Ef f ect of electroacupuncture on the mRNA and protein expression of Rho-A and Rho-associated kinase II in spinal cord injury rats
You-jiang Min, Li-li-qiang Ding, Li-hong Cheng, Wei-ping Xiao, Xing-wei He Hui Zhang Zhi-yun Min Jia Pei
1 Af filiated Hospital of Jiangxi University of Traditional Chinese Medicine, Nanchang, Jiangxi Province, China
2 Shanghai University of Traditional Chinese Medicine, Shanghai, China
Ef f ect of electroacupuncture on the mRNA and protein expression of Rho-A and Rho-associated kinase II in spinal cord injury rats
You-jiang Min1,#, Li-li-qiang Ding1,2,#, Li-hong Cheng1,*, Wei-ping Xiao1,*, Xing-wei He1, Hui Zhang1, Zhi-yun Min1, Jia Pei1
1 Af filiated Hospital of Jiangxi University of Traditional Chinese Medicine, Nanchang, Jiangxi Province, China
2 Shanghai University of Traditional Chinese Medicine, Shanghai, China
Electroacupuncture is benef i cial for the recovery of spinal cord injury, but the underlying mechanism is unclear. The Rho/Rho-associated kinase (ROCK) signaling pathway regulates the actin cytoskeleton by controlling the adhesive and migratory behaviors of cells that could inhibit neurite regrowth after neural injury and consequently hinder the recovery from spinal cord injury. Therefore, we hypothesized electroacupuncture could af f ect the Rho/ROCK signaling pathway to promote the recovery of spinal cord injury. In our experiments, the spinal cord injury in adult Sprague-Dawley rats was caused by an impact device. Those rats were subjected to electroacupuncture at Yaoyangguan (GV3), Dazhui (GV14), Zusanli (ST36) and Ciliao (BL32) and/or monosialoganglioside treatment. Behavioral scores revealed that the hindlimb motor functions improved with those treatments. Real-time quantitative polymerase chain reaction, fl uorescence in situ hybridization and western blot assay showed that electroacupuncture suppressed the mRNA and protein expression of Rho-A and Rho-associated kinase II (ROCKII) of injured spinal cord. Although monosialoganglioside promoted the recovery of hindlimb motor function, monosialoganglioside did not af f ect the expression of Rho-A and ROCKII. However, electroacupuncture combined with monosialoganglioside did not further improve the motor function or suppress the expression of Rho-A and ROCKII. Our data suggested that the electroacupuncture could specif i cally inhibit the activation of the Rho/ROCK signaling pathway thus partially contributing to the repair of injured spinal cord. Monosialoganglioside could promote the motor function but did not suppress expression of RhoA and ROCKII. There was no synergistic ef f ect of electroacupuncture combined with monosialoganglioside.
nerve regeneration; spinal cord injury; electroacupuncture; Rho/Rho-associated kinase signaling pathway; monosialoganglioside; motor function; cytoskeleton; real-time quantitative polymerase chain reaction; western blot assay; hybridization in situ; neural regeneration
Graphical Abstract

Introduction
Spinal cord injury (SCI) is a serious neurological injury that oThen results in profound disability (Yoshimura et al., 2006; Gao et al., 2014, 2015). SCI results in high mortality and there is no ef f ective treatment at present (Wu et al., 2014; Lin and Zhao, 2015). The regeneration of neurons in the adult central nervous system (CNS) is limited and their axons are unable to regenerate after severe injury (Domeniconi et al., 2005; Zhou and Snider, 2005; Chiba et al., 2010). The activities of the cytoskeleton influence the growth cone that is crucial for the growth of neural axons (Ito et al., 2004). The growth cone is susceptible to the surrounding environment (Monnier et al., 2003; James et al., 2008) and various signal pathways af f ect the cytoskeleton of the growth cone to regulate neuron axonal growth (Carmen et al., 2004; Lingor et al., 2008). The Rho/ROCK signaling pathway is a vital part in promoting the growth of neural axons and in the regulation of the cytoskeleton (Wettschureck et al., 2002; Doran et al., 2004; Liu et al., 2015). The two essential components of Rho/ ROCK signaling pathway are Rho-A and Rho-associated kinase II (ROCKII) (Wettschureck et al., 2002; Hou et al., 2015; Jia et al., 2016). Rho GTPases are important regulators of the actin cytoskeleton and thereby control the adhesive and migratory behaviors of cells (EtienneManneville and Hall, 2002; Govek et al., 2005). Within these subfamilies of Rho GTPases, Cdc42, Rac and Rho-A have been shown to participate in regulating the growth of neural axons; Cdc42 and Rac regulate the actin to promote axon growth and stability (Nobes and Hall, 1995). Rho-A activates the downstream signaling molecule, ROCKII, and then triggers a series of reactions that cause the growth cone to collapse and retraction that result in limited regeneration of neural axons (Dickson, 2001). Although there is low mRNA expression of Rho-A in the normal spinal cord, Rho-A expression is signif i cantly enhanced after SCI (Wu and Xu, 2016), which indicated that Rho/ROCK signaling pathway plays an essential role in the pathogenesis of SCI. How to promote the regeneration of axons is a key aim in treating SCI (Ng and Luo, 2004; Sun et al., 2008).
Electroacupuncture (EA) is widely used to treat SCI, and has been shown to be benef i cial for the recovery of SCI (Paola and Arnold, 2003; Min et al., 2013). However, the underlying mechanism of EA in the treatment of SCI remains unclear (Zhang et al., 2012). In the present study, we investigated the ef f ect of EA on the repair of SCI, and whether EA could inhibit the Rho/ROCK signaling pathway after SCI.
Materials and Methods
Animals
Eighty healthy, clean, male, Sprague-Dawley rats, aged 8 weeks, weighing 200 ± 20 g, were supplied by the Slack-Jingda Laboratory Animals Co., Ltd. of Hunan Province, China (certif i cate No. SCXK (Xiang) 2011-0003). The rats were fed with standard fodder and allowed free access to water and chow.
Following a 3-day adaptation, all rats were randomly divided into fi ve groups: sham surgery (sham, n = 16), modeltreatments, lasting for 14 days.
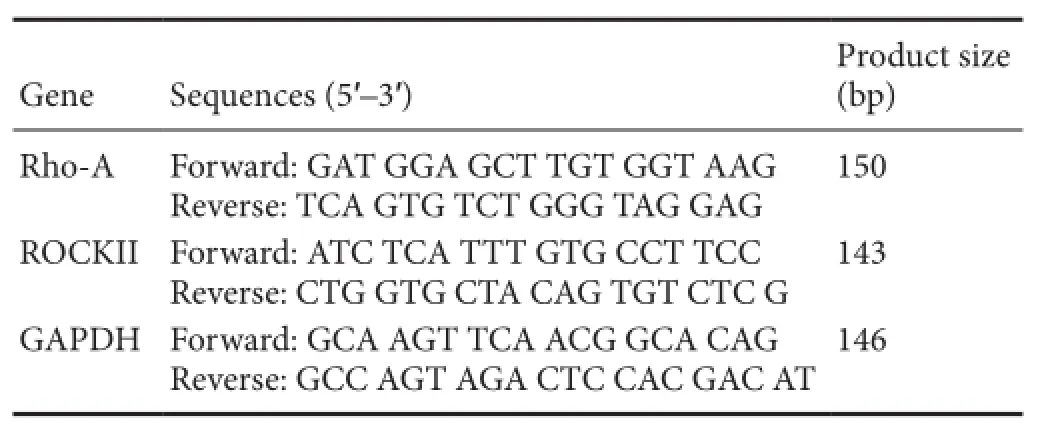
Table 1 Primer sequences for real-time quantitative polymerase chain reaction

Table 2 Primer sequences for hybridization in situ
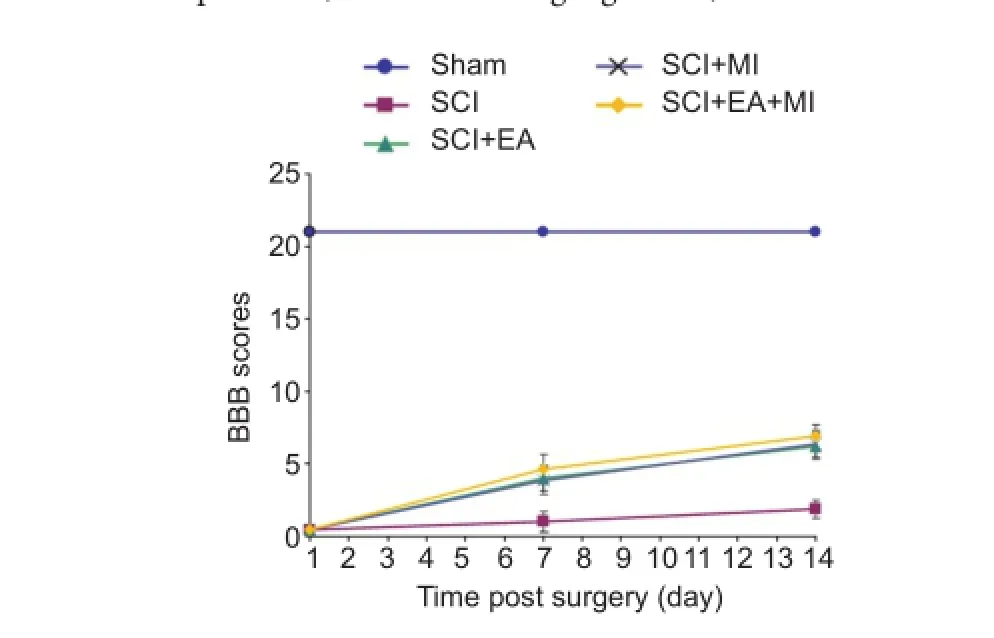
Figure 1 Ef f ect of electroacupuncture on the motor function of spinal cord injury rats.
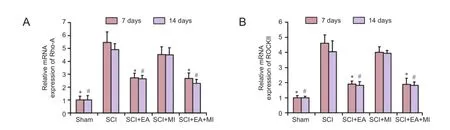
Figure 2 Ef f ect of electroacupuncture on changes in mRNA expression of Rho-A and ROCKII of injured spinal cords.
In the sham group, 16 rats received anesthesia, incision, removal of T10vertebral body and suture, but no strike injury.
Motor function assessment and tissue preparation
The hindlimb motor function of rats was assessed at 1, 7 and 14 days after SCI using the open fi eld locomotor test developed by Basso, Beattie, and Bresnahan (Basso et al., 1995).
Double independent BBB scores were recorded, and the average values are presented. A random selection of eight rats in each group were sacrif i ced at 7 and 14 days after SCI, and the impaired spinal cords were harvested for RT-qPCR, in situ hybridization and western blot assay. The tissues were frozen in liquid nitrogen and stored at —80°C.
RT-qPCR
RT-qPCR was performed using SYBR Green system. Total RNA was isolated from the spinal cords of rats in each group at 7 and 14 days using TRIzol solution (Invitrogen, Carlsbad, CA, USA). The mRNA expression levels of Rho-A and ROCKII were measured using a RT-qPCR system with SYBR Green (Thermo Fisher Scientific, Waltham, MA, USA). cDNA was amplif i ed by PCR using primers for each target gene. RT-qPCR conditions are as follows: 94°C for 5 minutes, followed by 40 cycles of 95°C for 15 seconds, 60°C for 45 seconds and 72°C for 30 seconds. Fluorescence signal was detected at 60°C and samples were fi nally extended at 72°C for 7 minutes. The amplification efficiency was compared between the target and reference control GAPDH (glyceraldehyde 3-phosphate dehydrogenase) using the delta-delta Ct (ΔΔCt) method (Min et al., 2016). Primers employed are listed in Table 1.
mRNA expression of Rho-A and ROCKII, as determined by fl uorescence in situ hybridization
?

Figure 3 mRNA expression of Rho-A and ROCKII in injured spinal cords at 7 and 14 days (in situ hybridization, × 200).

Figure 4 Ef f ect of Electroacupuncture on changes in protein expression of Rho-A and ROCKII of injured spinal cords.
The sections were dewaxed by xylene three times, 5 minutesper time. Xylene was removed in gradient alcohol of 100%, 96% and 70%. The sections were dried in the air, washed for 2—5 minutes with phosphate buf f ered saline (PBS) to remove protease, and then dehydrated in gradient alcohol. Each section was incubated in 50 μL hybridization buf f er containing oligonucleotide probe 10 μm at 95°C for 5 minutes and 37—40°C for 12 hours, washed three times with 5×, 1× and 0.2× saline sodium citrate respectively, and treated with blocking buf f er at 37°C for 15 minutes. The blocking buf f er was blotted of f with paper. Each section was then incubated in 30 μL biotinylated anti-digoxin (1:50) at 37°C for 1 hour, washed four times with 0.5 M PBS, incubated in streptavidin-biotin-peroxidase complex at 37°C for 30 minutes, and rinsed four times in 0.5 M PBS. Subsequently, the sections were developed in 3,3′-diaminobenzidine, counterstained with hematoxylin, dehydrated in alcohol, permeabilized in xylene, mounted with quench-proof mounting agent, and photographed with a fl uorescence microscope. Negative controls were incubated in 0.01 M PBS without primary antibody. Primers employed are displayed in Table 2.
Western blot assay
All spinal cord tissues obtained at 7 and 14 days in each group were homogenized in a lysis buffer (JRDUN Biotechnology, Shanghai, China). Equal amounts of proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The resolved proteins were transferred to polyvinylidene fl uoride membranes (Millipore, Bedford, MA, USA). The membranes were incubated with primary antibodies overnight at 4°C. Monoclonal antibodies included rat monoclonal anti-Rho-A (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rat monoclonal anti-ROCKII (1:1,000; Santa Cruz Biotechnology) and rat monoclonal anti-GAPDH (1:2,000; Santa Cruz Biotechnology). The membranes were washed 3 times for 5 minutes with Tris-buffered saline Tween, incubated with goat anti-rabbit IgG-horseradish peroxidase (HRP) (1:1,000; Beyotime Biotechnology, Shanghai, China) and goat anti-mouse IgG-HRP PS1 (C-20) (1:1,000; Beyotime Biotechnology) antibodies at 37°C for 1 hour. The immunoreactive bands were visualized using an enhanced chemiluminescence reagent (Beyotime Biotechnology). The grayscale values of bands were quantif i ed using Image J soThware (Fujif i lm, Tokyo, Japan). The relative expression of protein was calculated based on the grayscale value ratio of target to loading control.
Statistical analysis
All data, presented as the mean ± SD, were analyzed by a one-way analysis of variance, followed by a post hoc Student-Newman-Keuls test using the SPSS 13.0 soThware (SPSS, Chicago, IL, USA). A value of P < 0.05 was considered statistically signif i cant.
Results
Ef f ects of treatment on behavior in SCI rats
Behavioral scores (BBB scores) in each group are shown in Figure 1. BBB scores of SCI rats were lower than that of the normal rats (P < 0.05). after treatment with EA or MI, the hindlimb motor function of rats improved in each treatment group. BBB score was signif i cantly lower in the SCI group (P< 0.05) than in any of the treatment groups (SCI + EA, SCI + MI, and SCI + EA + MI). However, there was no signif i cant dif f erence in BBB score among those three treatment groups (P > 0.05).
EA suppressed mRNA and protein expression of Rho-A and ROCKII in injured spinal cords of rats
Relative mRNA expression of Rho-A and ROCKII in injured spinal cords was determined by RT-qPCR, in situ hybridization; and protein expression of Rho-A and ROCKII by western blot assay. As shown in Figures 2, 3, the mRNA expression levels of Rho-A and ROCKII were significantly increased in the SCI, SCI + EA, SCI + MI, and SCI + EA + MI groups compared with the sham group at 7 days (P < 0.05) and 14 days (P < 0.05). EA treatment signif i cantly down-regulated the mRNA expression of Rho-A and ROCKII compared with the SCI group (P < 0.05). The mRNA expression of Rho-A and ROCKII between SCI and SCI + MI groups was not significantly different (P > 0.05).The expression of mRNA for Rho-A and ROCKII showed no signif i cant dif f erence between 7 days and 14 days for each group (P > 0.05). Western blot assay results exhibited the same trend as did in the RT-qPCR (Figure 4). The above results showed that the Rho-A and ROCKII was activated rapidly after SCI and inhibited after EA treatment.
Discussion
In this study, we investigated the mechanism underlying the ef f ects of EA on SCI at the molecular level. Our results demonstrated that EA treatment improved hindlimb motor function of SCI rats. In our experiments, the mRNA and protein expression of Rho-A and ROCKII were enhanced after SCI compared with sham group. This confirms other studies showing that although there was low mRNA expression of Rho-A in the normal spinal cord, Rho-A expression was significantly enhanced after SCI (Conrad et al., 2005; Erschamer et al., 2005). This indicates that the Rho/ROCK signaling pathway plays an essential role in the pathogenesis of SCI. We found that EA treatment suppressed Rho-A and ROCKII mRNA and protein expression in the injured spinal cord of rats. These results suggest that a possible mechanism of inducing the recovery from SCI by EA treatment could be by down-regulating the expression of Rho-A and ROCKII. We also found that hindlimb motor function improved following inhibition of Rho-A and ROCKII in SCI models.
MI is frequently used in the treatment of SCI, and MI has a significant effect on promoting neural repair (Walker et al., 1993). Gangliosides are sialic acid derivatives of endogenous glycolipids and are present predominantly in the cell membrane in the central nervous system (Wang et al., 2015). MI is already used as a therapeutic option for treatment of central nervous system injuries because of its anti-neurotoxic, anti-inf l ammatory and neuroprotective ef f ects. Moreover,MI promotes the development, growth, differentiation and maturation of neurons (Barros et al., 2016). Our results indicated that although ganglioside promoted the recovery of hindlimb motor function of SCI rats, it did not af f ect the expression of Rho-A and ROCKII. SCI results in a complex pathophysiological process. Therefore different treatments may aid recovery from SCI in dif f erent ways. Although EA and MI each improved the hindlimb motor function, there was no additional effect with the combined treatment. Regeneration of the central nervous system is dif fi cult, and the recovery from SCI takes a much longer time in humans. In our experiment, it was not possible to observe the recovery after SCI rats for long enough to observe a better ef fi cacy or further recovery of motor function.
Our results suggest that the Rho/ROCK signal pathway is a target for EA in the treatment of SCI. The synergistic ef f ects of EA and other therapies could be explored in subsequent experiments.
Precisely where EA acts in inhibiting the Rho/ROCK pathway requires further research. Dent and Gertler (2003) indicated that the growth of axons is closely associated with cytoskeleton. The neurons in the adult central nervous system fail to regenerate their axons after an injury, which is a major dif ficulty in the repair of SCI. Axonal regeneration was inhibited in the glial scar after cerebral or spinal cord injury (Schwab et al., 1993). The amount of growth inhibitory molecules (growth-IMs), such as myelin-associated glycoproteins (McKerracher et al., 1994), Nogo (Chen et al., 2000) and oligodendrocytic myelin glycoprotein (Wang et al., 2002), is crucial in the promotion of axonal regeneration for treating SCI. Growth-IMs have been identif i ed as activators of the intracellular Rho/ROCK signaling pathway that induces reorganization of the actin cytoskeleton causing growth cone collapse and consequently inhibits axonal regeneration (Domeniconi et al., 2005). Many extracellular orientation information molecules, such as lysophosphatidic acid, thrombin receptor activator protein and activated prostaglandin E receptor, also affect Rho/ROCK signaling pathway leading to the collapse of the growth cone (Katoh et al., 1998).
After Rho-A has been activated through the receptor on the cell membrane by growth-IMs, the Rho-A activates the downstream signal molecule ROCKII. The ROCKII phosphorylates myosin light chain phosphatase so it is unable to dephosphorylate myosin. The elevated level of phosphorylated myosin increases its contractility, enhancing microf i lament retraction in the cytoskeleton of the axonal growth cone. Finally, it leads to the collapse of axonal growth cone and inhibits regrowth of the axon (Wettschureck et al., 2002).
In conclusion, after SCI, the Rho/ROCK signaling pathway is activated that contributes to the inhibition of regeneration of axons. EA could inhibit activation of Rho/ROCK signaling pathway after SCI by suppressing expression of Rho-A and ROCKII, thereby promoting axon regrowth and inducing recovery from SCI. These findings provide evidence to support the widespread clinical utilization of EA.
These research findings augment our understanding of the neuromodulatory ef f ects of EA and also provide us with an enhanced insight into the biology of SCI and repair.
Acknowledgments:Professor Jian-ming Wang from Jiangxi University of Traditional Chinese Medicine provided the guidance of model establishment and the equipment of electric cortical contusion impactor.
Author contributions:YJM conceived and designed the study and revised the paper. LLQD wrote the paper, provided data and revised the paper. WPX, HZ, ZYM, and JP participated in the experiment. WPX provided the guidance of model establishment. LHC and XWH provided some advices for the study. All authors approved the fi nal version of the paper.
Conf l icts of interest:None declared.
Plagiarism check:This paper was screened twice using CrossCheck to verify originality before publication.
Peer review:This paper was double-blinded and stringently reviewed by international expert reviewers.
Barros TE, Jr., Araujo FF, Higino Lda P, Marcon RM, Cristante AF (2016) The ef f ect of monosialoganglioside (GM-1) administration in spinal cord injury. Acta Ortop Bras 24:123-126.
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open fi eld testing in rats. J Neurotrauma 12:1-21.
Carmen V, Vasquez M, Einheber S (2004) Rho-kinase regulates Schwann cell Myelination and formation of associated axonal domains. Neurosci 24:3953-3963.
Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME (2000) Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 403:434-439.
Chiba Y, Kuroda S, Shichinohe H, Hokari M, Osanai T, Maruichi K, Yano S, Hida K, Iwasaki Y (2010) Synergistic ef f ects of bone marrow stromal cells and a Rho kinase (ROCK) inhibitor, fasudil on axon regeneration in rat spinal cord injury. Neuropathology 30:241-250.
Conrad S, Schluesener HJ, Trautmann K, Joannin N, Meyermann R, Schwab JM (2005) Prolonged lesional expression of RhoA and RhoB following spinal cord injury. J Comp Neurol 487:166-175.
Dent EW, Gertler F B (2003) Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron 40:209-227.
Dickson BJ (2001) Rho GTPases in growth cone guidance. Curr Opin Neurobiol 11:103-110.
Domeniconi M, Zampieri N, Spencer T, Hilaire M, Mellado W, Chao MV, Filbin MT (2005) MAG induces regulated intramembrane proteolysis of the p75 neurotrophin receptor to inhibit neurite outgrowth. Neuron 46:849-855.
Doran JD, Liu X, Taslimi P (2004) New insights into the structure-function relationships of Rho-associated kinase: a thermodynamic and hydrodynamic study of the dimmer-to-monomer transition and its kinetic implications. Biochem J 384:255.
EtienneManneville S, Hall A (2002) Rho GTPases in cell biology. Nature 420:629-635.
Erschamer MK, Hofstetter CP, Olson L (2005) RhoA, RhoB, RhoC, Racl, Cdc42, and Tc10 mRNA levels in spinal cord, sensory ganglia, and corticospinal tract neurons and long-lasting specif i c changes following spinal cord injury. J Comp Neurol 484:224-233.
Gao J, Zhang YJ, Min YJ, Cui J (2013) Application and research of acupoints on acupuncture treating paraplegina. Jiangxi Zhong Yi Yao 2:54-56.
Gao J, Cheng Lh, Min YJ (2015) Clinical Research in acupuncture treating the recovery period of spinal cord injury. Anmo yu Kangfu 6:18-21.
Gao LJ, Sun YC, Li JJ, Bai F, Li PK (2014) Ef f ects of electroacupuncture in dif f erent time on variations of fractional anisotropy mean value of dif f usion tensor tractography in spinal cord injured rats. Chin J Rehabil Theory Prac 20:728-733.
Govek EE, Newey SE, Van Aelst L (2005) The role of the Rho GTPases in neuronal development. Genes Dev 19:1-49.
Hou XL, Chen Y, Yin H, Duan WG (2015) Combination of fasudil and celecoxib promotes the recovery of injured spinal cord in rats better than celecoxib or fasudil alone. Neural Regen Res 10:1836-1840.
Ito M, Nakano T, Erdodi F, Hartshorne DJ (2004) Myosin phosphatase: structure, regulation and function. Mol Cell Biochem 259:197-209.
James SE, Burden H, Burgess R, Xie Y, Yang T, Massa SM, Longo FM, Lu Q (2008) Anti-cancer drug induced neurotoxicity and identif i cation of Rho pathway signaling modulators as potential neuro-protectants. Neurotoxicology 29:605-612.
Jia XF, Ye F, Wang YB, Feng DX (2016) ROCK inhibition enhances neurite outgrowth in neural stem cells by upregulating YAP expression in vitro. Neural Regen Res 11:983-987.
Katoh H, Aoki J, Ichikawa A, Negishi M (1998) p160 RhoA-binding kinase ROKalpha induces neurite retraction. J Biol Chem 273:2489-2492.
Lin SD, Zhao TB (2015) Surgical advance of pelvic viscera dysfunction after spinal cord injury. Zhongguo Jiaoxing Waike Zazhi 23:136-139.
Lingor P, Tönges L, Pieper N, Bermel C, Barski E, Planchamp V, Bähr M (2008) ROCK inhibition and CNTF interact on intrinsic signaling pathways and dif f erentially regulate survival and regeneration in retinal ganglion cells. Brain 131:250-263.
Liu J, Gao HY, Wang XF (2015) The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system. Neural Regen Res 10:1892-1896.
McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE (1994) Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron 13:805-811.
Min YJ, Cheng LH, Gao J (2013) Comparative observations on three-unblocking acupuncture for the treatment of spinal cord injury in convalescent patients with paraplegia. Shanghai Zhenjiu Zazhi 32:1010-1013.
Min YJ, Deng L, Hong ES (2016) Orthogonal study on dif f erent acupuncture factors based on hypothalamic-pituitary-adrenal axis in rats with kindey Yang deficiency. Shanghai Zhenjiu Zazhi 35:339-343.
Monnier PP, Sierra A, Schwab JM, Henke-Fahle S, Mueller BK (2003)The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neuresci 22:319-330.
Moon SY, Zheng Y (2003) Rho GTPase-activating proteins in cell regulation. Trends Cell Biol 13:13.
Ng J, Luo L (2004) Rho GTPases regulate axon growth through convergent and divergent signaling pathways. Neuron 44:779-793.
Nobes CD, Hall A (1995) Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fi bers, lamellipodia, and fi lopodia. Cell 81:53-62.
Paola FA, Arnold M (2003) Acupuncture and spinal cord medicine. Spinal Cord Med 26:12-20.
Schwab ME, KapThammer JP, Bandtlow CE (1993) Inhibitors of neurite growth. Ann Rev Neurosci 16:565-595.
Sun DR, Zhu Y, Zhu HT,Wang F (2008) Experimental study on axonal regeneration promoted by olfactory ensheathing cells after spinal cord injury. Zhongguo Yike Daxue Xuebao 37:727-729.
Walker J B, Harris M (1993) GM-1 ganglioside administration combined with physical therapy restores ambulation in humans with chronic spinal cord injury. Neurosci Lett 161:174-178.
Wang XH, Wang H, Cheng JH, Wang SP, Wang T, Zhu YL (2015) Ganglioside ef f ect on Nogo-A expression of rat oligodendrocytes in vitro after carbon monoxide poisoning. Zhongguo Zuzhi Gongcheng Yanjiu 19:945-949.
Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z (2002) Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature 417:941-944.
Wettschureck N, Of f eranns S (2002) Rho/Rho-kinase mediated signaling in physiology and pathophysiology. J Mol Med (Berl) 80:629-638.
Wu X, Xu XM (2016) RhoA/Rho kinase in spinal cord injury. Neural Regen Res 11:23-27.
Wu M, Yang HL (2014) Timing principles for spinal cord injury (review). Zhongguo Kangfu Lilun yu Shijian 20:738-741.
Yin MX, Shi SH, Song M, Song JN, Zheng GH, Li ZG (2010) Experimental study on the expression of Nogo-A after spinal cord injury in rats. Shanxi Zhongyi 31:371-373.
Yoshimura T, Arimura N, Kaibuchi K (2006) Molecular mechanisms of axon specification and neuronal disorders. Ann N Y Acad Sci 1086:116-125.
Zhang, Sun ZR, Chen C (2012) Experimental research progress on the mechanism of EA in the treatment of spinal cord injury in the recent 10 years. Zhenjiu Linchuang Zazhi 28:62-65.
Zhou FQ, Snider WD (2006) Intracellular control of developmental and regenerative axon growth. Philos Trans R Soc Lond B Biol Sci 361:1575-1592.
Copyedited by Dawes EA, Hindle A, Wang J, Li CH, Qiu Y, Song LP, Zhao M
How to cite this article: Min YJ, Ding LLQ, Cheng LH, Xiao WP, He XW, Zhang H, Min ZY, Pei J (2017) Ef f ect of electroacupuncture on the mRNA and protein expression of Rho-A and Rho-associated kinase II in spinal cord injury rats. Neural Regen Res 12(2):276-282.
Open access statement: This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Funding: This work was supported by the National Natural Science Foundation of China, No. 81360562.
*Correspondence to:
Li-hong Cheng or
Wei-ping Xiao, M.D.,
495466620@qq.com or
jxxiaowp@163.com.
#These authors contributed
equally to this study.
orcid:
0000-0003-0786-9510
(Li-hong Cheng)
0000-0002-8664-5437
(Wei-ping Xiao)
10.4103/1673-5374.200811 Accepted: 2016-12-29
- 中国神经再生研究(英文版)的其它文章
- Hyperbaric oxygen preconditioning improves postoperative cognitive dysfunction by reducing oxidant stress and inf l ammation
- Nonhuman primate models of focal cerebral ischemia
- The cortical activation pattern during bilateral arm raising movements
- Neuroprotective ef f ect of the Chinese medicine Tiantai No. 1 and its molecular mechanism in the senescence-accelerated mouse prone 8
- Adenyl cyclase activator forskolin protects against Huntington’s disease-like neurodegenerative disorders
- Edaravone protects against oxygen-glucose-serum deprivation/restoration-induced apoptosis in spinal cord astrocytes by inhibiting integrated stress response