ADT类[铁铁]氢化酶全羰基模型物的合成研究进展
李玉龙, 邹立科, 李瑶, 吴宇, 赵培华, 谢斌
ADT类[铁铁]氢化酶全羰基模型物的合成研究进展
李玉龙1,2, 邹立科1,2, 李瑶1,2, 吴宇1,2, 赵培华1, 谢斌1,2
(1.四川理工学院化学与环境工程学院, 四川自贡643000;2.绿色催化四川省高校重点实验室, 四川自贡643000)
自然界中的[铁铁]氢化酶高效催化质子还原产氢的能力为发展生物制氢技术解决能源危机带来了希望。合成[铁铁]氢化酶模型物并研究其催化性质,不仅有助于深入了解[铁铁]氢化酶的催化制氢机理,找到影响制氢效率的关键因素,还能够为设计合成高效、稳定的制氢催化剂提供有效途径,从而推动仿生制氢技术的发展,帮助人类解决所面临的能源紧缺和环境污染等问题。文章系统总结了ADT类[铁铁]氢化酶全羰基模型物的各种制备方法,描述了各种制备方法的优缺点和其应用范围。期望能够加深对ADT类[铁铁]氢化酶模型物这一研究领域的了解,为今后该类模型物的合成研究提供指导。
[铁铁]氢化酶;模型物;合成
引言
氢化酶是一类广泛存在于甲烷菌、醋酸菌、光合细菌和固氮菌等微生物体内的可以可逆地催化氢气氧化与生成的金属蛋白质。根据其所含过渡金属元素组成及结构不同,通常被分为三大类:[铁铁]氢化酶、[铁]氢化酶和[镍铁]氢化酶[1]。由于氢化酶具有高催化质子还原生成氢气的能力,引起科研工作者极大的研究兴趣,从而成为当今的研究热点之一[2]。例如,每摩尔从脱硫脱硫弧菌(Desulfovibrio desulfuricans)中分离得到的[铁铁]氢化酶以每秒9000 mol的速率催化质子还原生成氢气。
Fontecilla-Camps研究小组报道了从脱硫脱硫弧菌(Desulfovibrio desulfuricans ATCC 7757)中分离得到的DdH[铁铁]氢化酶单晶结构[3-4]。通过晶体结构解析发现[铁铁]氢化酶活性中心是由一个无机的[4Fe4S]立方烷子簇和一个[2Fe2S]蝶状子簇两部分构成,两者通过一个半胱氨酸残基的S原子连接起来(图1 )。通过红外光谱等研究发现[铁铁]氢化酶活性中心[2Fe2S]蝶状子簇的两个Fe原子周围含有CO和CN-配体。通过研究[铁铁]氢化酶活性中心的催化过程,科研工作者普遍认为[4Fe4S]立方烷子簇主要起传递电子的作用,而[2Fe2S]蝶状子簇是催化的活性中心。另外值得一提的是[2Fe2S]蝶状子簇中的两个Fe原子通过一个氮杂丙撑二硫桥(ADT,SCH2NHCH2S)结构相连。

图1 [铁铁]氢化酶活性中心结构图
国外Rauchfuss、Darensbourg、孙立成和国内宋礼成、吴骊珠、刘小明等多个研究小组长期致力于对[铁铁]氢化酶模型物的合成研究[5-14],取得了显著的成果,建立起了一系列制备[铁铁]氢化酶模型物的新方法,合成了大量[铁铁]氢化酶模型物。这些模型物根据二硫桥结构不同主要分为两大类:ADT和PDT类(丙撑二硫桥SCH2CH2CH2S)。
本文系统总结了ADT类[铁铁]氢化酶全羰基模型物的各种制备方法,描述了各种制备方法的优缺点和其应用范围。本文的讨论将加深人们对ADT类[铁铁]氢化酶模型物这一研究领域的了解,为今后该类模型物的合成研究提供指导,从而促进未来高效、稳定制氢催化剂的设计合成,推动仿生制氢技术的发展,进一步促进生物无机化学、金属有机化学和铁硫簇合物化学的发展和学科之间的交叉。
1 ADT类[铁铁]氢化酶全羰基模型物的合成
1.1 模型物Fe2[(SCH2NH](CO)6的合成
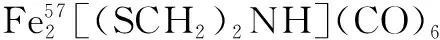
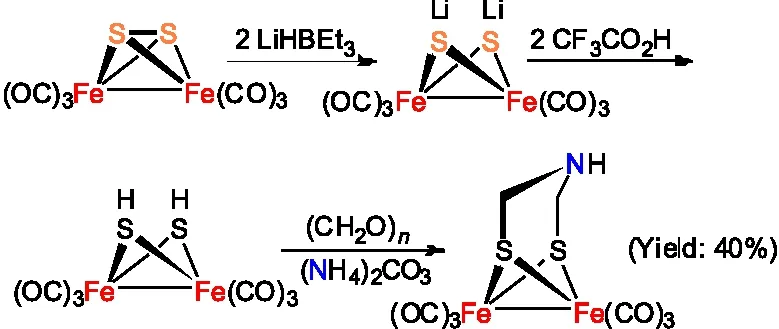
图2 Fe2[(SCH2NH](CO)6的合成路线一
模型物Fe2[(SCH2NH](CO)6还可以通过Fe2(SH)2(CO)6与乌洛托品(六亚甲基四胺)室温搅拌反应制备得到(图3 )[15]。与第一种合成路线相比较,虽然收率稍低(35%),但这种合成方法操作相对简单。
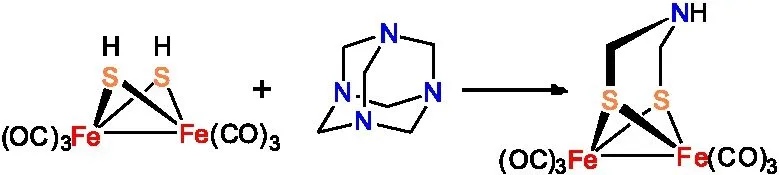
图3 Fe2[(SCH2NH](CO)6的合成路线二
Rauchfuss研究小组还通过将Fe3S2(CO)9、(NH4)2CO3和(CH2O)n等原料进行缩合反应制备得到ADT母体化合物(图4 )[15]。与前面两种合成路线相比较,这种合成方法收率相对较低,仅有28%。
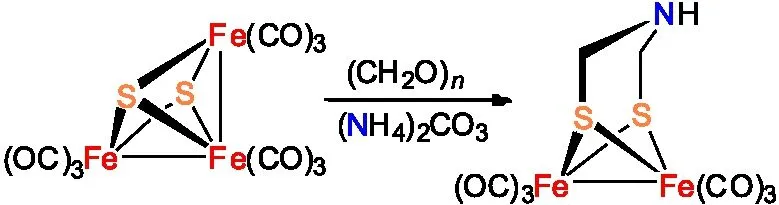
图4 Fe2[(SCH2NH](CO)6的合成路线三
孙立成研究小组设计了一个更多步骤的合成路线,首先合成ADT类[铁铁]氢化酶全羰基模型物Fe
2
[(SCH
2
NSiR
3
](CO)
6
(R
3
=Et
3
,Me
2
(
t
-Bu),(
i
-Pr)
3
),然后通过三氟醋酸脱掉SiR
3
基团分别以10%,32%和36%的收率制备得到模型物Fe
2
[(SCH
2
NH](CO)
6
(图5 )
[17]
。
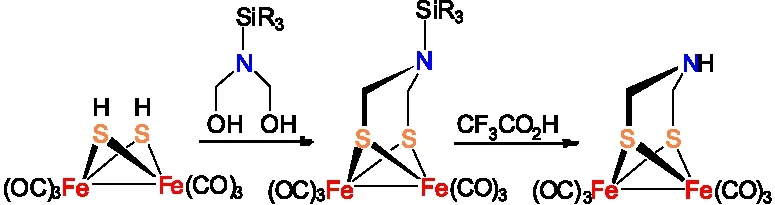
图5 Fe2[(SCH2NH](CO)6的合成路线四
1.2 模型物Fe2[(SCH2NR](CO)6的合成
与模型物Fe2[(SCH2NH](CO)6的合成策略一致,模型物Fe2[(SCH2NR](CO)6可以通过Fe2(SH)2(CO)6与RN(CH2OH)2或(RNCH3反应合成得到(图6 ),这是目前应用最广泛的合成路线之一,部分模型物的收率大于80%[15]。虽然利用Fe2S2(CO)9合成模型物Fe2[(SCH2NH](CO)6的收率较低,但是以Fe2S2(CO)9、t-BuNH2和(CH2O)n为原料合成模型物Fe2[(SCH2N-t-Bu](CO)6时可以得到相对较高的收率(60%)。

图6 Fe2[(SCH2NR](CO)6的合成路线一
模型物Fe2[(SCH2NR](CO)6还可以通过Fe2(SCH2OH)2(CO)6与RNH2缩合反应制备,这种方法与前面合成方法相比较具有操作更加简单、成本更低、原料易得等优点[18]。当R为烷基时,利用该合成路线通常能够得到满意的收率,比如通过Fe2(SCH2OH)2(CO)6与HOCH2CH2NH2制备模型物Fe2[(SCH2NCH2CH2OH](CO)6的收率高达80%。但是当R为芳基时,利用该方法合成ADT类[铁铁]氢化酶全羰基模型物的收率通常较低。
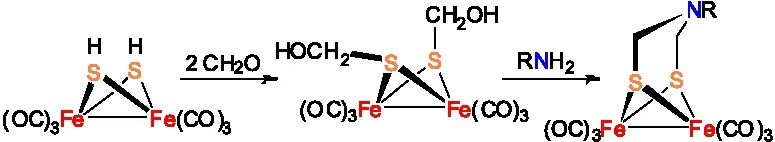
图7 Fe2[(SCH2NR](CO)6的合成路线二
对于R为芳基的ADT类[铁铁]氢化酶全羰基模型物Fe2[(SCH2NR](CO)6经常通过双负离子锂盐Fe2(SLi)2(CO)6与双卤代烃RN(CH2Cl)2缩合反应制备得到(图8 )[11,18-37]。该合成路线相对较长,但是通常能够得到较高的收率。
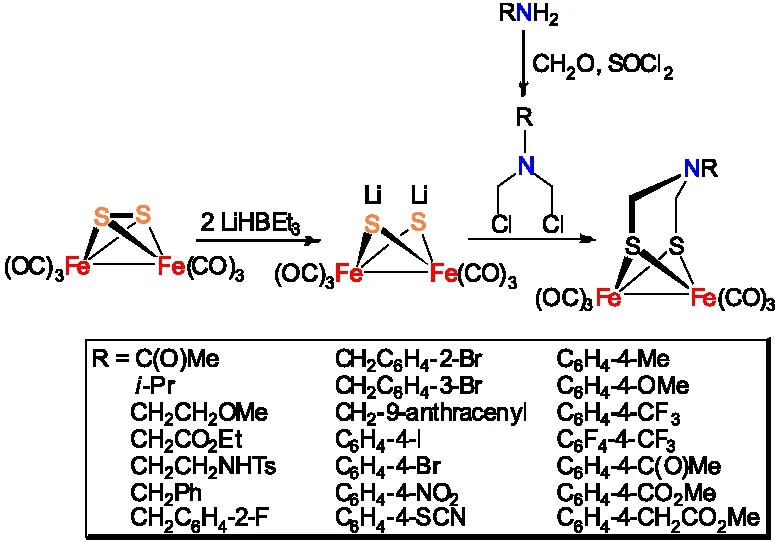
图8 Fe2[(SCH2NR](CO)6的合成路线三
此外,模型物Fe2[(SCH2NR](CO)6还可以通过(MeC5H4)2Ti(SCH2NR与Fe(bda)(CO)3(bda=苄叉丙酮)进行金属转移反应合成得到(图9 )[38]。需要指出的是,这条合成路线相对步骤较多,合成成本也比较高,实际应用范围受到很大限制。
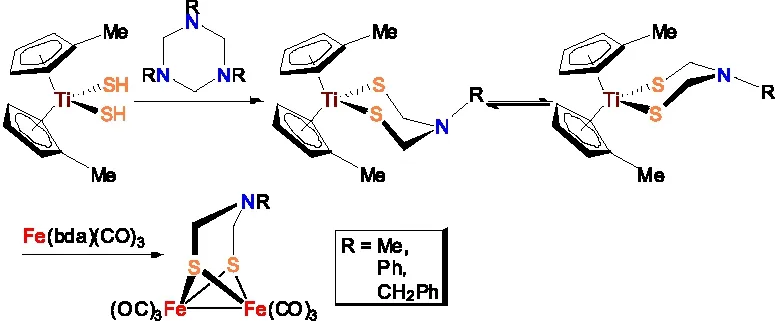
图9 Fe2[(SCH2NR](CO)6的合成路线四
1.3 复杂ADT模型物Fe2[(SCH2NR](CO)6的合成
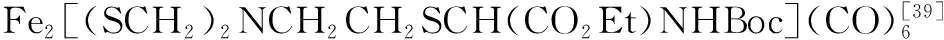
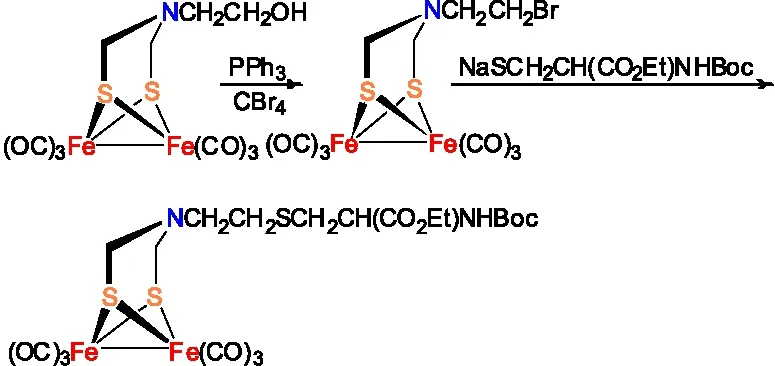
图10 Fe2[(SCH2NCH2CH2OH](CO)6的官能团转化反应
将含有硝基官能团的ADT类模型物Fe2[(SCH2NC6H4-4-NO2](CO)6在Pd/C存在的条件下催化加氢可合成含氨基官能团的ADT类模型物Fe2[(SCH2NC6H4-4-NH2](CO)6(图11 )[19]。
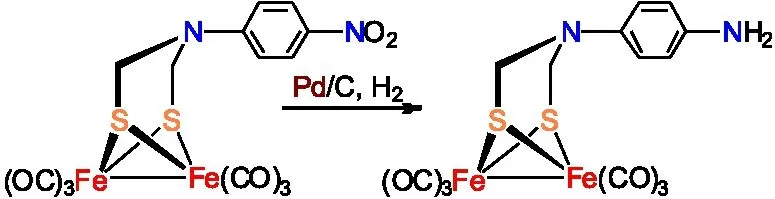
图11 Fe2[(SCH2NC6H4-4-NO2](CO)6的还原反应
孙立成研究小组通过将ADT类[铁铁]氢化酶全羰基模型物Fe2[(SCH2NC6H4-4-I](CO)6先与4-ethynyl-2,2′:6′,2″-terpyridine进行Sonogashira偶联反应,再与Ru(terpy)(DMSO)Cl2反应合成出含有吡啶钌骨架的ADT类[铁铁]氢化酶全羰基模型物(图12 )[20-21]。
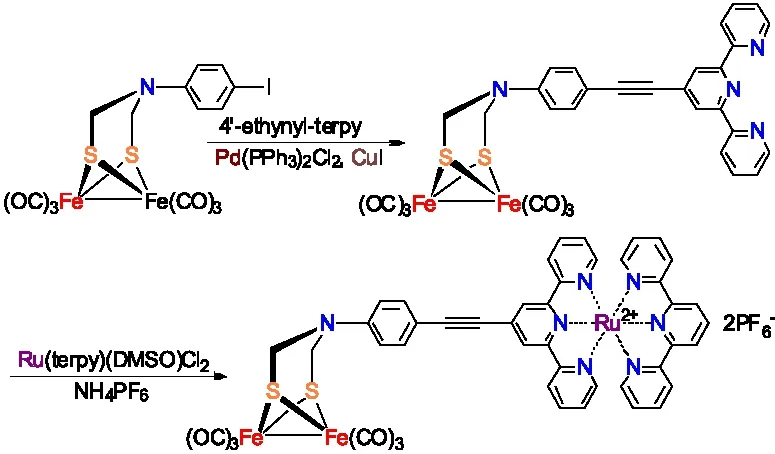
图12 含吡啶钌结构的[铁铁]氢化酶全羰基模型物
为了合成出具有光催化放氢能力的[铁铁]氢化酶模型物,宋礼成研究小组利用ADT类[铁铁]氢化酶全羰基模型物Fe2[(SCH2NC6H4-4-CHO](CO)6设计合成出含有光敏剂卟啉结构的ADT类[铁铁]氢化酶全羰基模型物(图13 )[11]。
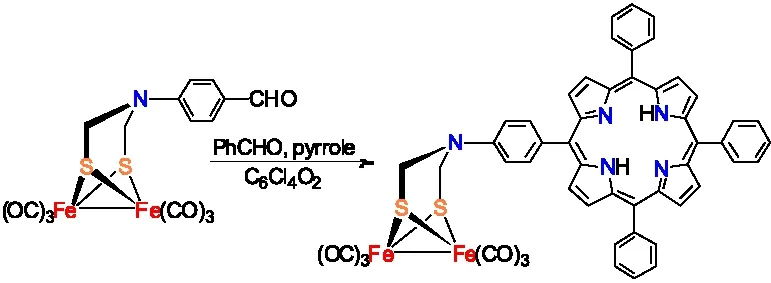
图13 含卟啉结构的[铁铁]氢化酶全羰基模型物
宋礼成研究小组利用吡啶氮原子的配位能力,将ADT母体化合物Fe2[(SCH2NH](CO)6与吡啶酰氯进行缩合反应,再将制备得到的中间体化合物Fe2[(SCH2NC(O)-4-Py](CO)6与金属卟啉ZnTPP进行反应,合成出了含有ZnTPP结构的ADT类[铁铁]氢化酶全羰基模型物(图14)[40]。
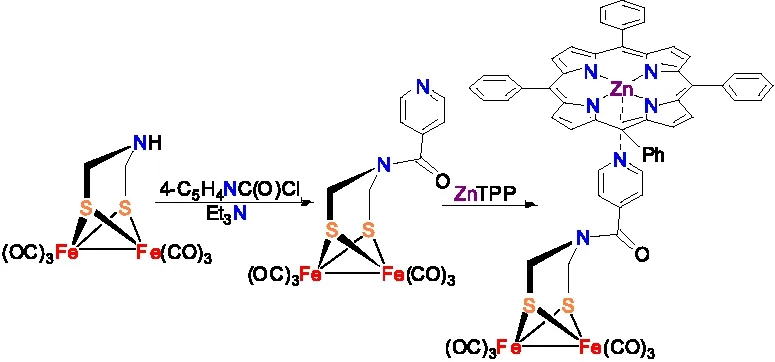
图14含有ZnTPP结构的[铁铁]氢化酶全羰基模型物
2 展望
本文总结了ADT类[铁铁]氢化酶全羰基模型物的各种制备方法,概述了各种制备方法的优缺点和其应用范围。主要方法包括:
(1)以Fe2(SH)2(CO)6、(NH4)2(CO)3和(CH2O)n等为原料,根据Sharpless等基于Mannich反应原理制备。
(2)通过双负离子锂盐Fe2(SLi)2(CO)6与双卤代烃RN(CH2Cl)2缩合反应制备。
本文还介绍了通过简单ADT类[铁铁]氢化酶全羰基模型物制备复杂结构ADT类[铁铁]氢化酶全羰基模型物的方法。希望通过本文的介绍能够加深人们对ADT类[铁铁]氢化酶模型物这一研究领域的了解,为今后该类模型物的合成研究提供指导。
众所周知,近年来围绕[铁铁]氢化酶活性中心结构和功能模拟而展开的仿生化学研究取得了一定的成果,促进了与之相关的生物无机化学、无机配位化学和金属有机化学等学科的迅速发展。未来对于[铁铁]氢化酶仿生化学研究将进一步围绕如何提高[铁铁]氢化酶模型物的催化制氢效率,如何利用[铁铁]氢化酶模型物实现光催化高效制氢,如何为设计制氢效率高、成本低的制氢催化剂提供理论与实践基础等方面的问题展开。可以预见,对于[铁铁]氢化酶的仿生化学研究将会得到更加振奋人心的结果,为新型高效、稳定的制氢催化剂的设计合成提供新的思路和途径,推动仿生制氢技术的发展,帮助人类解决所面临的能源危机和环境污染等问题。
[1]LUBITZ W,OGATA H,RUDIGER O,et al.Hydrogenases.Chemical Reviews,2014,114:4081-4148.
[2]TARD C,PICKETT C.Structural and functional analogues of the active sites of the,and -hydrogenases.Chemical Reviews,2009,109:2245-2274.
[3]PETERS J W,LANZILOTTA W N,LEMON B J,et al.X-Ray crystal structure of the Fe-only hydrogenase(Cpl)from clostridium pasteurianum to 1.8 angstrom resolution.Science,1998,282:1853-1858.
[4]NICOLET Y,PIRAS C,LEGRAND P,et al.Desulfovibrio desulfuricans iron hydrogenase:The structure shows unusual coordination to an active site Fe binuclear center.Structure,1999,7:13-23.
[5]LI Y L,RAUCHFUSS T B.Synthesis of diiron(I)dithiolato carbonyl complexes.Chemical Reviews,2016,116:7043-7077.
[6]WANG W,NILGES M J,RAUCHFUSS T B,et al.Isolation of a mixed valence diiron hydride:evidence for a spectator hydride in hydrogen evolution catalysis.Journal of the American Chemical Society,2013,135:3633-3639.
[7]WANG W,RAUCHFUSS T B,ZHU L,et al.New reactions of terminal hydrides on a diiron dithiolate.Journal of the American Chemical Society,2014,136:5773-5782.
[8]BETHEL R D,DARENSBOURG M Y.Bioinorganic chemistry:Enzymes activated by synthetic components.Nature,2013,499:40-41.
[9]EKSTROM J,ABRAHAMSSON M,OLSON C,et al.Bio-inspired,side-on attachment of a ruthenium photosensitizer to an iron hydrogenase active site model.Dalton Transactions,2006,4599-4606.
[10]ZHENG D H,WANG N,WANG M,et al.Intramolecular iron-mediated C-H bond heterolysis with an assist of pendant base in a -Hydrogenase model.Journal of the American Chemical Society,2014,136:16817-16823.
[11]SONG L C,TANG M Y,SU F H,et al.A biomimetic model for the active site of iron-only hydrogenases covalently bonded to a porphyrin photosensitizer.Angewandte Chemie International Edition,2006,45:1130-1133.
[12]WANG F,LIANG W J,JIAN J X,et al.Exceptional poly(acrylic acid)-based artificial -hydrogenases for photocatalytic H2production in water.Angewandte Chemie International Edition,2013,52:8134-8138.
[13]SONG L C,WANG L X,YIN B S,et al.The N-acylated derivatives of parent complex as active site models of Fe-only Hydrogenases:synthesis,characterization,and related properties.European Journal of Inorganic Chemistry,2008,2:291-297.
[14]XIAO Z Y,WEI Z H,LI L,et al.Diiron carbonyl complexes possessing a {Fe(II)Fe(II)} core:synthesis,characterisation,and electrochemical investigation.Dalton Transactions,2011,40:4291-4299.
[15]LI H X,RAUCHFUSS T B.Iron carbonyl sulfides,formaldehyde,and amines condense to give the proposed azadithiolate cofactor of the Fe-only hydrogenases.Journal of the American Chemical Society,2002,124:726-727.
[16]GILBERT-WILSON R,SIEBEL J F,ADAMSKA-VENKATESH A,et al.Spectroscopic investigations of hydrogenase maturated with 57Fe2(adt)(CN)2(CO)2-.Journal of the American Chemical Society,2015,137:8998-9005.
[17]WANG Z,LIU J H,HE C J,et al.Azadithiolates cofactor of the iron-only hydrogenase and its PR3-monosubstituted derivatives:synthesis,structure,electrochemistry and protonation.Journal of Organometallic Chemistry,2007,692:5501-5507.
[18]STANLEY J L,RAUCHFUSS T B,WILSON S R.Studies on the condensation pathway to and properties of diiron azadithiolate carbonyls.Organometallics,2007,26:1907-1911.
[19]LIU T B,WANG M,SHI Z,et al.Synthesis,structures and electrochemical properties of nitro- and amino-functionalized diiron azadithiolates as active site models of Fe-only hydrogenases.Chemistry-A European Journal,2004,10:4474-4479.
[20]OTT S,KRITIKOS M,AKERMARK B,et al.Synthesis and structure of a biomimetic model of the iron hydrogenase active site covalently linked to a ruthenium photosensitizer.Angewandte Chemie International Edition,2003,42:3285-3288.
[21]OTT S,BORGSTROM M,KRITIKOS M,et al.Model of the iron hydrogenase active site covalently linked to a ruthenium photosensitizer:synthesis and photophysical properties.Inorganic Chemistry,2004,43:4683-4692.
[22]SI G,WU L Z,WANG W G,et al.Synthesis,structure and electrochemical property of diphenylacetypene-substituted diiron azadithiolates as active site of Fe-only hydrogenases.Tetrahedron Letters,2007,48:4775-4779.
[23]GAO W M,LIU J H,MA C B,et al.Synthesis,structures and electrochemical properties of amino-derivatives of diiron azadithiolates as active site models of Fe-only hydrogenase.Inorganica Chimica Acta,2006,359:1071-1080.
[24]DONG W B,WANG M,LIU X Y,et al.An insight into the protonation property of a diiron azadithiolate complex pertinent to the active site of Fe-only hydrogenases.Chemical Communications,2006,305-307.
[27]SONG L C,GE J H,LIU X F,et al.Synthesis,Structure and Electrochemical Properties of N-Substituted Diiron Azadithiolates as Active Site Models of Fe-Only Hydrogenases.J Organomet Chem,2006,691,5701-5709.[28]SONG L C,GE J H,ZHANG X G,et al.Methoxyphenyl-functionalized diiron azadithiolates as models for the active site of Fe-only hydrogenases:synthesis,structures,and biomimetic H2evolution.European Journal of Inorganic Chemistry,2006,3204-3210.
[29]STANLEY J L,HEIDEN Z M,RAUCHFUSS T B,et al.Desymmetrized diiron azadithiolato carbonyls:a step toward modeling the iron-only hydrogenases.Organometallics,2008,27:119-125.
[30]HOU J,PENG X J,LIU J F,et al.A binuclear isocyanide azadithiolatoiron complex relevant to the active site of Fe-only hydrogenases:synthesis,structure and electrochemical properties.European Journal of Inorganic Chemistry,2006,4679-4686.
[31]WANG W G,WANG H Y,SI G,et al.Fluorophenyl-substituted Fe-only hydrogenases active site ADT models:different electrocatalytic process for proton reduction in HOAc and HBF4/Et2O.Dalton Transactions,2009,2712-2720.
[32]VIJAIKANTH V,CAPON J F,GLOAGUEN F,et al.Carboxy-functionalized dithiolate di-iron complexes related to the active site of Fe-only hydrogenase.Journal of Organometallic Chemistry,2007,692:4177-4181.
[33]GAO S,DUAN Q,JIANG D Y.{μ-2-(1,3-Benzothiazol-2-yl)phenyl〗-2-azapropane-1,3-dithiolato-k4S,S':S,S'}bisicarbonyliron(I)〗.Acta Crystallorgraphica Section E,2012,68:m248.
[34]GAO S,DUAN Q,JIANG D Y.{μ-2-(Benzothiazol-2-yl)benzyl〗-2-azapropane-1,3-dithiolato-1:2k4S,S':S,S'}bisicarbonyliron(I)〗.Acta Crystallorgraphica Section E,2012,68:m315.
[35]GAO S,PENG X J.2-(9-Anthrylmethyl)-2-azapropane-1,3-dithiolato-k4S:S'〗bisicarbonyliron(I)〗.Acta Crystallorgraphica Section E,2007,63:m1997.
[36]CAPON J F,EZZAHER S,GLOAGUEN F,et al.Electrochemical and theoretical investigations of the reduction of2(CO)5L{μ-SCH2XCH2S}〗 complexes related to hydrogenase.New Journal of Chemistry,2007,31:2052-2064.
[37]DEY S,RANA A,DEY S G,et al.Electrochemical hydrogen production in acidic water by an azadithiolate bridged synthetic hydrogenese mimic:role of aqueous solvation in lowering overpotential.ACS Catalysis,2013,3:429-436.
[38]ANGAMUTHU R,CARROLL M E,RAMESH M,et al.A new route to azadithiolato complexes.European Journal of Inorganic Chemistry,2011,1029-1032.
[39]SONG L C,YIN B S,LI Y L,et al.Synthesis,structural characterization,and some properties of new N-functionally substituted diiron azadithiolate complexes as biomimetic models of iron-only hydrogenases.Organometallics,2007,26:4921-4929.
[40]SONG L C,TANG M Y,MEI S Z,et al.The active site model for iron-only hydrogenases coordinatively bonded to a metalloporphyrin photosensitizer.Organometallics,2007,26:1575-1577.
Progress in the Synthesis of Diiron Azadithiolato Hexcarbonyls as Models for the [FeFe]-Hydrogenase
LIYulong1,2,ZOULike1,2,LIYao1,2,WUYu1,2,ZHAOPeihua1,XIEBin1,2
(1.School of Chemistry and Environmental Engineering, Sichuan University of Science & Engineering, Zigong 643000,China;2.Key Laboratory of Green Catalysis of Sichuan Institute of High Education, Zigong 643000, China)
[FeFe]-Hydrogenase brings the hope to develop biological hydrogen production technology and solve the problem of energy crisis because of its catalytic ability and high efficiency of reduction of proton. Studies on the syntheses and catalytic properties of [FeFe]-hydrogenase models will not only enrich a systematic understanding of the mechanism of enzyme-catalyzed reaction of producing hydrogen and find the key factors of affecting catalytic efficiency, but also provide an effective route to design and synthesize high efficient and stable catalysts for hydrogen production. Furthermore, it will promote the development of biological hydrogen production technology and help us to solve the problems of energy crisis and environmental pollution. This paper systematically summarizes the syntheses of diiron azadithiolato hexcarbonyls as active site models for the [FeFe]-hydrogenase, and the various advantages as well as disadvantages of those methods. This review is anticipated to deepen the understanding of the research field where diiron azadithiolato hexcarbonyls as active site models for the [FeFe]-hydrogenase and hence provide guidance for the syntheses of those models.
[FeFe]-hydrogenase; models; synthesis
2016-09-06
国家自然科学基金项目(21501124);四川省科技厅项目(16JC0099);自贡市科技局项目(2014HX02);材料腐蚀与防护四川省重点实验室项目(2014CL14);四川理工学院引进人才项目(2014RC05)
李玉龙(1983-),男,四川遂宁人,副教授,博士,主要从事仿生物酶制氢催化剂方面的研究,(E-mail)yu_longli@aliyun.cn
1673-1549(2017)01-0001-07
10.11863/j.suse.2017.01.01
O614.121;O625.621
A

