Snail基因沉默对胰腺癌PANC1细胞侵袭和增殖的影响
杨静 吴洪玉 李理想 任洪波
·论著·
Snail基因沉默对胰腺癌PANC1细胞侵袭和增殖的影响
杨静 吴洪玉 李理想 任洪波
目的 观察Snail基因沉默后对胰腺癌PANC1细胞侵袭和增殖能力的影响。方法 构建针对Snail的小发卡RNA(shRNA-Snail)的慢病毒载体和不针对任何已知mRNA序列的shRNA(shRNA-NC)慢病毒载体,分别感染PANC1细胞,以未感染细胞作为对照组。应用实时荧光定量PCR法和蛋白质印迹法检测Snail、α-平滑肌肌动蛋白(α-SMA)及E-钙黏素(E-cadherin)mRNA和蛋白的表达;Transwell小室检测细胞体外侵袭能力,CCK-8法检测细胞增殖能力。结果 与shRNA-NC组比较,shRNA-Snail组细胞Snail mRNA和蛋白表达水平显著下降[(0.27±0.02)比(0.92±0.03),(0.26±0.02)比(0.80±0.02)],α-SMA mRNA和蛋白表达水平亦显著下降[(0.33±0.04)比(0.97±0.07),(0.31±0.04)比(0.74±0.06)],E-cadherin mRNA和蛋白表达水平则显著升高[(1.57±0.45)比(0.95±0.08),(0.86±0.03)比(0.20±0.03)],穿膜细胞数显著减少[(6.80±0.73)比(26.80±2.52)个/400倍视野],细胞增殖明显被抑制[(0.74±0.05)比(1.47±0.04)],差异均有统计学意义(P值均<0.01)。shRNA-NC组与对照组细胞各指标的差异均无统计学意义。结论 沉默Snail基因表达可在一定程度上抑制胰腺癌PANC1细胞的侵袭和增殖能力。
胰腺肿瘤; Snail因子; RNA,小分子干扰; 肿瘤转移; 细胞增殖
Fund program:Outstanding Yong and Middle-aged Scientific Research Fund of Shandong Province(BS2011YY023)
转录因子Snail可通过抑制E-钙黏素(E-cadherin)的表达促进上皮-间质转化(epithelial-to-mesenchymal transition,EMT)及细胞存活,促进肿瘤的增殖、侵袭和扩散[1]。已有研究证明,伴随淋巴结和远处转移的胰腺癌组织高表达Snail[2]。将Snail基因转染不表达Snail的高分化胰腺癌细胞BxPC3,结果该株细胞发生EMT,且在体内的生长能力和侵袭性明显增强[3]。本研究通过shRNA-慢病毒载体干扰技术沉默胰腺癌PANC1细胞Snail基因表达,观察其对细胞侵袭和增殖的影响。
材料与方法
一、材料与试剂
人胰腺癌细胞株PANC1由第二军医大学长海医院消化内科实验室惠赠;胰酶、胎牛血清及DMEM培养液购自美国Gibco公司;CCK-8试剂盒购自日本同仁公司;Transwell小室购自美国Corning公司;实时荧光定量PCR相关试剂均购自日本Takara公司;相关引物由Invitrogen公司设计合成;羊抗人Snail多克隆抗体(sc-10432)、兔抗人E-cadherin多克隆抗体(sc-7870)、羊抗人GAPDH单克隆抗体以及辣根过氧化物酶标记相关二抗均购自Santa Cruz 公司。
二、shRNA合成与慢病毒载体构建
体外合成针对Snail的shRNA(shRNA-Snail),正义序列为5′-GATCCGGCCACTCAGATGTCAA-GAAGTAGGTACCTACTTCTTGACATCTGAGTGGTTT-TTG-3′,反义序列为5′-AATTCAAAAACCACTCAGATGTCAAGAAGTA-GGTACCTACTTCTTGACATCTGAGTGGCCG-3′,两端分别带EcoRⅠ和BamHⅠ酶切位点;另设不针对任何已知mRNA序列的阴性对照shRNA(shRNA-NC)。采用双酶切方法将shRNA插入质粒载体,重组质粒转化GeneHogs化学感受态细菌,常规扩增、收集细菌,提取质粒mRNA,应用RT-PCR鉴定插入片段正确后应用慢病毒载体包装重组质粒,按试剂盒说明书操作,然后将慢病毒表达载体感染293T细胞,收集上清病毒悬液,分装保存。
三、细胞感染与分组
PANC1细胞常规培养传代。取104个细胞接种96孔板,培养24 h待细胞融合达80%时换含有5 μg/ml Polybrene的新鲜培养液,分别加入插有Snail-shRNA、shRNA-NC的慢病毒感染24 h,更换新鲜培养液继续培养72 h,以未感染慢病毒的细胞作为对照组。荧光显微镜下观察荧光信号,荧光细胞超过90%视为感染成功。
四、实时荧光定量PCR检测
提取各组感染细胞总RNA,先反转录为cDNA,再行实时荧光定量PCR。Snail、α-SMA、E-cadherin及内参GAPDH引物序列如下:Snail正义序列5′-TCGGAAGCCTAACTACAGCGA-3′,反义序列5′-AGATGAGCATTGGCAGCGAG-3′;α-SMA正义序列5′-CCAGCTATGTGAAGAAGAAGAGG- 3′,反义序列5′-GTGATCTCCTTCTGCATTCGGT-3′;E-cadherin正义序列5′ -ATCCAAAGCCTCAGGTCATAAACA- 3′,反义序列5′-AAGAAACAGCAAGAGCAGCAGAAT-3′;GAPDH正义序列5′-CGGGAAACTGTGGCGTGAT-3′,反义序列5′-CAAAGGTGGAGGAGTGGGT-3′。PCR反应体系:2×荧光定量SYBR Green mix 10 μl,上下游引物各0.5 μl,DyeⅡ0.4 μl,cDNA 5 μl,加双蒸水至20 μl。PCR反应条件:95℃ 10 min;95℃ 15 s、60℃ 30 s、72℃ 30 s,40个循环;最后72℃延伸10 min。每个样本设3个复孔。采用公式2-ΔΔCt计算mRNA相对表达量。
五、蛋白质印迹法检测
收集各组感染细胞,离心并用预冷PBS冲洗后加入RIPA及蛋白酶抑制剂置冰上裂解细胞,所得细胞匀浆以12 000 r/min 4℃离心20 min,取上清定量蛋白。取30 μg蛋白常规行蛋白质印迹法检测Snail、α-SMA、E-cadherin蛋白表达,以GAPDH为内参,实验重复3次。应用ImageJ软件扫描各条带灰度值,以目的条带与内参条带灰度值的比值表示蛋白相对表达量。
六、细胞侵袭实验
采用聚碳酸酯膜孔径为8 μm的Transwell小室,小室隔膜表面预先用Matrigel包被后分别在上室和下室加入无血清DMEM培养液4℃过夜,弃培养液。取各组对数生长期细胞,用PBS和无血清培养液先后洗涤1次,以无血清培养液重悬细胞,调整细胞密度为5×104/L。上室加细胞悬液100 μl,下室加含10%血清的培养液600 μl,培养24 h后取出小室,用棉签擦掉膜上方的Matrigel及未穿膜细胞,置95%乙醇溶液中固定10 min,结晶紫染色8 min,封固于载玻片上。显微镜下随机取8个高倍镜视野(400倍),计数每个视野的穿膜细胞。实验重复3次,取均值。
七、CCK-8法检测
取各组对数生长期细胞,以5×103个细胞/孔接种96孔板(100 μl),每组设4个复孔,置37℃、5% CO2条件下培养4 h,加入10 μl CCK-8溶液,继续培养4 h,上酶标仪测定各孔450 nm处的吸光度值(A450值)。实验重复3次,取均值。
八、统计学处理
结 果
一、各组PANC1细胞Snail、α-SMA、E-cadherin mRNA表达
shRNA-Snail组、shRNA-NC组、对照组PANC1细胞的Snail mRNA表达量分别为0.27±0.02、0.92±0.03、0.93±0.04;α-SMA mRNA表达量为0.33±0.04、0.97±0.07、0.98±0.06;E-cadherin mRNA为1.57±0.45、0.95±0.08、0.96±0.05。shRNA-Snail组Snail、α-SMA的mRNA表达量较shRNA-NC组显著下降(t值分别为19.66、7.97),而E-cadherin mRNA表达量显著增加(t=6.77),差异均有统计学意义(P值均<0.01)。shRNA-NC组与对照组间的差异均无统计学意义。
二、各组PANC1细胞Snail、α-SMA、E-cadherin蛋白表达
shRNA-Snail组、shRNA-NC组、对照组PANC1细胞的Snail 蛋白表达量分别为0.26±0.02、0.80±0.02、0.83±0.03;α-SMA蛋白为0.31±0.04、0.74±0.06、0.82±0.02;E-cadherin蛋白为0.86±0.03、0.20±0.03、0.19±0.01。shRNA-Snail组Snail蛋白、α-SMA蛋白表达量较shRNA-NC组显著下降(t值分别为19.02、5.85),而E-cadherin蛋白表达量显著增加(t=14.62),差异均有统计学意义(P值均<0.01)。shRNA-NC组与对照组间的差异均无统计学意义。
三、各组PANC1细胞体外侵袭能力
shRNA-Snail组、shRNA-NC组、对照组PANC1细胞的穿膜细胞数分别为(6.80±0.73)、(26.80±2.52)、(31.20±2.48)个/400倍视野(图1),shRNA-Snail组较shRNA-NC组显著减少,差异有统计学意义(t=7.63,P<0.01),而shRNA-NC组与对照组之间差异无统计学意义。
四、各组PANC1 细胞的增殖能力
shRNA-Snail组、shRNA-NC组、对照组PANC1细胞培养4 h后A450值分别为0.74±0.05、1.47±0.04、1.68±0.01。shRNA-Snail组细胞增殖较shRNA-NC组显著被抑制,差异有统计学意义(t=11.23,P<0.01),而shRNA-NC组与对照组之间差异无统计学意义。
讨 论
胰腺癌是消化系统恶性度极高的肿瘤,总体病死率高达85%[4],主要原因是其具有高侵袭性,早期即可发生转移[5-7]。EMT能使细胞失去极性和细胞间连接,获得游走能力,是胰腺癌发生侵袭转移的主要生物学机制[8]。Yamada等[9]报道,EMT可作为预测胰腺癌预后的独立危险因素。EMT过程的始动因素为E-cadherin表达缺失[10]。转录因子Snail作为E-cadherin的直接抑制子促进EMT的发生[11-14],被视为EMT的关键控制因素。
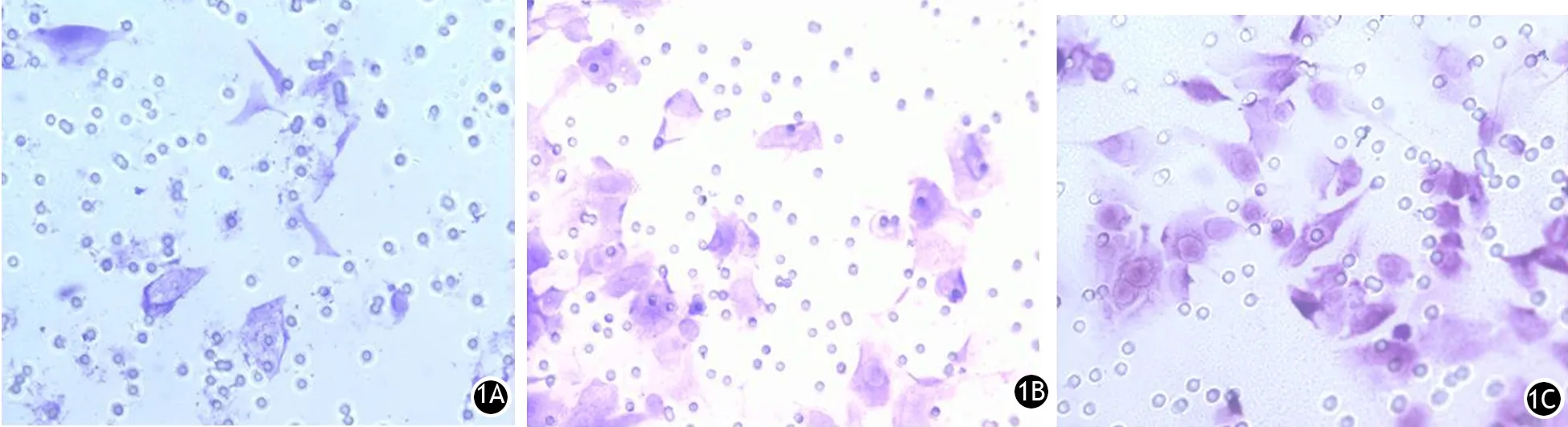
图1 shRNA-Snail组(1A)、shRNA-NC组(1B)、对照组(1C)的PANC1细胞侵袭能力(×400)
Tao和Tagare[15]报道,约36%的胰腺癌Snail 阳性表达,同时伴随E-cadherin低表达,而且有淋巴结转移和远处转移的胰腺癌的Snail阳性表达率明显升高。Hotz等[10]报道,低分化的胰腺癌细胞株(如PANC1)Snail的阳性表达率明显高于高分化的胰腺癌细胞株(如HPAF-2、AsPC-1),而E-cadherin表达则被抑制。 Nishioka等[3]将Snail基因转染不表达Snail的高分化胰腺癌细胞株BxPC3,结果该细胞株的细胞形态发生了EMT,将转染Snail基因的细胞种植于裸鼠后,种植瘤的侵袭性明显增强,早期即发生转移。此外有报道,抑制Snail表达可以提高胰腺癌对放化疗的敏感性[16]。
本研究选择低分化的PANC1胰腺癌细胞株进行实验,结果显示PANC1细胞Snail基因高表达,与Hotz等[10]的研究一致。应用插入shRNA-Snail的慢病毒载体沉默PANC1细胞Snail基因表达后,该细胞的α-SMA表达显著下降,而E-cadherin表达显著升高,说明抑制Snail表达后细胞恢复上皮表型。此外,沉默Snail基因表达后细胞的增殖及侵袭能力下降,与α-SMA和E-cadherin表达变化趋势相吻合。由于本研究仅应用PANC1一种细胞株,因此尚不能说明在所有胰腺癌细胞中抑制Snail表达均可以作为有效的干预措施。
[1] Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer[J]. Development, 2005,132(14):3151-3161.DOI:10.1242/dev.01907.
[2] Zhuo W, Wang Y, Zhuo X, et al. Knockdown of Snail, a novel zinc finger transcription factor, via RNA interference increases A549 cell sensitivity to cisplatin via JNK/mitochondrial pathway[J]. Lung cancer, 2008,62(1):8-14.DOI: 10.1016/j.lungcan.2008.02.007.
[3] Nishioka R, Itoh S, Gui T, et al. SNAIL induces epithelial-to-mesenchymal transition in a human pancreatic cancer cell line (BxPC3) and promotes distant metastasis and invasiveness in vivo[J]. Exp Mol Pathol, 2010,89(2):149-157.DOI: 10.1016/j.yexmp.2010.05.008.
[4] Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths[J]. CA Cancer J Clin, 2011,61(4):212-236.DOI:10.3322/caac.20121.
[5] Singh D, Upadhyay G, Srivastava RK, et al. Recent advances in pancreatic cancer: biology, treatment, and prevention[J]. Biochim Biophys Acta, 2015,1856(1):13-27.DOI:10.1016/j.bbcan.2015.04.003.
[6] 赵玉沛.外科医师要重视胰腺癌的临床研究[J].中华消化外科杂志,2016,15(6):534-536.DOI:10.3760/cma.j.issn.1673-9752.2016.06.002.
[7] 张太平,曹喆,赵玉沛.胰腺癌的化疗与放疗[J].中华消化外科杂志,2015,14(8):619-622.DOI:10.3760/cma.j.issn.1673-9752.2015.08.006.
[8] 刘志容. 上皮-间质转化及其调控基因Snail在肿瘤侵袭转移中的作用[J]. 中国普通外科杂志, 2010,19(8):916-920.
[9] Yamada S, Fuchs BC, Fujii T, et al. Epithelial-to-mesenchymal transition predicts prognosis of pancreatic cancer[J]. Surgery, 2013,154(5):946-954.DOI: 10.1016/j.surg.2013.05.004.
[10] Hotz B, Arndt M, Dullat S, et al. Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer[J]. Clin Cancer Res, 2007,13(16):4769-4776.DOI: 10.1158/1078-0432.CCR-06-2926.
[11] Batlle E, Sancho E, Franci C, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells[J]. Nat Cell Biol,2000,2(2):84-89.DOI:10.1038/35000034.
[12] Cano A, Perez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression[J]. Nat Cell Biol, 2000,2(2):76-83.DOI: 10.1038/35000025.
[13] Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression[J]. Curr Opin Cell Biol,2005,17(5):548-558.DOI:10.1016/j.ceb.2005.08.001.
[14] Takano S, Kanai F, Jazag A, et al. Smad4 is essential for down-regulation of E-cadherin induced by TGF-beta in pancreatic cancer cell line PANC-1[J]. J Biochem, 2007,141(3):345-351. DOI: 10.1093/jb/mvm039.
[15] Tao Z, Tagare HD. Tunneling descent level set segmentation of ultrasound images[J]. Inf Process Med Imaging, 2005, 19:750-761.
[16] Zhang K, Jiao X, Liu X, et al. Knockdown of snail sensitizes pancreatic cancer cells to chemotherapeutic agents and radiation[J]. Int J Mol Sci, 2010,11(12):4891-4904. DOI:10.3390/ijms11124891.
(本文编辑:冀凯宏)
The effect of Snail gene silencing on cell invasion and proliferation in human pancreatic cancer cell line PANC1
YangJing,Wuhongyu,LiLixiang,RenHongbo.
DepartmentofGastroenterology,QiluHospital,ShandongUniversity,Jinan250012,China
RenHongbo,Email:rhb2229@medmail.com.cn
Objective To observe the effect of silencing Snail gene on the invasion and proliferation ability of human pancreatic cancer cell line PANC1. Methods Lentiviral vectors that can express small hairpin RNA(shRNA) targeting human Snail gene(shRNA-Snail) or shRNA sequence that did not match any known mRNA(shRNA-NC) were constructed,and transfected into PANC1 cells. Untransfected cells served as control. mRNA and protein expression of Snail,α-smooth muscle actin(α-SMA) and E-cadherin was determined by real time quantitative PCR and Western blotting, respectively. In vitro invasion ability was tested by Transwell model. Proliferation ability was measured by CCK-8 assay. Results Compared with those in shRNA-NC group, Snail mRNA (0.27±0.02vs0.92±0.03) and protein level (0.26±0.02vs0.80±0.02),and α-SMA mRNA (0.33±0.04vs0.97±0.07) and protein level (0.31±0.04vs0.74±0.06) in shRNA-Snail group were obviously decreased, but E-cadherin mRNA (1.57±0.45vs0.95±0.08) and protein level (0.86±0.03vs0.20±0.03) were greatly increased. The number of cells permeating the septum of transwell [(6.80±0.73)/400 magnificationvs(26.80±2.52)/400 magnification,P<0.01] was significantly decreased, and cell proliferation was inhibited(0.74±0.05vs1.47±0.04,P<0.01). All the differences above were statistically significant (allP<0.01). No significant differences were observed between shRNA-NC and normal control group. Conclusions Silencing Snail gene may restrain the invasion and proliferation ability of PANC1 cells to a certain degree.
Pancreatic neoplasms; Transcriptional factor Snail; RNA, Small interfering; Neoplasms metastasis; Cell proliferation
10.3760/cma.j.issn.1674-1935.2017.01.003
250012 济南,山东大学齐鲁医院消化内科(杨静、李理想、任洪波);第二军医大学长海医院消化内科(吴洪玉)
任洪波,Email: rhb2229@medmail.com.cn
山东省优秀中青年科研奖励基金(BS2011YY023)
2015-10-23)

