N-[2-(4-吗啉基)乙基]苯甲酰胺类单胺氧化酶抑制剂的合成及其生物活性研究
周石洋,郭香琴,杨 柳,杨善彬
·研究论文·
N-[2-(4-吗啉基)乙基]苯甲酰胺类单胺氧化酶抑制剂的合成及其生物活性研究
周石洋1a,1b,郭香琴1a,1b,杨 柳1a,杨善彬1a,1b*
(1.重庆师范大学 a.化学学院;b.活性物质生物技术教育部工程研究中心制剂工程研究所,重庆 401331)
以取代苯甲酸为原料,经缩合反应制得N-(2-溴乙基)苯甲酰胺类化合物(4a~4i);4a~4i经N-烷基化反应合成N-[2-(4-吗啉基)乙基]苯甲酰胺类化合物(1a~1i),其结构经1H NMR,13C NMR,MS和元素分析表征。并对其生物活性进行了初步实验。实验结果表明:1a~1i对单胺氧化酶(MAO-A)有良好的抑制作用,其中1a最好,当IC50=3.652 μmol·L-1时,SI=42.32。
R-苯甲酸;苯甲酰胺;生物活性;MAO-A;合成
单胺氧化酶(MAO)是一种催化氧化单胺类递质脱氨基代谢反应失活的酶,单胺氧化酶抑制剂(MAOI)可以通过抑制去甲肾上腺素、肾上腺素、多巴胺和5-羟色胺[1-2]等单胺类递质的代谢失活,而减少脑内5-HT和NA的氧化脱胺代谢,使脑内受体部位神经递质5-HT或NA的浓度增加促使突触的神经传递而达到抗抑制的目的。MAO有两种亚型:MAO-A和MAO-B[3-4],在肝脏和大多数神经元中都有单胺存在,具有宽广和重叠的底物谱,都代谢多巴胺和5-HT,但MAO-A偏重于儿茶酚胺(去甲肾上腺素和肾上腺素)和5-HT的代谢有关,所以如果特异性地与MAO-A作用,则能提高药物的选择性而增强抗抑制作用;而MAO-B则偏重于酪胺、苯乙胺、苯乙醇胺和苄胺[5-6]。第一代单胺氧化酶抑制剂苯乙肼和反苯环丙胺对MAO具有不可逆的抑制作用,对MAO-A和MAO-B缺乏选择性,与拟交感药物和富含酪胺的食物具有广泛相互作用,可引起高血压危险,即所谓的“奶酪反应”[7]。于是开发了可逆性MAO-A抑制剂吗氯贝胺,吗氯贝胺通过可逆和选择性地抑制MAO-A,增加细胞外去甲肾上腺素、多巴胺和5-HT的浓度,发挥药效,由于保留了MAO-B降减单胺的功能,以及抑制酶作用的可逆性[8-9]。
本课题组结合吗氯贝胺的分子结构,设计合成类似结构的一系列化合物。以取代苯甲酸(2)为原料,经缩合反应生成N-(2-溴乙基)苯甲酰胺类化合物(4a~4i);4a~4i经N-烷基化反应合成N-[2-(4-吗啉基)乙基]苯甲酰胺类化合物(1a~1i,Scheme 1),其结构经1H NMR,13C NMR,MS和元素分析表征。并对其生物活性进行了初步测试。
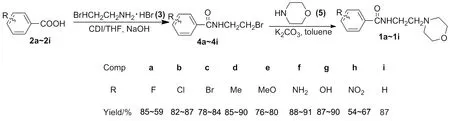
Scheme 1
1 实验部分
1.1 仪器与试剂
ZRD-1型全自动熔点仪(温度未校正);Brucker ARX-300型核磁共振仪(CDCl3为溶剂,TMS为内标);Agilent 1100LC/MSD型质谱仪。
MAO[1]和3[10]按文献方法制备;其余所用试剂均为分析纯或化学纯。
1.2 合成
(1) 4a~4i的合成通法
在烧瓶中加入2 0.10 mol,3 2.46 g(0.12 mol),氢氧化钠4.8 g(0.12 mol),N,N′-羰基二咪唑(CDI) 16.2 g(0.10 mol)和THF 100 mL,回流反应4 h。冷却至室温,过滤,滤饼用母液洗涤,经丙酮重结晶得白色固体4a~4i,m.p.135~137 ℃(136~138 ℃[11])。
(2) 1a~1i的合成
在烧瓶中加入4a~4i 0.10 mol,吗啉9.6 mL(0.12 mol),无水碳酸钾6.9 g(0.05 mol)和甲苯100 mL,搅拌下于115 ℃回流反应4 h。冷却至室温,过滤,滤饼用母液洗涤,用丙酮50 mL重结晶得晶体1a~1i。
o-氟-N-[2-(4-吗啉基)乙基]苯甲酰胺(o-1a):白色晶体,产率87%,m.p.112~114 ℃;1H NMRδ:8.00(s,1H,NH),7.93(s,1H,PhH),7.49(s,1H,PhH),7.21(s,1H,PhH),7.15(s,1H,PhH),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(t,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2);13C NMRδ:165.2,160.4,131.9,131.8,124.7,121.6,115.8,66.3,56.7,53.1,36.0;HR-MS(ESI)m/z:Calcd for C13H17N2O2F{[M+H]+}252.182 0,found 252.281 0。
m-氟-N-[2-(4-吗啉基)乙基]苯甲酰胺(m-1a):白色晶体,产率85%,m.p.109~110 ℃;1H NMRδ:8.00(s,1H,NH),7.72(s,1H,PhH),7.66(s,1H,PhH),7.42(s,1H,PhH),7.22(s,1H,PhH),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(t,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2);13C NMRδ:165.7,162.9,137.1,130.5,122.4,117.4,114.5,66.3,56.7,53.1,36.0;HR-MS(ESI)m/z:Calcd for C13H17N2O2F{[M+H]+}252.182 0,found 252.281 0。
p-氟-N-[2-(4-吗啉基)乙基]苯甲酰胺(p-1a):白色晶体,产率89%,m.p.120~121 ℃;1H NMRδ:8.00(s,1H,NH),7.93(s,2H,PhH),7.15(s,2H,PhH),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(t,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2);13C NMRδ:165.7,163.4,133.1,129.4,115.7,66.3,56.7,53.1,36.6;HR-MS(ESI)m/z:Calcd for C13H17N2O2F{[M+H]+}252.182 0,found 252.281 0。
o-氯-N-[2-(4-吗啉基)乙基]苯甲酰胺(o-1b):白色结晶体,产率82%,m.p.131~133 ℃,1H NMRδ:8.00(s,1H,NH),7.89(s,1H,PhH),7.51(s,1H,PhH),7.45(s,1H,PhH),7.32(s,1H,PhH),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(t,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2);13C NMRδ:165.2,135.6,130.6,130.5,129.8,129.7,127.1,66.3,56.7,53.1,36.6;HR-MS(ESI)m/z:Calcd for C13H17N2O2Cl{[M+H]+}268.598 0,found 268.739 0。
m-氯-N-[2-(4-吗啉基)乙基]苯甲酰胺(m-1b):白色结晶体,产率87%,m.p.123~125 ℃;1H NMRδ:8.00(s,1H,NH),7.96(s,1H,PhH),7.83(s,1H,PhH),7.52(s,1H,PhH),7.38(s,1H,PhH),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(t,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2);13C NMRδ:165.7,134.7,14.6,130.1,129.6,127.5,126.1,66.3,56.7,53.1,36.6;HR-MS(ESI)m/z:Calcd for C13H17N2O2Cl{[M+H]+}268.598 0,found 268.739 0。
o-溴-N-[2-(4-吗啉基)乙基]苯甲酰胺(o-1c):黄色晶体,产率80%,m.p.140~141 ℃;1H NMRδ:8.00(s,1H,NH),7.84(s,1H,PhH),7.61(s,1H,PhH),7.40(s,1H,PhH),7.38(s,1H,PhH),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(t,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2);13C NMRδ:165.2,139.2,132.1,130.6,127.8,127.6,120.7,66.3,56.7,53.1,36.6;HR-MS(ESI)m/z:Calcd for C13H17N2O2Br{[M+H]+}314.052 0,found 314.281 0。
m-溴-N-[2-(4-吗啉基)乙基]苯甲酰胺(m-1c):黄色晶体,产率78%,m.p.135~136 ℃;1H NMRδ:8.12(s,1H,PhH),8.00(s,1H,NH),7.89(s,1H,PhH),7.68(s,1H,PhH),7.33(s,1H,PhH),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(t,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2);13C NMRδ:165.7,134.6,133.0,132.1,129.9,127.5,122.4,66.3,56.7,53.1,36.6;HR-MS(ESI)m/z:Calcd for C13H17N2O2Br{[M+H]+}314.052 0,found 314.281 0。
p-溴-N-[2-(4-吗啉基)乙基]苯甲酰胺(p-1c):黄色晶体,产率84%,m.p.148~150 ℃;1H NMRδ:8.00(s,1H,NH),7.84(s,2H,PhH),7.61(s,2H,PhH),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(t,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2);13C NMRδ:165.7,133.1,129.7,124.0,66.3,56.7,53.1,36.6;HR-MS(ESI)m/z:Calcd for C13H17N2O2Br{[M+H]+}314.052 0,found 314.281 0。
o-甲基-N-[2-(4-吗啉基)乙基]苯甲酰胺(o-1d):白色晶体,产率87%,m.p.135~136 ℃;1H NMRδ:8.00(s,1H,NH),7.83(s,1H,PhH),7.39(s,1H,PhH),7.25(s,1H,PhH),7.24(s,1H,PhH),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(t,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2),2.35(m,3H,CH3);13C NMRδ:169.4,136.4,135.3,130.0,129.7,129.2,126.8,66.3,56.7,53.1,36.6,19.6;HR-MS(ESI)m/z:Calcd forC14H20N2O2{[M+H]+}248.213 0,found 248.404 0。
m-甲基-N-[2-(4-吗啉基)乙基]苯甲酰胺(m-1d):白色晶体,产率85%,m.p.130~132 ℃;1H NMRδ:8.00(s,1H,NH),7.76(s,1H,PhH),7.75(s,1H,PhH),7.32(s,1H,PhH),7.31(s,1H,PhH),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(t,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2),2.35(m,3H,CH3);13C NMRδ:165.7,137.5,133.8,130.5,128.4,128.2,127.7,66.3,56.7,53.1,36.6,20.9;HR-MS(ESI)m/z:Calcd for C14H20N2O2{[M+H]+}248.213 0,found 248.404 0。
p-甲基-N-[2-(4-吗啉基)乙基]苯甲酰胺(p-1d):白色晶体,产率90%,m.p.139~140 ℃;1H NMRδ:8.00(s,1H,NH),7.83(s,2H,PhH),7.24(s,2H,PhH),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(t,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2),2.35(m,3H,CH3);13C NMRδ:165.7,139.8,131.0,128.6,127.6,66.3,56.7,53.1,36.6,21.3;HR-MS(ESI)m/z:Calcd for C14H20N2O2{[M+H]+}248.213 0,found 248.404 0。
o-甲氧基-N-[2-(4-吗啉基)乙基]苯甲酰胺(o-1e):白色晶体,产率77%,m.p.140~141 ℃;1H NMRδ:8.00(s,1H,NH),7.84(s,1H,PhH),7.40(s,1H,PhH),7.00(s,1H,PhH),6.95(s,1H,PhH),3.73(m,3H,CH3O),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(t,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2);13C NMRδ:165.7,157.4,131.9,131.3,122.0,121.8,111.3,66.3,56.7,55.9,53.1,36.6;HR-MS(ESI)m/z:Calcd for C14H20N2O3{[M+H]+}264.198 0,found 264.304 0。
m-甲氧基-N-[2-(4-吗啉基)乙基]苯甲酰胺(m-1e):白色晶体,产率78%,m.p.138~139 ℃;1H NMRδ:8.00(s,1H,NH),7.51(s,1H,PhH),7.46(s,1H,PhH),7.33(s,1H,PhH),7.02(s,1H,PhH),3.73(m,3H,CH3O),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(m,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2);13C NMRδ:165.7,159.6,134.6,129.3,127.5,114.9,109.3,66.3,56.7,55.5,53.1,36.6;HR-MS(ESI)m/z:Calcd for C14H20N2O3{[M+H]+}264.198 0,found 264.304 0。
p-甲氧基-N-[2-(4-吗啉基)乙基]苯甲酰胺(p-1e):白色晶体,产率80%,m.p.144~146 ℃;1H NMRδ:8.00(s,1H,NH),7.84(s,2H,PhH),6.95(s,2H,PhH),3.73(m,3H,CH3O),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(t,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2);13C NMRδ:165.7,160.4,133.1,130.0,113.7,66.3,56.7,55.5,53.1,36.6;HR-MS(ESI)m/z:Calcd for C14H20N2O3{[M+H]+}264.098 0,found 264.304 0。
o-氨基-N-[2-(4-吗啉基)乙基]苯甲酰胺(o-1f):白色晶体,产率89%,m.p.137~139 ℃;1H NMRδ:8.00(s,1H,NH),7.70(s,1H,PhH),7.26(s,1H,PhH),6.80(s,1H,PhH),6.64(s,1H,PhH),4.00(t,J=7.2 Hz,2H,NH2),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(t,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2);13C NMRδ:166.4,146.5,130.8,128.1,125.1,120.4,115.7,66.3,56.7,55.1,36.0;HR-MS(ESI)m/z:Calcd for C13H19N3O2{[M+H]+}249.108 0,found 249.374 0。
m-氨基-N-[2-(4-吗啉基)乙基]苯甲酰胺(m-1f):白色晶体,产率88%,m.p.136~137 ℃;1H NMRδ:8.00(s,1H,NH),7.31(s,1H,PhH),7.19(s,1H,PhH),7.15(s,1H,PhH),6.71(s,1H,PhH),4.00(t,J=7.2 Hz,2H,NH2),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(t,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2);13C NMRδ:165.7,146.7,127.5,127.4,127.1,116.4,115.5,66.3,56.7,55.1,36.0;HR-MS(ESI)m/z:Calcd for C13H19N3O2{[M+H]+}249.108 0,found 249.374 0。
p-氨基-N-[2-(4-吗啉基)乙基]苯甲酰胺(p-1f):白色晶体,产率91%,m.p.140~141 ℃;1H NMRδ:8.00(s,1H,NH),7.70(s,2H,PhH),6.64(s,2H,PhH),4.00(t,J=7.2 Hz,2H,NH2),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(t,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2);13C NMRδ:165.7,149.1,129.3,126.7,113.2,66.3,56.7,55.1,36.0;HR-MS(ESI)m/z:Calcd for C13H19N3O2{[M+H]+}249.108 0,found 249.374 0。
o-羟基-N-[2-(4-吗啉基)乙基]苯甲酰胺(o-1g):白色晶体,产率88%,m.p.137~139 ℃;1H NMRδ:8.00(s,1H,NH),7.78(s,1H,PhH),7.34(s,1H,PhH),7.00(s,1H,PhH),6.91(s,1H,PhH),5.00(s,1H,OH),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(t,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2);13C NMRδ:165.7,159.1,131.9,128.6,122.0,117.3,115.1,66.3,56.7,55.1,36.0;HR-MS(ESI)m/z:Calcd for C13H18N2O3{[M+H]+}250.120 0,found 250.392 0。
m-羟基-N-[2-(4-吗啉基)乙基]苯甲酰胺(m-1g):白色晶体,产率87%,m.p.135~137 ℃;1H NMRδ:8.00(s,1H,NH),7.51(s,1H,PhH),7.42(s,1H,PhH),7.27(s,1H,PhH),6.98(s,1H,PhH),5.00(s,1H,OH),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(t,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2);13C NMRδ:165.7,157.7,134.6,129.9,127.5,116.6,114.6,66.3,56.7,55.1,36.0;HR-MS(ESI)m/z:Calcd for C13H18N2O3{[M+H]+}250.120 0,found 250.392 0。
p-羟基-N-[2-(4-吗啉基)乙基]苯甲酰胺(p-1g):白色晶体,产率90%,m.p.140~141 ℃;1H NMRδ:8.00(s,1H,NH),7.78(s,2H,PhH),6.91(s,2H,PhH),5.00(s,1H,OH),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(t,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2);13C NMRδ:165.7,157.8,133.1,127.7,115.7,66.3,56.7,55.1,36.0;HR-MS(ESI)m/z:calcd for C13H18N2O3{[M+H]+}250.120 0,found 250.392 0。
o-硝基-N-[2-(4-吗啉基)乙基]苯甲酰胺(o-1h):黄色晶体,产率60%,m.p.165~166 ℃;1H NMRδ:8.37(s,1H,PhH),8.21(s,1H,PhH),8.00(s,1H,NH),7.83(s,1H,PhH),7.77(s,1H,PhH),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(t,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2);13C NMRδ:166.4,148.4,129.4,128.6,128.1,125.1,120.4,66.3,56.7,55.1,36.0;HR-MS(ESI)m/z:Calcd for C13H17N3O4{[M+H]+}279.263 0,found 279.405 0。
m-硝基-N-[2-(4-吗啉基)乙基]苯甲酰胺(m-1h):黄色晶体,产率54%,m.p.160~161 ℃;1H NMRδ:8.88(s,1H,PhH),8.44(s,1H,PhH),8.34(s,1H,PhH),8.00(s,1H,NH),7.70(s,1H,PhH),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(t,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2);13C NMRδ:165.7,140.5,127.5,127.4,127.1,117.3,116.4,66.3,56.7,55.1,36.0;HR-MS(ESI)m/z:Calcd for C13H17N3O4{[M+H]+}279.263 0,found 279.405 0。
p-硝基-N-[2-(4-吗啉基)乙基]苯甲酰胺(p-1h):黄色晶体,产率67%,m.p.169~170 ℃;1H NMRδ:8.37(s,2H,PhH),8.32(s,2H,PhH),8.00(s,1H,NH),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(t,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2);13C NMRδ:165.7,140.5,128.7,126.7,117.3,66.3,56.7,55.1,36.0;HR-MS(ESI)m/z:Calcd for C13H17N3O4{[M+H]+}279.263 0,found 279.405 0。
N-[2-(4-吗啉基)乙基]苯甲酰胺(1i):白色晶体,产率87%,m.p.101~102 ℃;1H NMRδ:8.00(s,1H,NH),7.95(s,2H,PhH),7.51(s,2H,PhH),7.44(s,1H,PhH),3.67(t,J=5.1 Hz,2H,NHCH2),3.30(t,J=5.0 Hz,4H,CH2NCH2),2.66(t,J=5.3 Hz,2H,NCH2),2.37(t,J=7.1 Hz,4H,CH2OCH2);13C NMRδ:165.7,133.8,128.9,128.6,127.7,66.3,56.7,55.1,36.0;HR-MS(ESI)m/z:Calcd for C13H18N2O2{[M+H]+}234.093 0,found 234.280 0。
1.3 生物活性测试
设置空白对照和阳性药物对照(以雷沙吉兰为阳性药物),MAO抑制药物的体外筛选参考文献[2]方法进行。
2 结果与讨论
2.1 合成
考察了不同取代基及取代位置对1a~1i合成的影响。选择了两类取代基,供电子基和吸电子基(Scheme 1)。供电子基的产率明显比吸电子基的产率高(1a:85%~89%;1h:54%~67%)。这是因为供电子基能使苯环活化,从而影响产率。就供电子基而言,其产率也有点差异(1b:82%~87%;1g:87%~90%),主要是因为各取代基供电能力差异,比如NH2的供电子能力最强,其产率最高(1f:88%~91%)。
2.2 生物活性
评价了1a~1h对MAO的抑制活性,其IC50见表1。

表1 1a~1h对MAO的抑制活性
注:*没有抑制作用;-没有相关值
由表1可见,1a~1i对MAO-A的抑制活性比对MAO-B的抑制活性要高。1a对MAO-A的抑制活性最好(IC50=3.652 μmol·L-1,选择性指数(SI)=42.32),1b、1f、1g、1i的抑制活性也较好,IC50分别为5.741 μmol·L-1,7.451 μmol·L-1,8.548 μmol·L-1和10.653 μmol·L-1,SI分别为35.90,17.03,15.92和13.23。 取代基不同其抑制效果也不同,供电子基团的抑制活性明显高于吸电子基团的抑制活性,主要是由于基团的供电子能力和空间构象等原因所导致。Rasagiline作为标准参照物,对MAO-A和MAO-B的抑制性都比较好,但其SI值比较低,说明其选择性不如1a~1i对MAO-A选择性好。
以取代苯甲酸为原料合成了N-[2-(4-吗啉基)乙基]苯甲酰胺类化合物(1a~1i)。并对其生物活性进行了初步试验,试验结果表明:1a~1i对单胺氧化酶(MAO-A)有良好的抑制作用,其中1a最好,当IC50=3.652 μmol·L-1时,SI=42.32。
[1] Moussa B H,Youdim,A G.Rasagiline [N-propargyl-1R(+)-amin-oindan],a selective and potent inhibitor of mitochondrial monoamine oxidase[J].British Journal of Pharmacology,2001,132(2):500-506.
[2] Chachignon H,Scalacci N,Petricci E,etal.Synthesis of 1,2,3-substituted pyrroles from propargylaminesviaa one-pot tandem enyne cross metathesis-cyclization reaction[J].Organic Chemistry,2015,80(10):5287-5295.
[3] 朱岩,吴健勇,杨小乐,等.新型氮杂异香豆素化合物的合成[J].合成化学,2016,24(6):494-498.
[4] Ming Z,Wotton C,Appleton R,etal.Systemic lipopolysaccharide-mediated alteration of cortical neuromodulation involves increases in monoamine oxidase-A and acetylcholinesterase activity[J].Neuroinflammation,2015,12(2):37.
[5] Xie S,Chen J,Li X,etal.Synthesis and evaluation of selegiline derivatives as monoamine oxidase inhibitor,antioxidant and metal chelator against Alzheimer′s disease[J].Bioorganic &Medicinal Chemistry,2015,23(13):3722-3729.
[6] 周石洋,陈玲,杨善彬.4-乙酰氨基苯酚的合成工艺研究[J].贵州师范大学学报(自然科学版),2016,(34):65-68.
[7] Wang Y,Sun Y,Guo Y,etal.Dual functional cholinesterase and MAO inhibitors for the treatment of Alzheimer′s disease:Synthesis,pharmacological analysis and molecular modeling of homoisoflavonoid derivatives[J].Enzyme Inhibition and Medicinal Chemistry,2016,31(3):389-397.
[8] DeMunck L,Monleon A,Vila C,etal.Enantioselective alkynylation of benzo[e][1,2,3]-oxathiazine 2,2-dioxides catalysed by (R)-VAPOL-Zn complexes:Synthesis of chiral propargylic cyclic sulfamidates[J].Organic &Biomolecular Chemistry,2015,13(27):7393-7396.
[9] 谢晓阳,蔡远鸿,王晓琴,等.4-氯-2-(2-硝基苯基)喹唑啉的合成[J].合成化学,2016,24(6):544-546.
[10] Kam A,Tangella Y,Manasa K,etal.PhI(OAc)2-mediated one-pot oxidative decarboxylation and aromatization of tetrahydro-beta-carbolines:Synthesis of norharmane,harmane,eudistomin U and eudistomin I[J].Organic &Biomolecular Chemistry,2015,13(32):8652-8662.
[11] Khattab S,Haiba N,Asal A,etal.Synthesis and evaluation of quinazoline amino acid derivatives as mono amine oxidase (MAO) inhibitors[J].Bioorganic &Medicinal Chemistry,2015,23(13):3574-3585.
Study On Synthesis and Bioactivities ofN-[2-(4-morpholinyl)ethyl] Benzamides as Monoamine Oxidase Inhibitor
ZHOU Shi-yang1a,1b,GUO Xiang-qin1a,1b,YANG-Liu1a,YANG Shan-bing1a,1b*
(a.College of Chemistry;b.Pharmaceutical Engineering Institute of Engineering Research Center of Biotechnology of Active Materials(Minsitry of Education),1.Chongqing Normal University,Chongqing 401331,China)
N-(2-bromoethyl) benzamide compounds (4a~4i) were synthesized by the condensation reaction of substituted benzoic acids.N-[2-(4-morpholinyl)ethyl] benzamide compounds(1a~1i) were synthesized byN-alkylation reaction of 4a~4i.The structures were characterized by1H NMR,13C NMR,MS and elemental analysis.The bioactivities were investgated.The results showed that 1a~1i have good inhibitory effect for MAO-A,1a exhibited best inhibitory with IC50=3.652 μmol·L-1,SI=42.32.
R-benzoic acid;benzamide;bioactivity;MAO-A;synthesis
2016-05-31
重庆市社会民生科技创新专项资助项目(cstc2015shmszx80060);活性物质生物技术教育部工程研究中心开放基金资助项目(20150408)
周石洋(1986-),男,汉族,湖南怀化人,硕士,主要从事药物设计与合成的研究。 E-mail:zhoushiyang520@126.com
杨善彬,博士,教授,E-mail:shanbiny@126.com
O623.626;O621.3
A
10.15952/j.cnki.cjsc.1005-1511.2016.12.16135

