多金属氧酸盐修饰的过渡金属配合物的催化效果和电化学行为
叶丹丹 王蕾 顾晓敏 倪良 张文莉
(江苏大学化学化工学院,镇江212013)
多金属氧酸盐修饰的过渡金属配合物的催化效果和电化学行为
叶丹丹 王蕾 顾晓敏 倪良 张文莉*
(江苏大学化学化工学院,镇江212013)
基于水热技术,合成了多金属氧酸盐修饰的2种新的过渡金属配合物[Cu(dmbipy)2(SiW12O40)][Cu(dmbipy)(H2O)3]·3H2O(1)和{(Hdmphen)2[Ag2(dmphen)2(SiW12O40)]}n(2)(dmbipy=4,4′-二甲基-2,2′-联吡啶,dmphen=2,9-二甲基-1,10-菲咯啉)。配合物1的最小不对称单元包含2种不同的Cu(Ⅱ)单元,其中Cu(Ⅱ)离子都采用了五配位的模式,并且水分子也参与了配位。氢键将不同的Cu(Ⅱ)单元连接形成了一维链,并延伸至二维层。在配合物2中,多金属氧酸盐连接Ag(Ⅱ)离子形成一维链。氢键和π…π堆积作用将一维链拓展成了二维层。
多金属氧酸盐;过渡金属配合物;催化性能;电化学行为
0 Introduction
Polyoxometalates(POMs),as a class of functional inorganic building blocks,have attracted extensive attention because of their affluent structures and potential applications[1-4].Up to now,a booming branchof POMs field is POMs-modified transition metal compounds(TMCs),which incorporate the merits of both inorganic and organic components.So,diverse techniques have been used to construct POMs-TMC compounds.The hydrothermal technique has been verified as a common but efficientsynthetic method[5-6]. Tuning hydrothermal conditions,such as the nature of reactants,ratio of reactants,reaction time,pH value, to obtain target compounds is attractive and challenging.In this work,we try to obtain POMs-TMC compounds by environment-friendly hydrothermal technique with an appropriate pH value range.
The suitable selection of organic ligands is extremely important for construction of POMs-TMC compounds.Nowadays,various organic ligands have been used to construct novel topologies[7-9].In our work,rigid ligands with simple coordination modes are introduced,such as 4,4′-dimethyl-2,2′-bipyridine (dmbipy),2,9-dimethyl-1,10-phenanthroline(dmphem), to explore whether these simple organic ligands can also construct new POMs-TMC compounds with high dimensionalities.As expected,the most common feature of these ligands is chelating coordination mode.But the binding angles of the 2,2′-substitution and 1,10-substitution account for an easy stabilization of metal complexes[10].Utilizing these ligands containing-CH3groups to construct new compounds also want to discuss the-CH3steric hindrance influencing on the structures[5].Furthermore,phenyl group in these simply rigid organic ligands is in favor of forming abundantπ…πstacking interactions and hydrogen bonding inducing high dimensional structures.
Fortunately,two POMs-TMC compounds,namely [Cu(dmbipy)2(SiW12O40)][Cu(dmbipy)(H2O)3]·3H2O(1), and{(Hdmphen)2[Ag2(dmphen)2(SiW12O40)]}n(2) (dmbipy=4,4′-dimethyl-2,2′-bipyridine,dmphen=2,9-dimethyl-1,10-phenanthrolin)were obtained under hydrothermal conditions with the pH value of 3.52 or 4.56.Furthermore,the two compounds extended to high dimensional structures through noncovalent interactions.The photocatalytic and electrochemical properties of these two compounds were also discussed.
1 Experimental
1.1 Materials and methods
All reagents and solvents for syntheses were commercially available and used as received without further purification.Transmission mode FTIR spectra were recorded on a Nicolet Nexus 470 infrared spectrometer between 400 and 4 000 cm-1using KBr disks.The carbon,nitrogen,and hydrogen contents of the solid compounds were determined by a Perkin-Elmer 240C elemental analyzer.Thermogravimetric analysis(TGA)was performed on a Germany Netzsch STA449C at a heating rate of 10℃·min-1from ambient temperature to 1 000℃in nitrogen.
Electrochemical signals were recorded using CHI660B electrochemical analyzer.The electrochemical curves were recorded by a conventional three-electrode system where glassy carbon electrode(GCE,3 mm in diameter)was used as working electrode,Ag/AgCl(saturated KClsolution) as reference electrode and platinum wire as counter electrode,respectively.The electrochemical measurements were performed with the scan rate range from 30 to 300 mV·s-1under quiescent conditions.
1.2 Synthesis of the compounds 1~2
[Cu(dmbipy)2(SiW12O40)][Cu(dmbipy)(H2O)3]·3H2O (1):A mixture of CuCl2(0.2 g,0.19 mmol),H4SiW12O40(0.288 g,0.10 mmol)and dmbipy(0.022 g,0.12 mmol)was dissolved in 18 mL distilled water at room temperature.The mixture was stirred for about 30 min.And the pH value of the solution was then adjusted to about 3.52 with 1.0 mol·L-1NaOH.The suspension was sealed in a 25 mL Teflon-lined stainless steel vessel at 160℃for five days.After cooling to room temperature,blue block crystals of compound 1 were collected by filtration and washed with distilled water in 58%yield(based on dmbipy ligand).Anal.Calcd.for C36H48Cu2N6O46SiW12(%):C, 11.83;H,1.32;N,2.30.Found(%):C,11.71;H,1.09; N,2.44.IR(KBr,cm-1):3 391(m),2 975(m),1 619(s), 1 559(w),1 496(m),1 445(m),1 042(m),1 014(m),969(s),921(s),879(m),797(m),518(w),406(w).
{(Hdmphen)2[Ag2(dmphen)2(SiW12O40)]}n(2):The synthesis of compound 2 was similar to that of 1 except that AgNO3(0.033 g,0.19 mmol)and dmphen (0.025 g,0.12 mmol)were used instead of CuCl2(0.2 g,0.19 mmol)and dmbipy(0.022 g,0.12 mmol).The pH value of suspension was adjusted to about 4.56. Colorless block crystals of compound 2 were collected by filtration and washed with distilled water in 56% yield(based on dmphen ligand).Anal.Calcd.for C56H52Ag2N8O40SiW12(%):C,17.14;H,1.33;N,2.85. Found(%):C,17.01;H,1.28;N,2.92.IR(KBr, cm-1):3 547(m),3 065(m),1 611(s),1 535(s),1 502 (w),1 463(m),1423(m),1 377(m),1 330(m),1 218 (m),1 151(m),1012(m),972(s),922(s),863(m),798 (m),720(m),680(m),636(m),533(w),404(w).
1.3 Catalysis experiments
The photocatalytic activities of compounds 1~2 were tested by degradation of rhodamine B(RhB) under the irradiation of UV light at room temperature. In the process of photocatalysis,100 mg of the compounds were suspended in 100 mL of 0.02 mmol· L-1RhB aqueous solution and magnetically stirred for about 30 min to ensure the equilibrium in the dark. Then with continuous stirring,the solution was exposed to UV irradiation from an Hg lamp.Every interval(45 min),5.0 mL samples were taken out for the analysis by UV visible spectroscopy(UV-2450 Shimadzu).
1.4 X-ray crystallography
X-ray diffraction data for compounds 1~2 were collected on a Bruker SMART Apex CCD diffractometer,equipped with a graphite monochromatic Mo Kαradiation(λ=0.071 073 nm)at 293(2)K.The structures were solved by direct methods implemented in SHELXS-97[11]and refined by a full-matrix leastsquares procedure based on F2using SHELXL-97[12]. All non-hydrogen atoms were refined anisotropically. The hydrogen atoms attached to coordinated water molecules were located from difference Fourier maps. The hydrogen atoms attached to uncoordinated water molecules were not treated.All the other hydrogen atoms were placed at calculated positions and treated using appropriate riding models.The detailed crystallographic data and structure refinement parameters for the two compounds are summarized in Table 1.Selected bond lengths and angles are listed in Table S1~S4(Supporting Information).
CCDC:1033952,1;1053009,2.
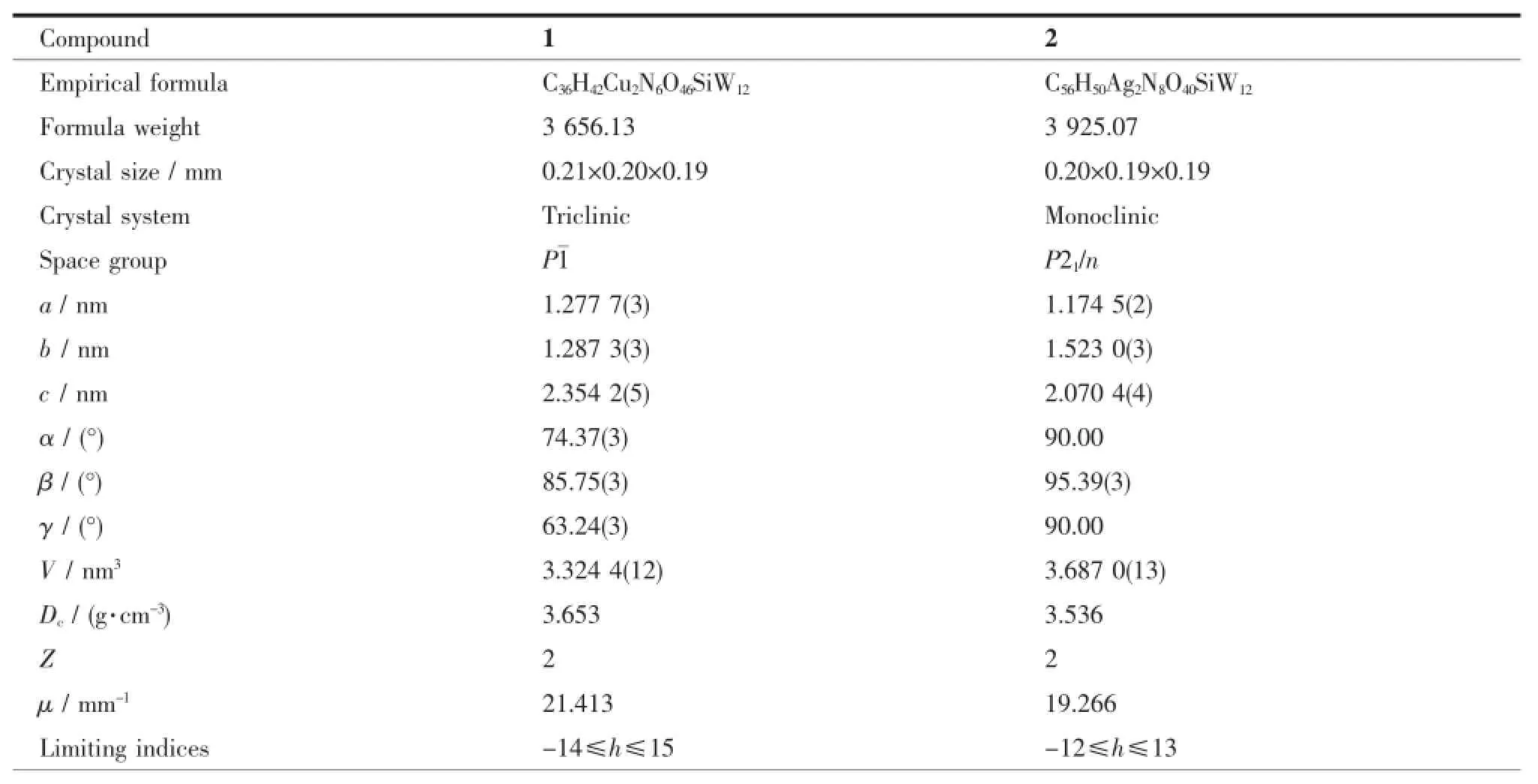
Table 1 Crystal data and structure refinement for compounds 1~2
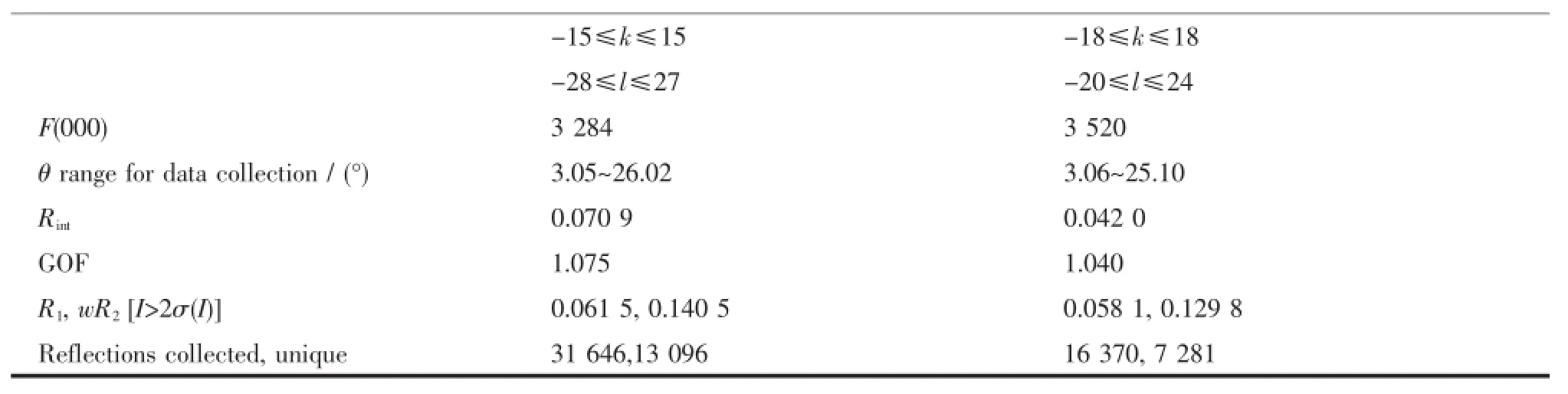
Continued Table 1
1.5 Fabrication of modified electrodes
Prior to modification,the GCE was first polished with sand paper,and followed by 1.0,0.3,and 0.05 μm alumina slurry,respectively.After successively sonicated in ethanol and double distilled water,the electrode was rinsed with double distilled water and allowed to dry at room temperature.5 mg compound 1 was dispersed in 5 mL dimethylformamide solution to make a homogeneous suspension of compound 1.And 2μL of this suspension was cast on the pretreated GCE surface and dried in air at room temperature to form compound 1 modified GCE(denoted as 1-GCE). The modified electrode was rinsed by water for several times prior to use.The fabrication of compound 2 modified glassy carbon electrodes is similar to that described for the preparation of 1-GCE.
2 Results and discussion
2.1 Structuralanalysis of compounds 1~2
2.1.1 Crystal structure of[Cu(dmbipy)2(SiW12O40)] [Cu(dmbipy)(H2O)3]·3H2O(1)
Single-crystal X-ray diffraction analysis reveals that the asymmetric unit of compound 1 includes two Cu(Ⅱ)ions,three dmbipy ligands,one[SiW12O40]4-anion and six water molecules,as depicted in Fig.1a. Of the two Cu(Ⅱ)centers,Cu(1)is penta-coordinated by two nitrogen atoms(N1,N2)from a dmbipy ligand, three oxygen atoms(O41,O42,O43)from three coordinated water molecules.It exhibits a distorted square pyramidal geometry.The second Cu(Ⅱ)ion, Cu(2),is surrounded by four N atoms(N3,N4,N5, N6)from two dmbipy ligands and one oxygen atoms (O36)from one[SiW12O40]4-polyoxoanion,respectively. The distances Cu-O and Cu-N fall in the normal ranges,and also can be observed in other Cucontaining compounds[13].
The coordinated water molecules in compound 1 play an important role in the formation of complementary hydrogen bonding,as shown in Fig.1b. Such strong hydrogen bonding interactions between coordinated water molecules and terminal oxygen atoms from[SiW12O40]4-anions cross-link each other asymmetric unit and lead to the formation of onedimensional(1D)chain(Fig.1c)(the main hydrogen bondings included in the 1D chain:O41-H41B…O18 0.229 6(2)nm,O42-H42A…O14 0.209 0(5)nm,O43-H43A…O12 0.207 4(4)nm).Moreover,there are hydrogen bonding interactions between coordinated water molecules and[SiW12O40]4-anions in adjacent chains(Fig.1c),such as O42-H42B…O6(0.217 5(3) nm),further forming two-dimensional layer.
2.1.2 Crystal structure of{(Hdmphen)2[Ag2(dmphen)2(SiW12O40)]}n(2)
The asymmetric unit of compound 2 includes one Ag(Ⅱ)ions,one coordinated dmphen ligands,one uncoordinated Hdmphen ligands and a half[SiW12O40]4-anion,as depicted in Fig.2a.Each Ag(Ⅱ)center exhibits a distorted quadrangular geometry[14]consisting of two nitrogen donors(N3,N4)from one dmphen ligand and two oxygen atoms from two[SiW12O40]4-anions,as depicted in Fig.2b.
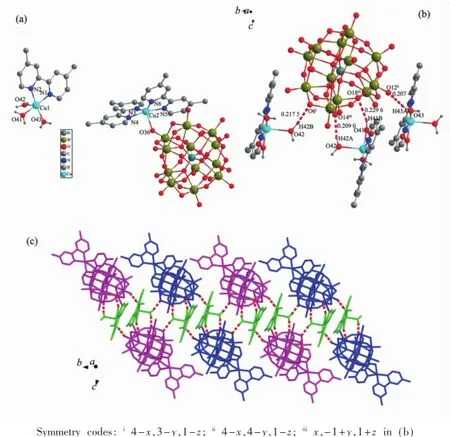
Fig.1(a)Coordination environment of Cu(Ⅱ)ions in compound 1;(b)Hydrogen bonds of compound 1; (c)Layer structure formed by hydrogen bonding interactions of compound 1
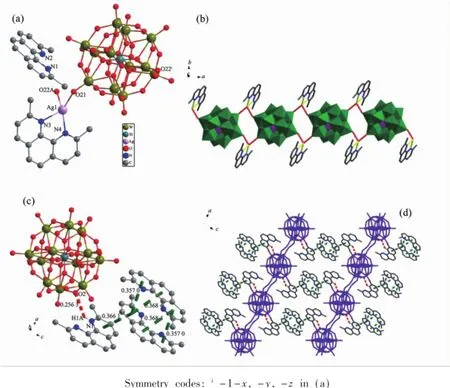
Fig.2(a)Coordination environment of Ag(Ⅱ)atom in compound 2;(b)One dimensional chain structure of compound 2;(c)Hydrogen bonding andπ…πstacking interactions of compound 2;(d)Two dimensional layer formed by hydrogen bonding andπ…πstacking interactions in compound 2
The neighboring Ag(Ⅱ)centers are separated at a distance of 0.839 nm,and are bridged by the terminal oxygen atoms from[SiW12O40]4-anions to exhibit onedimensional infinite zigzag coordination chain(Fig.2b). The chain forming is attributed to the-CH3groupsteric hindrance.Namely,the steric hindrance reduce the coordination ability of Ag(Ⅱ)ion,which only exhibits a tetra-coordinated mode to form 1D chain. The packing of the chains is dominated by face-tofaceπ…πstacking interactions between phenanthroline rings existing inside the chains and separated at a centroid-to-centroid distance of 0.357 0(0)and 0.368 4(4)nm(Fig.2c).Such interactions lead the formation of two-dimensional layer as shown in Fig. 2d.Uncoordinated Hdmphen ligandsare also connected to the two-dimensional layer through face-to-faceπ…πstacking interactions(centroid-to-centroid distance of 0.366 1(3)nm)and strong hydrogen bonding(the main hydrogen bonding:N1-H1A…O2 0.256 2(9) nm).
2.2 FTIR analysis
Fig.S1 exhibits the IR spectra of compounds 1~2. In the spectra of 1~2,the characteristic bands at 1 014,969,921 and 797 cm-1for 1,as well as 1 012, 972,922,and 798 cm-1for2 are attributed toν(Si-Oa),ν (W-Od),corner-sharingν(W-Oc-W)and edge-sharingνas(W-Oe-W)of the[SiW12O40]4-polyoxoanions[15],respectively.Furthermore,the characteristic absorption bands corresponding to theν(C=N)vibration of the organic ligands dmbipy and dmphen shift to lower wavenumber of 1 559 cm-1for compound 1,and 1 535 cm-1for 2,respectively.This negative shifts indicate the coordination ofpyridine nitrogen to the metalatom[16].The band of3 319 cm-1for 1 is associated with the ν(O-H)stretching mode of water molecules.The band of 3 547 cm-1for 2 is attributed to theν(N-H) stretching mode of the pyridine ring.The bands observed at 535~518 and 406~404 cm-1are assigned to theν(M-N)andν(M-O)vibrations,respectively.
2.3 Thermalstability
In order to characterize the thermal stabilities about the compounds 1~2,thermogravimetric(TGA) analyzer was performed under nitrogen atmosphere (Fig.S2).In compound 1,the TGA curve shows a twostep weight loss.The first weight loss of 2.81% (Calcd.2.94%)occurs from 20 to 150℃,which corresponds to the loss of all water molecules.The second weight loss of 16.27%(Calcd.15.01%)occurs from 460 to 810℃that may be due to the loss of dmbipy ligand.Compound 2 is stable until 410℃. After that temperature,the host framework then collapses rapidly.A major weight loss of 23.74% (Calcd.22.10%)takes place,due to the decomposition of the organic ligand dmphen.The weight loss is in agreement with the theoretical value,which further confirms the structuralformula ofthe title compounds.
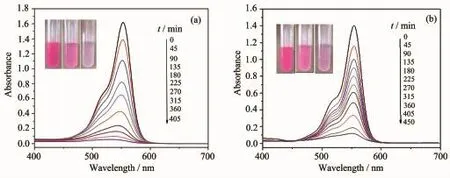
Fig.3 Photocatalytic degradation of RhB by compound 1(a)or 2(b)
2.4 Photocatalytic activity
As known,the POMs-modified transition metal compounds have been proved good photocatalysts in degrading organic dyes[17].In our work,the photocatalytic performance of compounds 1~2 was evaluated by the photocatalytic degradation of RhB,as shown in Fig.3.It can be clearly observed from Fig.3 that only 10%of RhB can be degraded without any compounds after the irradiation of UV light for 450 min.After compound 1 or 2 was added,the photocatalytic activities greatly improve.The degradation efficiency can reach 90.3%for 1,and 66.0%for 2,after 315 min.Changes in the concentration ratios(C/C0)of RhB solutions versus reaction time of the two compounds are plotted in Fig.4.Clearly,thephotocatalytic efficiency of compound 1 is higher than compound 2,probably because of their different metal centers and structures.
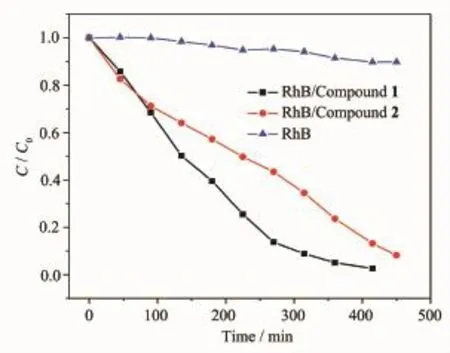
Fig.4 Plots of the concentration ratios of RhB (C/C0)vs irradiation time(min)in the absence and presence of 1 or 2 during the decomposition reaction under UV irradiation
2.5 Cyclic voltammetry
In order to investigate the electrochemical properties of the compounds 1~2,bulk-modified GCEs of the title compounds are the optimal choice,due to their high thermal stability and low solubility in water and common organic solvents.The cyclic voltammograms for 1-GCE and 2-GCE in 1 mol·L-1H2SO4aqueous solution at different scan rates were presented in Fig.5.In the potential range of-800~200 mV for 1-GCE,there are two reversible redox peaks:Ⅰ-Ⅰ′,Ⅱ-Ⅱ′.The mean peak potentials,E1/2= (Epa+Epc)/2,are-481(Ⅰ-Ⅰ′)and-646(Ⅱ-Ⅱ′)mv (scan rate:120 mV·s-1).For 2-GCE,two reversible redox peaks appear in the potential range of-600~600 mV and the half-wave potentials are at 60(Ⅰ-Ⅰ′)and-450(Ⅱ-Ⅱ′)mV(scan rate:120 mV·s-1), respectively.These redox peaks(Ⅰ-Ⅰ′andⅡ-Ⅱ′) for 1-GCE and 2-GCE correspond to two consecutive one-electron processes of SiW12anions[18].The peak potentials of cyclic voltammograms for 1-GCE and 2-GCE change gradually following the scan rates from 30 to 300 mV·s-1.Namely,with increasing scan rates, the cathodic peak potentials shift toward the negative direction and the corresponding anodic peak potentials to the positive direction.That is to say the peak currents are proportional to the scan rates up to 300 mV·s-1(Fig.5,inset),which indicate that the redox process of 1-GCE and 2-GCE are surfaceconfined.
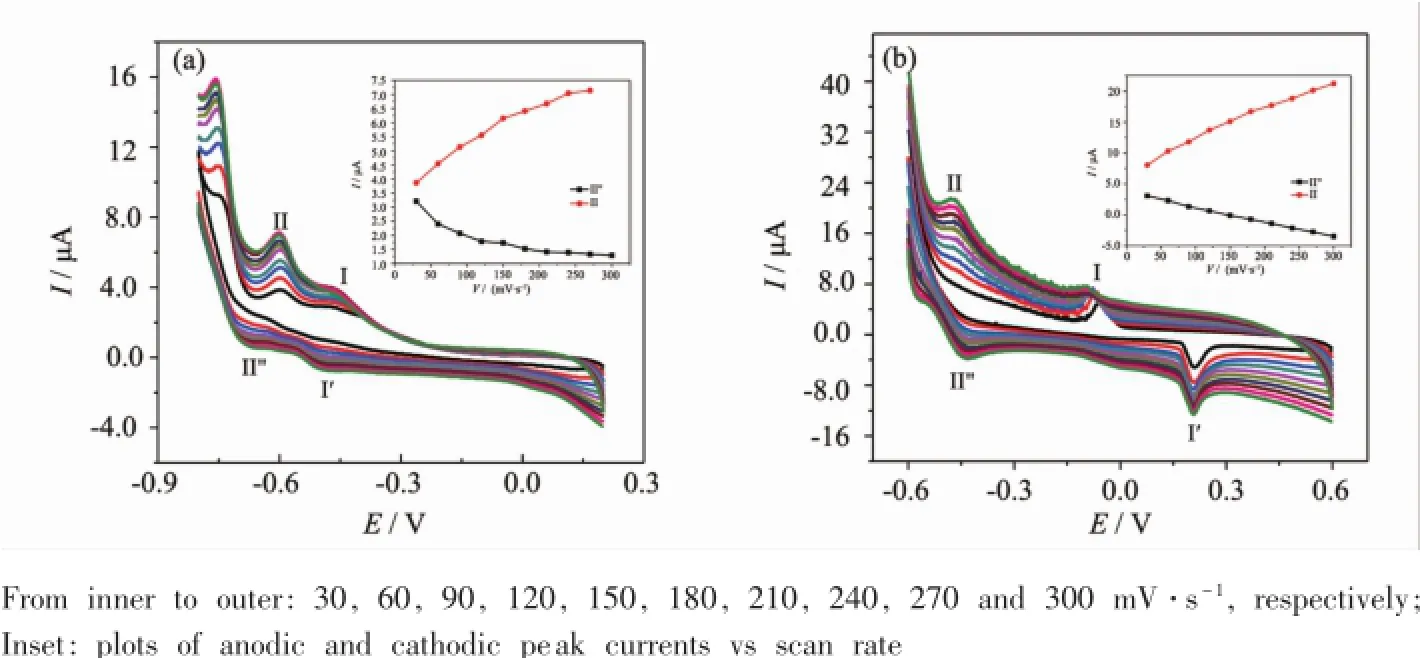
Fig.5 Cyclic voltammograms of 1-GCE(a)and 2-GCE(b)in 1 mol·L-1H2SO4at different scan rates
3 Conclusions
In this work,two polyoxometalates-modified transition metal compounds(POMs-TMC)based on two kinds of simply rigid organic ligands have been constructed.Compounds 1 and 2 are binuclear cluster and 1D structure,respectively.They extend to a 2D framework by noncovalent interactions.These two compounds present good photocatalytic activities.The electrochemical behavior of the two compounds indicates that the redox ability of the SiW12anions can stillbe preserved in the finalstructure.
Supporting information is available athttp://www.wjhxxb.cn
[1]Zhou J,Zhao J W,Wei Q,et al.J.Am.Chem.Soc.,2014, 136(13):5065-5071
[2]Han X B,Li Y G,Zhang Z M,et al.J.Am.Chem.Soc., 2015,137(16):5486-5493
[3]Suzuki K,Tang F,Kikukawa Y,et al.Angew.Chem.Int. Ed.,2014,53(21):5356-5360
[4]Tian A X,Ning Y L,Ying J,et al.Dalton Trans.,2015,44 (22):10499-10507
[5]Tian A X,Yang Y,Ying J,et al.Dalton Trans.,2014,43(22): 8405-8413
[6]Wang L,Shan Y X,Jiang Y H,et al.J.Inorg.Organomet. Polym.,2014,24(6):954-962
[7]Ma H X,Du J,Zhu Z M,et al.Dalton Trans.,2016,45(4): 1631-1637
[8]Artetxe B,Reinoso S,San Felices L,etal.Inorg.Chem.,2015, 54(1):241-252
[9]Wang X L,Li N,Tian A X,et al.Inorg.Chem.,2014,53 (14):7118-7129
[10]Wang X,Duan X Y,Kong C Y,et al.J.Coord.Chem., 2016,69(5):779-787
[11]Sheldrick G M.SHELXS-97,Program for Automatic Solution of Crystal Structure,University of Göttingen, Germany,1997.
[12]Sheldrick G M.SHELXL-97,Program for Crystal Structure Refinement,University of Göttingen,Germany,1997.
[13]Wang X L,Xu C,Lin H Y,et al.CrystEngComm,2012,14 (18):5836-5844
[14]WANG Lei(王蕾),GU Xiao-Ming(顾晓敏),YE Dan-Dan (叶丹丹),et al.Chinese J.Inorg.Chem.(无机化学学报), 2016,32(4):691-698
[15]Zhang Z,Yang J,Liu Y Y,et al.CrystEngComm,2013,15 (19):3843-3853
[16]Wang L,Shan Y X,Gu X M,et al.J.Coord.Chem.,2015, 68(11):2014-2028
[17]Tian A X,Ning Y L,Ying J,et al.CrystEngComm,2015,17 (29):5569-5578
[18]Tian A X,Hou X,Ying J,et al.RSC Adv.,2015,5(66): 53757-53765
Two POMs-Modified Transition Metal Compounds Constructed by Rigid Ligands: Structures,Catalytic and Electrochemical Properties
YE Dan-Dan WANG Lei GU Xiao-Min NI Liang ZHANG Wen-Li*
(School of Chemistry and Chemical Engineering,Jiangsu University,Zhenjiang,Jiangsu 212013,China)
By utilizing two kinds of simply rigid organic ligands,two new polyoxometalates-modified transition metal compounds(POMs-TMC),[Cu(dmbipy)2(SiW12O40)][Cu(dmbipy)(H2O)3]·3H2O(1),and{(Hdmphen)2[Ag2(dmphen)2(SiW12O40)]}n(2)(dmbipy=4,4′-dimethyl-2,2′-bipyridine,dmphen=2,9-dimethyl-1,10-phenanthrolin),were synthesized through hydrothermal technique.In compound 1,there are two crystallographically independent Cu(Ⅱ)ions.They all exhibit penta-coordinated modes,with three water molecules embedding through Cu-O bonds. Different Cu(Ⅱ)subunits cross-link each other to build a 1D chain by hydrogen bonding interactions between coordinated water molecules and[SiW12O40]4-anions.Adjacent chains also pursue to construct a 2D layer by such hydrogen bonding.In compound 2,the Keggin anions are fused by Ag(Ⅱ)ions to form a 1D chain.The adjacent chains are further linked byπ…πstacking and hydrogen bonding interactions.So,2D polyoxometalates-modified organic layer is constructed.The main traits of compounds 1 and 2 are that these simply rigid organic ligands employ noncovalent interactions to form the 2D layer.CCDC:1033952,1;1053009,2.
polyoxometalates;transition metal compound;catalytic activity;electrochemical property
O614.121;O614.122
A
1001-4861(2016)09-1629-08
10.11862/CJIC.2016.189
2016-05-05。收修改稿日期:2016-06-23。
国家自然科学基金(No.21507047)、中国博士后基金(No.2015M571695)、江苏省博士后科学基金(No.1401176C)和江苏大学高级人才启动基金项目(No.14JDG053)资助。
*通信联系人。E-mail:wlzhang@ujs.edu.cn

