, Gao Y, Ruan L, Xiao Z, Qin C (2013) Recombinant DNA vaccine against neurite outgrowth inhibitors attenuates behavioral def i cits and decreases Abeta in an Alzheimer’s disease mouse model. Neuropharmacology 70:200-210.
Copyedited by Slone-Murphy J, de Souza M, Wang J, Li CH, Qiu Y, Song LP, Zhao M
Nathanael J. Yates1, Marcus K. Giacci1, Ryan L. O’Hare Doig1,2, Wissam Chiha1,2, Bethany E. Ashworth1, Jade Kenna1, Carole A. Bartlett1, Melinda Fitzgerald1,*
1 Department of Experimental and Regenerative Neurosciences, School of Animal Biology, The University of Western Australia, Crawley, Western Australia, Australia
2 Department of Experimental and Regenerative Neurosciences, School of Anatomy, Physiology and Human Biology, The University of Western Australia, Crawley, Western Australia, Australia
How to cite this article: Li YH, Wang XS, Chen XL, Jin Y, Chen HB, Jia XQ, Zhang YF, Wu ZZ (2017) Neuroprotective ef f ect of the Chinese medicine Tiantai No. 1 and its molecular mechanism in the senescence-accelerated mouse prone 8. Neural Regen Res 12(2):301-306.
Open access statement: This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Funding: This study was funded by the National Natural Science Foundation of China, No. 81473742; the Guangdong Science and Technology Foundation, No. 2013B021800101; the Shenzhen Major Project of Science and Technology Planning, No. JCYJ20130401115231337.
*Correspondence to: Ying-hong Li or
Zheng-zhi Wu,
yinghongli@163.com or szwzz001@163.com.
#These authors contributed
equally to this study.
orcid:
0000-0002-1963-8560
(Ying-hong Li)
0000-0002-8819-609X
(Zheng-zhi Wu)
10.4103/1673-5374.200813
Accepted: 2016-12-28
- 中国神经再生研究(英文版)的其它文章
- Hyperbaric oxygen preconditioning improves postoperative cognitive dysfunction by reducing oxidant stress and inf l ammation
- Nonhuman primate models of focal cerebral ischemia
- The cortical activation pattern during bilateral arm raising movements
- Adenyl cyclase activator forskolin protects against Huntington’s disease-like neurodegenerative disorders
- Edaravone protects against oxygen-glucose-serum deprivation/restoration-induced apoptosis in spinal cord astrocytes by inhibiting integrated stress response
- Ef f ect of electroacupuncture on the mRNA and protein expression of Rho-A and Rho-associated kinase II in spinal cord injury rats