干旱和臭氧浓度升高对元宝枫早生和晚生叶片色素和脱落酸含量的影响
李 丽, 牛俊峰, 文 志, 崔 健, 王效科,*
1 中国科学院生态环境研究中心城市与区域国家重点实验室, 北京 100085 2 中国科学院华南植物园, 广州 510650 3 中国农业科学院农业资源与农业区划研究所, 北京 100081
干旱和臭氧浓度升高对元宝枫早生和晚生叶片色素和脱落酸含量的影响
李 丽1, 牛俊峰2, 文 志1, 崔 健3, 王效科1,*
1 中国科学院生态环境研究中心城市与区域国家重点实验室, 北京 100085 2 中国科学院华南植物园, 广州 510650 3 中国农业科学院农业资源与农业区划研究所, 北京 100081
臭氧和干旱是威胁我国北方城市植物生长的两大重要因素。于2012年利用开顶式气室,通过设置4个处理(AW-大气环境和水分充足;AW+60-大气增加60 nL/L臭氧+水分充足;AD-大气+干旱处理;AD+60-大气增加60 nL/L臭氧+干旱处理),开展了大气臭氧浓度升高(以下简称“臭氧”)和干旱对元宝枫秋季变色期主要色素含量及脱落酸(ABA)含量的影响研究。结果表明:(1)早生叶在臭氧处理后,总叶绿素和类胡萝卜素分别下降了21%和29.6%、花青苷和类黄酮相对含量显著升高了34.1%和7.3%、脱落酸含量增加了19.8%。干旱处理后,早生叶总叶绿素显著下降了18.7%、花青苷和类黄酮相对含量分别显著升高了37%和7.4%、脱落酸含量显著升高了13%。叶片的上述生理变化将会导致叶片提前变红、叶片早衰和提前脱落。(2)晚生叶在干旱处理后总叶绿素含量减少了18.8%,脱落酸含量增加了33.4%,臭氧以及与干旱共同处理未对晚生叶产生显著影响。(3)臭氧和干旱共同处理后,早生叶总叶绿素含量、花青苷和叶片脱落酸含量存在显著交互作用,交互作用缓解了叶片总叶绿素的下降和花青苷的上升,但未缓解叶片脱落酸含量的增加。综上,早生叶和晚生叶对臭氧和干旱处理的响应不同,早生叶对臭氧处理响应大于晚生叶,而晚生叶对干旱更敏感。臭氧和干旱处理均加速了叶片衰老,二者共同处理后叶片脱落风险增加。
元宝枫;臭氧;干旱;光合色素;花青苷;类黄酮;脱落酸
臭氧是一种重要的具有氧化性的污染气体,过高的浓度会威胁植物的生长、农作物产量及人类健康[1- 2]。由于化石燃料的使用和城市化的快速发展,大量臭氧前体物体NOx被排放到大气中,导致了我国各大城市空气中臭氧浓度不断升高[3- 5]。据文献报道,2012年北京城郊7—8月份臭氧极值达到195.8 nL/L[6],如此高的臭氧浓度将会对植物生长造成伤害,如叶片出现黄褐色、黑色或紫红色集中于叶脉两侧的密集斑点,在北京城区及郊区已发现多种植物叶片受害症状[6-7]。同时,干旱也是限制我国北方树木生长的重要因素。在全球气候变化背景下,频繁出现的干旱和不断升高的大气臭氧浓度将会共同对城市树木生长产生严重威胁,增加了植物响应的不确定性和复杂性。
叶片色素含量对植物生长具有重要意义。叶绿素和类胡萝卜素不仅是主要的光合色素,同时也与花青苷和类黄酮共同参与调节叶片颜色,叶片叶绿素和类胡萝卜素的降解,花色素苷的积累,使元宝枫等彩叶树的叶片在秋季由绿色转为红色[8-9]。因此叶片秋季色素含量和脱落酸含量的变化能够反映着叶片秋季变色的时间和长度,也影响着城市的秋季景观。已有研究表明,臭氧会通过植物气孔进行叶片内部,直接攻击原生质膜上的类脂或蛋白质,造成光合色素合成降低,叶绿素分解[10-11]。花青苷是一种水溶性的黄酮多酚类化合物,是秋季红叶植物呈现红色的主要色素,也是一些彩叶树种叶片衰老的标志[12-13]。类黄酮也是植物体内重要的色素,用于吸收UV-B,调控叶片颜色和亮度[14-15]。花青苷具有很强的抗氧化性,能够清除臭氧等污染物导致的活性氧自由基,一些研究结果表明臭氧浓度升高后叶片花青苷和类黄酮的含量增加[16-17]。因为花青苷具有水溶性,干旱胁迫后叶片花青苷含量上升以降低细胞渗透势[16,18]。但目前对臭氧和干旱复合影响植物叶片花青苷和类黄酮的研究还很少。
元宝枫(AcertruncatumBunge)是我国特有的槭树科落叶乔木,因其秋季叶片鲜艳的红色,叶片能够吸附粉尘,苗木易栽培等特点在北方城市广泛种植,是重要的具有观赏价值,生态和经济价值的城市绿化树种。目前对于秋色叶树种-元宝枫叶色表达影响的研究有光照影响[19],K元素影响[20],季节变化[21],温湿度及外源蔗糖[9]以及转色机理[8]研究等,目前未见有关于元宝枫色素含量对于大气污染物臭氧,干旱及其协同作用响应的相关研究。
本研究选择一年生元宝枫幼苗为研究对象,于2012年在北京市昌平区利用开顶式气室,设置两个臭氧浓度和两个水分梯度,对叶片总叶绿素、花青苷和类黄酮相对含量以及叶片脱落酸含量进行了测定。本实验目的是研究臭氧浓度升高和干旱对元宝枫叶片的主要色素和脱落酸含量的变化,为评价我国北方城市面临的大气污染和干旱共同对树木生长影响提供数据支持,研究结果对于模拟和预测未来气候变化对城市树木生长及观赏价值影响具有重要意义。
1 材料与方法
1.1 实验地点
实验地点位于北京市昌平区种子管理站(40°12′N,116°08′E),属暖温带大陆性季风气候,年均降水量为550.3 mm[22]。土壤类型为潮土,质地为砂壤,有机质含量为16.4 g/kg,全氮0.9 g/kg,速效磷38.1 mg/kg,速效钾102.1 mg/kg,pH 8.3。
1.2 实验设计和实验材料
实验在开顶式气室内进行,气室的设计在之前的基础上有所改进[5]。气室材料为阳光板(透光率85%),形状为圆柱体 (直径2 m,高2.5 m),顶部有高于顶端的透明挡板用于防止雨水进入,罩子内外气体自由流通,每个罩子周围挖有25 cm宽,75 cm深的水沟,内铺设透明薄膜用于防止外部雨水进入以及内外水分交换。共12个罩子,每两个之间的距离为2 m (开顶式气室及细节描述见文献[10])。实验设置4个处理,分别为:AW-大气臭氧+水分充足,AW+60-大气增加60 nL/L臭氧+水分充足,AD-大气臭氧+干旱,AD+60-大气加60 nL/L臭氧+干旱。共12个开顶式气室,每个处理3个重复,因此每个罩子为1个重复。干旱设置为土壤田间持水量的50%,实验开始后所有罩子停止浇水,待土壤水分下降到相同值后进行不同水量灌溉。通过土壤水分(EC- 5,Decagon Device,UK)和土壤水势(MPS- 2,Decagon Device,UK)监测,定量浇水的方法控制干旱。对照处理土壤水分为田间持水量,不存在干旱胁迫。臭氧熏气时间为7月28日开始,10月25日结束,每天熏气时间8:30—17:30,阴雨天气停止熏气,总处理时间为57d,熏气期间大气臭氧均值为34 nL/L,臭氧累计量AOT40为28.2 μL L-1h。
实验材料为1年生元宝枫(Acertruncatum)幼苗,由北京本地苗圃购入,2012年4月份移栽到气室外缓苗,5月30日选取长势一致的120株幼苗移入开顶式气室内进行适应,每个罩子内种植10株元宝枫。每个罩子内种植10株元宝枫。为避免花盆限制根系生长,幼苗直接种植于开顶式气室内。元宝枫为落叶树种,选择植株顶端向阳位置,将7月1日—7日间展开的叶片作为早生叶进行挂牌标记。将9月1日—7日间展开的叶片作为晚生叶进行挂牌标记。从叶片展开到采样结束,早生叶臭氧累计量AOT40约为28.2 μL L-1h,晚生叶臭氧累积量AOT40约为14.2 μL L-1h。
1.3 指标测定
采样时间为10月10日上午,采样时植株株高为(80±7) cm,基径为(11±1) mm,处理间株高,基径无显著差异。每个开顶式气室内选择3株长势良好的植株,每个植株分别选择2—3片标记的早生叶和晚生叶进行采样,取样时叶片避开叶脉分为两部分,一部分用保温箱快速转移到4 ℃冰箱中,于2—3d内完成叶绿素a和b、类胡萝卜素、花青苷和类黄酮的测定,另一部分用液氮罐转移到-80 ℃冰箱内用于生长激素测定。
叶绿素的测定取50 mg新鲜叶片,在4℃环境下剪碎后用4 mL 95% 的乙醇溶液浸泡提取,取提取液用分光光度计(型号)测定664、648、470 nm 波长吸光度,根据Lichtenthaler[23]的修正公式计算叶绿素a、b总量以及类胡萝卜素含量。
花青苷和类黄酮相对含量的测定分别参照Pirie[24]和Caldwell[25]的方法并略作改进,取100 mg新鲜叶片,用5mL 0.1% HCl甲醇4 ℃浸泡提取24 h,取提取液用分光光度计(型号)于530 nm(花色素苷)和 300 nm(类黄酮)处测定吸光值。用每g叶片在530 nm处的吸收值OD530/g鲜重表示叶片中花色素苷相对含量,OD300/g鲜重作为叶片中类黄酮相对含量[26]。
脱落酸(ABA)含量的测定酶联免疫吸附测定法(ELISA)[27]。
1.4 数据分析
臭氧和干旱分别作为影响因子对各指标的作用以及交互作用利用双因素方差Two-way ANOVA分析进行。处理间多因素比较采用LSD法。所有数据在处理前均进行了方差齐性和正态分布检验。数据分析采用SPSS 17.0 软件进行,并利用Sigmaplot 11.0进行绘图。
2 结果
2.1 总叶绿素和类胡萝卜素含量
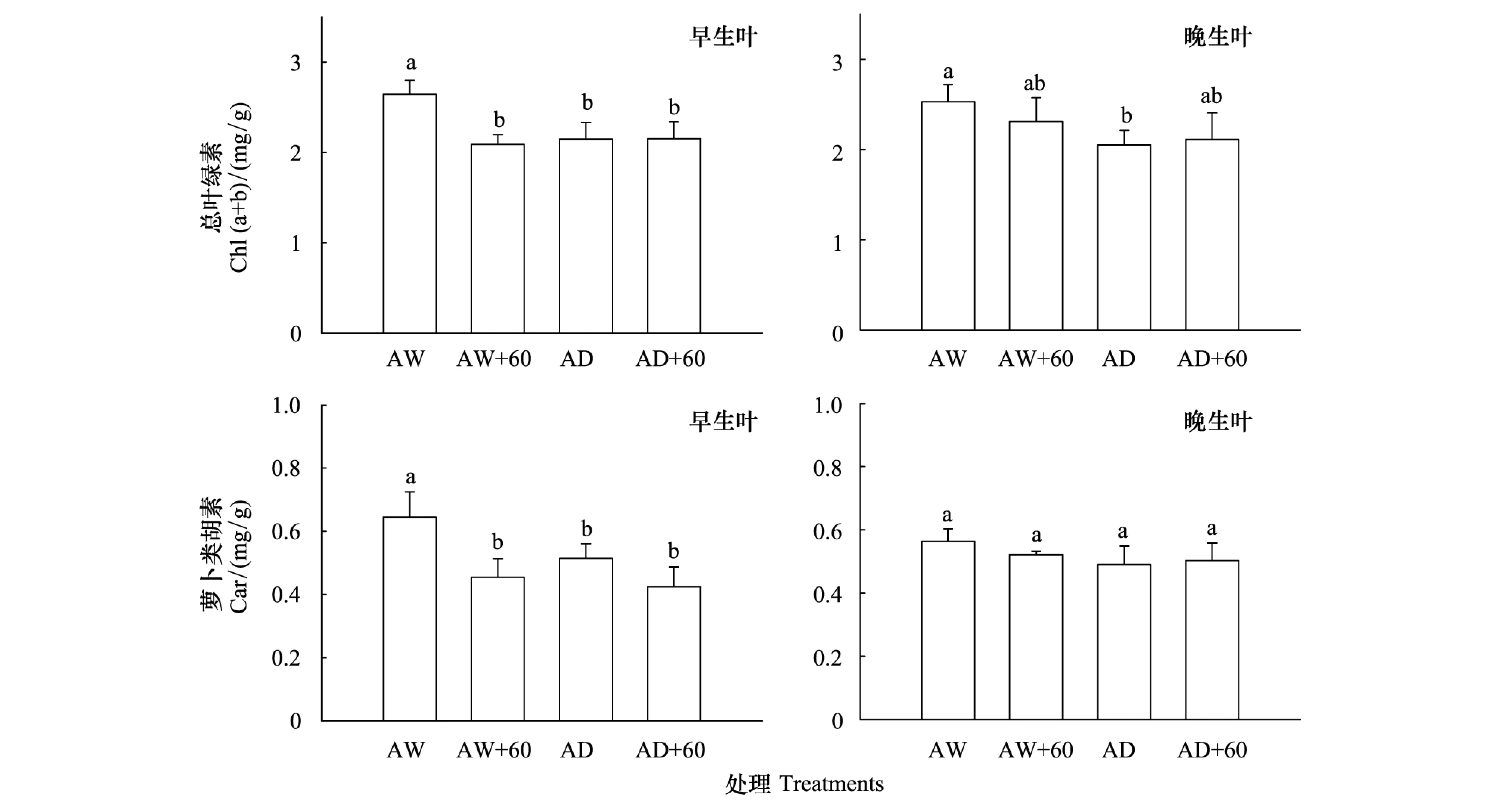
图1 臭氧浓度增加和干旱处理对早生叶和晚生叶总叶绿素和类胡萝卜素含量的影响 Fig.1 The effects of elevated ozone and drought stress on total chlorophyll (Chl (a+b)) and carotenoid (Car) contents in early-flush and late-flush leaves图中不同字母表示处理间多重比较差异显著 (P<0.05)
早生叶总叶绿素和类胡萝卜素含量在臭氧处理后显著降低,降低百分比分别为21%和29.6%(图1,表1)。早生叶总叶绿素含量在干旱处理后显著降低了18.7%。共同处理对早生叶片总叶绿素含量具有显著交互作用,交互作用表明总叶绿素下降了18.6%,介于单独臭氧和干旱处理之间(图1,表1)。晚生叶总叶绿素在干旱处理后显著降低了18.8%,臭氧单独或共同处理未对晚生叶上述指标产生显著影响(图1,表1)。
2.2 花青苷和类黄酮色素相对含量
早生叶花青苷和类黄酮相对含量在臭氧处理后分别显著增加了34.1%和7.3%,在干旱处理后分别显著增加了37%和7.4%。共同处理对早生叶中花青苷相对含量产生了显著的交互作用,处理后花青苷相对含量升高了34.2%,介于单独臭氧和干旱处理之间(图2,表1)。臭氧、干旱以及共同处理未对晚生叶叶片花青苷和类黄酮相对含量产生显著影响。

图2 臭氧增加和干旱处理对早生叶和晚生叶花色素苷和类黄酮相对含量的影响Fig.2 The effects of elevated ozone and drought stress on anthocyanin and flavonoid relative contents in early-flush and late-flush leaves图中不同小写字母表示处理间多重比较差异显著 (P<0.05)
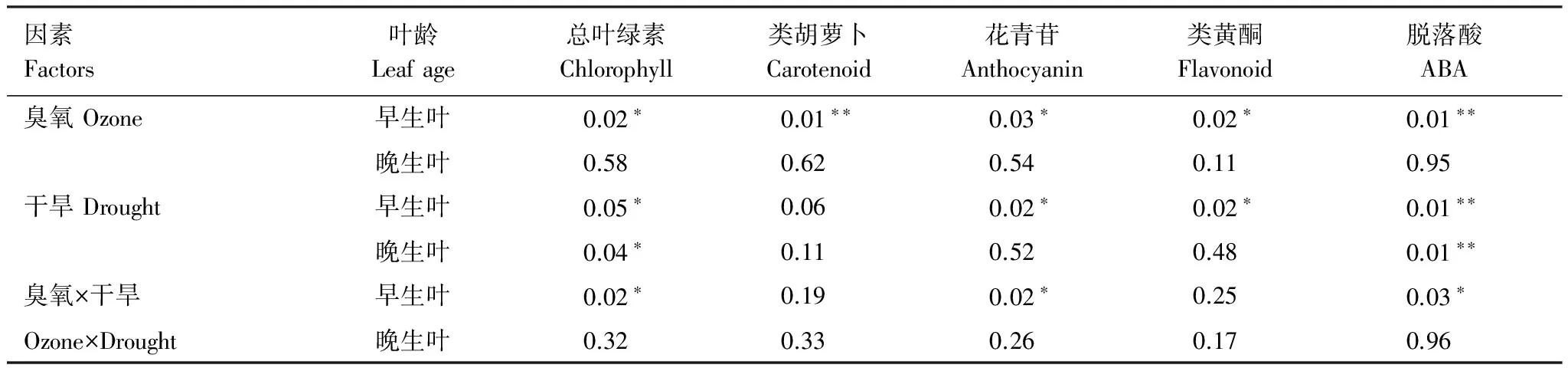
因素Factors叶龄Leafage总叶绿素Chlorophyll类胡萝卜Carotenoid花青苷Anthocyanin类黄酮Flavonoid脱落酸ABA臭氧Ozone早生叶0.02∗0.01∗∗0.03∗0.02∗0.01∗∗晚生叶0.580.620.540.110.95干旱Drought早生叶0.05∗0.060.02∗0.02∗0.01∗∗晚生叶0.04∗0.110.520.480.01∗∗臭氧×干旱早生叶0.02∗0.190.02∗0.250.03∗Ozone×Drought晚生叶0.320.330.260.170.96
表中数值为P值,*P<0.05,**P<0.01,***P<0.001
2.3 脱落酸含量
早生叶脱落酸含量在臭氧和干旱单独处理后均显著升高,升高幅度分别为19.8%和13%。共同处理对早生叶脱落酸含量有显著交互作用,处理后脱落酸含量升高了20.8% (图3,表1),略高于单独的臭氧和干旱处理。晚生叶在干旱处理后脱落酸含量显著上升了33.4%。臭氧和共同处理未对晚生叶叶片脱落酸含量产生显著影响。
3 讨论

图3 臭氧增加和干旱处理对早生叶 和晚生叶 叶片脱落酸(ABA)含量的影响Fig.3 The effects of elevated ozone and drought stress on abscisic acid (ABA) contents in early-flush and late-flush leaves图中不同小写字母表示处理间多重比较差异显著 (P<0.05)
叶绿素和类胡萝卜是重要的光合色素也同时影响着叶片的颜色,光合色素含量的下降意味着叶片光合能力的降低。臭氧和干旱胁迫对叶绿素的分解作用已经被大量研究证实[5,10,28],本实验结果也表明臭氧浓度升高和干旱处理导致早生叶总叶绿素含量下降(图1)。交互作用表明叶片早生叶总叶绿素含量介于单独臭氧和干旱处理之间,说明干旱保护和减缓了臭氧对叶片叶绿素的下降作用(图1,表1)。可能的解释是气孔是臭氧进入叶片的主要通道,而干旱能够降低叶片的气孔导度[12],从而减少了叶片对臭氧的摄入量[29-30]。
元宝枫是一种具有秋季观赏价值的彩叶树种,花青苷和相关的可溶性糖结合形成红色,树木秋季叶片呈现的鲜红色便是叶绿素含量减少花青苷的合成增加的结果[12,31],类黄酮也能在秋季提升叶片的亮度[14]。本实验结果表明,臭氧和干旱处理后早生叶叶片花青苷和类黄酮相对含量均显著增加(图2,表1),研究表明花青苷作为一种可溶性黄酮类物质,其含量的增加能够降低渗透压减轻干旱胁迫[16,18],增加叶片对臭氧抗氧化性[17]。花青苷交互作用的结果表明,臭氧的存在缓解了干旱造成的早生叶花青苷含量的上升,目前未发现臭氧和干旱复合影响对花青苷影响的研究,所以具体响应机制仍不明确。
脱落酸是能够降低叶片气孔导度、加速叶片衰老、脱落的主要植物激素[32- 34]。本实验结果表明,臭氧和干旱处理均导致叶片脱落酸含量升高,交互作用表现脱落酸含量大于单独臭氧和干旱处理,变化趋势与之前发表的光合测量结果-气孔导度在处理后的下降趋势相符合[10]。对于元宝枫等不敏感树种,叶片气孔臭氧、干旱等胁迫的反应是ABA含量升高以调控气孔关闭,减少水分散失和臭氧进入,交互作用的结果也与一些文献结果一致[35-36],脱落酸含量升高会导致叶片早衰,提前脱落,变色期变短。
晚生叶和早生叶对臭氧浓度升高和干旱处理的响应不同。晚生叶总叶绿素和脱落酸含量对干旱处理较为敏感,这可能与晚生叶蒸腾作用较为强烈有关。在早生叶中,除了类胡萝卜素外,臭氧浓度升高和干旱处理均对早生叶相关指标产生了显著影响。早生和晚生叶对臭氧处理的响应差异可能与以下原因有关:1)臭氧对叶片的伤害具有明显的时间累积效应[37],受害程度与臭氧累积量正相关,而早生叶接受臭氧熏蒸的时间较长,因此臭氧累积量大于晚生叶。2)晚生叶对臭氧的适应性。有研究表明,植物在受到臭氧威胁后植株会产生更多的抗氧化类物质如抗坏血酸等输送到更重要的幼嫩器官如晚生叶片中[38],因此晚生叶抗氧化能力可能强于早生叶,这也可能是晚生叶受臭氧影响较小的原因。在本实验中,由于未对叶片开始变红、变红持续时间、落叶时间和叶片颜色色度进行监测和分析,所以难以详细评价臭氧和干旱对元宝枫秋季物候变化的影响程度。
4 结论
(1)元宝枫早生叶和晚生叶对干旱和臭氧的响应不同,早生叶对臭氧处理响应大于晚生叶,而晚生叶对干旱处理更敏感。
(2)臭氧浓度升高和干旱均加速了叶片衰老。处理后早生叶光合色素减少、花青苷和类黄酮增加、叶片ABA含量增加,这些变化将会改变元宝枫秋季叶片秋季变色时间,影响元宝枫秋季物候和作为彩叶树的观赏价值。
(3)共同处理后叶片脱落酸含量增加,叶片提前脱落风险增加。
致谢:北京城市生态站宋文质工程师和姚余辉老师在OTC设计,搭建和运行过程中给予帮助,张红星老师在设备安装中提供帮助,冯兆忠老师帮助写作,特此致谢。
[1] Matyssek R, Kozovits A R, Schnitzler J P, Pretzsch H, Dieler J, Wieser G. Forest trees under air pollution as a factor of climate change // Tausz M, Grulke N, eds. Trees in a Changing Environment. Netherlands: Springer, 2014, 9: 117- 163.
[2] Matyssek R, Sandermann H Jr. Impact of ozone on trees: an ecophysiological perspective // Esser K, Lüttge U, Beyschlag W, Hellwig F, eds. Progress in Botany. Heidelberg: Springer, 2003, 64: 349- 404.
[3] Wang X K, Manning W, Feng Z W, Zhu Y G. Ground-level ozone in China: distribution and effects on crop yields. Environmental Pollution, 2007, 147(2): 394- 400.
[4] Zhang W W, Wang G H, Liu X B, Feng Z Z. Effects of elevated O3exposure on seed yield, N concentration and photosynthesis of nine soybean cultivars (Glycinemax(L.) Merr.) in Northeast China. Plant Science, 2014, 226: 172- 181.
[5] Zheng F X, Wang X K, Lu F, Hou P Q, Zhang W W, Duan X N, Zhou X P, Ai Y P, Zheng H, Ouyang Z Y, Feng Z W. Effects of elevated ozone concentration on methane emission from a rice paddy in Yangtze River Delta, China. Global Change Biology, 2011, 17(2): 898- 910.
[6] Wan W X, Manning W J, Wang X K, Zhang H X, Sun X, Zhang Q Q. Ozone and ozone injury on plants in and around Beijing, China. Environmental Pollution, 2014, 191: 215- 222.
[7] Feng Z Z, Sun J S, Wan W X, Hu E Z, Calatayud V. Evidence of widespread ozone-induced visible injury on plants in Beijing, China. Environmental Pollution, 2014, 193: 296- 301.
[8] 陈建芳. 温湿度及外源蔗糖对元宝枫秋叶变色的影响研究[D]. 北京: 北京林业大学, 2014.
[9] 钱见平. 元宝枫转色机理的初步研究[D]. 泰安: 山东农业大学, 2013.
[10] Li L, Manning W J, Tong L, Wang X K. Chronic drought stress reduced but not protected Shantung maple (AcertruncatumBunge) from adverse effects of ozone (O3) on growth and physiology in the suburb of Beijing, China. Environmental Pollution, 2015, 201: 34- 41.
[11] 列淦文, 叶龙华, 薛立. 臭氧胁迫对植物主要生理功能的影响. 生态学报, 2014, 34(2): 294- 306.
[12] Feild T S, Lee D W, Holbrook N M. Why leaves turn red in autumn. The role of anthocyanins in senescing leaves of red-osier dogwood. Plant Physiology, 2001, 127(2): 566- 574.
[13] Lee D W, Gould K S. Anthocyanins in leaves and other vegetative organs: an introduction. Advances in Botanical Research, 2002, 37: 1- 16.
[14] 葛雨萱, 王亮生, 周肖红, 甘长青. 香山黄栌叶色和色素组成的相互关系及时空变化. 林业科学, 2011, 47(4): 38- 42.
[15] Liu L, Gitz III D C, McClure J W. Effects of UV-B on flavonoids, ferulic acid, growth and photosynthesis in barley primary leaves. Physiologia Plantarum, 1995, 93(4): 725- 733.
[16] Chalker-Scott L. Do anthocyanins function as osmoregulators in leaf tissues?. Advances in Botanical Research, 2002, 37: 103- 127.
[17] Foot J P, Caporn S J M, Lee J A, Ashenden T W. The effect of long-term ozone fumigation on the growth, physiology and frost sensitivity ofCallunavulgaris. New Phytologist, 1996, 133(3): 503- 511.
[18] Balakumar T, Vincent V H B, Paliwal K. On the interaction of UV-B radiation (280315 nm) with water stress in crop plants. Physiologia Plantarum, 1993, 87(2): 217- 222.
[19] 梁峰, 蔺银鼎. 光照强度对彩叶植物元宝枫叶色表达的影响. 山西农业大学学报: 自然科学版, 2009, 29(1): 41- 45.
[20] 王志红, 蔺银鼎. K元素对元宝枫秋叶变色的影响研究. 山西农业大学学报: 自然科学版, 2009, 29(2): 139- 142.
[21] 楚爱香, 张要战, 田永芳. 几种秋色叶树种秋冬转色期叶色变化的生理特性. 东北林业大学学报, 2012, 40(11): 40- 43.
[22] 佟磊, 冯宗炜, 苏德, 毕力格, 王琼, 耿春梅, 逯非, 王玮, 殷宝辉, 王效科. 冬小麦气孔臭氧通量拟合及通量产量关系的比较分析. 生态学报, 2012, 32(9): 2890- 2899.
[23] Lichtenthaler H K. Chlorophyll fluorescence signatures of leaves during the autumnal chlorophyll breakdown. Journal of Plant Physiology, 1987, 131(1/2): 101- 110.
[24] Pirie A, Mullins M G. Changes in anthocyanin and phenolics content of grapevine leaf and fruit tissues treated with sucrose, nitrate, and abscisic acid. Plant Physiology, 1976, 58(4): 468- 472.
[25] Caldwell M M. The effects of solar UV-B radiation (280- 315 nm) on higher plants: implications of stratospheric ozone reduction // Castellani A, ed. Research in Photobiology. New York: Springer, 1977: 597- 607.
[26] 郑有飞, 徐卫民, 吴荣军, 张金恩, 刘瑞娜, 姚娟, 胡会芳. 地表臭氧浓度增加和UV-B 辐射增强及其复合处理对大豆光合特性的影响. 生态学报, 2012, 32(8): 2515- 2524.
[27] Yang Y M, Xu C N, Wang B M, Jia J Z. Effects of plant growth regulators on secondary wall thickening of cotton fibres. Plant Growth Regulation, 2001, 35(3): 233- 237.
[28] Wallin G, Karlsson P E, Selldén G, Ottosson S, Medin E L, Pleijel H, Skärby L. Impact of four years exposure to different levels of ozone, phosphorus and drought on chlorophyll, mineral nutrients, and stem volume of Norway spruce,Piceaabies. Physiologia Plantarum, 2002, 114(2): 192- 206.
[29] Garnier E, Berger A. The influence of drought on stomatal conductance and water potential of peach trees growing in the field. Scientia Horticulturae, 1987, 32(3/4): 249- 263.
[30] Matyssek R, Le Thiec D, Löw M, Dizengremel P, Nunn A J, Häberle K H. Interactions between Drought and O3Stress in Forest Trees. Plant Biology, 2006, 8(1): 11- 17.
[31] Matile P. Biochemistry of Indian summer: physiology of autumnal leaf coloration. Experimental Gerontology, 2000, 35(2): 145- 158.
[32] Conklin P L, Barth C. Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant, Cell & Environment, 2004, 27(8): 959- 970.
[33] Pourtau N, Marès M, Purdy S, Quentin N, Ru⊇l A, Wingler A. Interactions of abscisic acid and sugar signalling in the regulation of leaf senescence. Planta, 2004, 219(5): 765- 772.
[34] Miller J D, Arteca R N, Pell E J. Senescence-associated gene expression during ozone-induced leaf senescence in Arabidopsis. Plant Physiology, 1999, 120(4): 1015- 1024.
[35] Wittig V E, Ainsworth E A, Long S P. To what extent do current and projected increases in surface ozone affect photosynthesis and stomatal conductance of trees? A meta-analytic review of the last 3 decades of experiments. Plant, Cell & Environment, 2007, 30(9): 1150- 1162.
[36] Wieser G, Havranek W M. Environmental control of ozone uptake inLarixdeciduaMill.: a comparison between different altitudes. Tree Physiology, 1995, 15(4): 253- 258.
[37] Craker L E, Starbuck J S. Leaf age and air pollutant susceptibility: Uptake of ozone and sulfur dioxide. Environmental Research, 1973, 6(1): 91- 94.
[38] Severino J F, Stich K, Soja G. Ozone stress and antioxidant substances inTrifoliumrepensandCentaureajacealeaves. Environmental Pollution, 2007, 146(3): 707- 714.
The effects of elevated ozone and chronic drought on leaf pigments and abscisic acid contents in early and late-flush leaves of Shantung maple (AcertruncatumBunge)
LI Li1, NIU Junfeng2, WEN Zhi1, CUI Jian3, WANG Xiaoke1,*
1StateKeyLaboratoryofUrbanandRegionalEcology,ResearchCenterforEco-EnvironmentalSciences,ChineseAcademyofSciences,Beijing100085,China2SouthChinaBotanicalGarden,ChineseAcademyofSciences,Guangzhou510650,China3InstituteofAgricultureResourcesandRegionalPlanning,ChineseAcademyofAgricultureSciences,Beijing100081,China
The changing color of leaves in autumn is not only necessary for the growth of foliage trees but is also important for urban landscape interests. Red color in maple leaves relates to the synthesis of anthocyanin and the degraded pigments. Ozone (O3) is an important phytotoxic pollutant, which may affect the basic physiological functions of plants due to its highly reactive oxidative characteristics. The increased consumption of fossil fuels in China has led to increased emission of O3precursors and visible plant injury induced by high O3concentration frequently occurs around Beijing. In addition, trees are often subjected to periodic drought in North China with the frequency and the severity of drought events projected in the background of climate change. Drought in summer usually coincides with episodes of high O3concentrations, which, together, may affect plant growth including color expression and leaf senescence of foliage tree species in fall. Although numerous publications have reported the effects of elevated O3concentration or drought stress on trees, little is known about the possible interactions between anthocyanin and flavonoid in Asian maple species. To investigate the effects of chronic drought stress and elevated O3concentrations on leaf pigments and abscission of Shantung maple (AcertruncatumBunge), we set up 12 open-top chambers with four treatments (AW: non-filtered ambient air and well watered; AW + 60: non-filtered ambient air plus 60 nL/L O3and well watered; AD: non-filtered ambient air and drought; AD + 60: non-filtered ambient air plus 60 nL/L O3and drought) in a suburb of Beijing, China. Total chlorophyll (total Chl), carotenoid (Car), anthocyanin, flavonoid, and abscisic acid (ABA) contents in early and late-flush leaves were measured in October of the first year. Leaves that unfolded from July 1 to 7 were marked as early-flush leaves and the AOT40 (The cumulative O3 exposure, which was described as the accumulated hourly mean O3concentrations over 40 ppb during O3 fumigation period) was 28.2 μL L-1h when harvested. Late-flush leaves unfolded from September 1 to 7 and the AOT40 was 14.2 μL L-1h when harvested. The results showed: (1) For early-flush leaves, elevated O3significantly decreased the total Chl and Car contents by 21% and 29.6%, respectively, increased anthocyanin and flavonoid relative contents by 34.1% and 7.3%, respectively, and increased ABA contents by 19.8%. Drought stress decreased the total Chl by 18.7%, increased the relative contents of anthocyanin and flavonoid by 37% and 7.4%, respectively, and increased ABA contents by 13%. These physiology changes would collectively lead to leaf reddening, senescence, and abscission in advance of autumn. (2) Late-flush leaves only responded to drought treatments as indicated by a significant decrease of 18.8% in total Chl and an increase of 33.4% in ABA contents. (3) Significant interactions were found in total Chl, anthocyanin, and ABA contents of early-flush leaves, as indicated by the fact that the decrease in total Chl and increase in anthocyanin were mitigated, but the increase in ABA contents was aggravated. In conclusion, early and late-flush leaves responded differently to elevated O3and drought stress. Early-flush leaves responded more to ozone treatment, while late-flush leaves were more sensitive to drought stress. Both treatments lead to leaf early senescence and their interactive treatments may increase the risk of early abscission. This study may provide a better understanding of autumnal leaf growth and the phenology response to elevated O3and drought stress in the coming future.
AcertruncatumBunge; ozone; drought; photosynthetic pigment; anthocyanin; flavonoid; ABA
国家自然科学基金项目(31170424, 41571053, 71533005)
2015- 05- 06;
日期:2016- 03- 03
10.5846/stxb201505060933
*通讯作者Corresponding author.E-mail: wangxk@rcees.ac.cn
李丽, 牛俊峰, 文志, 崔健, 王效科.干旱和臭氧浓度升高对元宝枫早生和晚生叶片色素和脱落酸含量的影响.生态学报,2016,36(21):6804- 6811.
Li L, Niu J F, Wen Z, Cui J, Wang X K.The effects of elevated ozone and chronic drought on leaf pigments and abscisic acid contents in early and late-flush leaves of Shantung maple (AcertruncatumBunge).Acta Ecologica Sinica,2016,36(21):6804- 6811.

