宫颈鳞癌组织中促红细胞生成素及其受体蛋白表达、微血管密度变化
胡湘麟,周铁军
(1西南医科大学临床医学院,四川泸州 646000;2西南医科大学附属医院)
·临床研究·
宫颈鳞癌组织中促红细胞生成素及其受体蛋白表达、微血管密度变化
胡湘麟1,周铁军2
(1西南医科大学临床医学院,四川泸州 646000;2西南医科大学附属医院)
目的 观察宫颈鳞癌组织促红细胞生成素(EPO)、促红细胞生成素受体(EPO-R)蛋白表达及微血管密度(MVD)变化,并探讨三者与宫颈鳞癌临床病理参数的关系。方法 收集宫颈鳞癌组织标本76例份(观察组),宫颈高度鳞状上皮内病变(HSIL)组织标本25例份(HSIL组),宫颈低度鳞状上皮内病变(LSIL)组织标本20例份(LSIL组),正常宫颈组织标本20例份(正常组)。用免疫组化SP法检测各组宫颈组织EPO、EPO-R蛋白及CD31,根据CD31免疫组化染色结果测算MVD。比较各组EPO、EPO-R蛋白表达及MVD,分析观察组EPO、EPO-R蛋白表达及MVD与宫颈鳞癌患者年龄、肿瘤直径、组织学分级、FIGO分期、脉管腔侵犯、淋巴结转移的关系,分析观察组宫颈鳞癌组织EPO、EPO-R表达与MVD的相关性。结果 观察组、HSIL组、LSIL组、正常组EPO阳性表达率分别为76.32%、52.00%、25.00%、10.00%,EPO-R阳性表达率分别为82.89%、60.00%、30.00%、15.00%,观察组与HSIL组、LSIL组、正常组相比,HSIL组与正常组相比,P均<0.05。观察组、HSIL组、LSIL组、正常组MVD分别为(45.46±5.62)、(25.64±3.80)、(12.35±2.70)、(6.90±1.62)条/5 HP,各组两两相比,P均<0.05。观察组EPO、EPO-R蛋白的表达与患者年龄、肿瘤直径、组织学分级、FIGO分期、脉管腔侵犯、淋巴结转移无关(P均>0.05);MVD与患者肿瘤直径、组织学分级、脉管腔侵犯、淋巴结转移有关(P均<0.05)。观察组MVD与EPO、EPO-R表达均呈正相关(r分别为0.623、0.575,P均<0.05)。结论 宫颈鳞癌组织EPO、EPO-R蛋白表达及MVD升高,EPO、EPO-R可能通过促进肿瘤血管形成而促进宫颈鳞癌的进展。
子宫颈鳞状细胞癌;促红细胞生成素;促红细胞生成素受体;微血管密度
宫颈癌是女性最常见的恶性肿瘤之一。宫颈癌组织中丰富的新生微血管不仅为肿瘤的生长提供了充足的营养和氧气,也是癌细胞发生转移的重要途径[1,2]。促红细胞生成素(EPO)是一种分泌性的糖蛋白细胞因子,通常由胎儿肝脏及成人肾脏合成,机体合成的EPO通过与其特异性受体促红细胞生成素受体(EPO-R)相互作用,促进红系祖细胞的增殖分化。大量研究[3]表明,EPO、EPO-R广泛地表达于多种恶性实体肿瘤组织,并在肿瘤的血管形成方面发挥作用。微血管密度(MVD)为评价肿瘤血管生成的“金标准”[4]。本研究观察了宫颈鳞癌组织EPO、EPO-R蛋白表达及MVD变化,并探讨了三者与宫颈鳞癌临床病理参数的关系。
1 材料与方法
1.1 标本来源 收集西南医科大学附属医院2010年1月~2011年12月存档的宫颈鳞癌组织石蜡标本76例份(观察组),宫颈高度鳞状上皮内病变(HSIL)组织标本25例份(HSIL组),宫颈低度鳞状上皮内病变(LSIL)组织标本20例份(LSIL组),因子宫肌瘤行全子宫切除的正常宫颈组织标本20例份(正常组)。所有标本均经HE染色后由病理学专家确诊,所有患者病历资料保存完整,均为首次发病,术前均未接受放、化疗,患者无贫血及红细胞增多症。观察组患者年龄28~62(44.9±7.0)岁,>40岁者46例、≤40岁者30例;依据国际妇产科联盟(FIGO,2000年)的临床分期标准Ⅰ期42例、Ⅱ期34例;肿瘤直径>4 cm者28例、肿瘤直径≤4 cm者48例;按WHO组织学分类标准,角化型25例、非角化型51例;有淋巴结转移31例、无淋巴结转移45例;有脉管腔侵犯33例、无脉管腔侵犯43例。所有标本均经4%中性甲醛固定,石蜡包埋,4 μm连续切片。
1.2 宫颈组织EPO、EPO-R蛋白检测及MVD测算方法 采用免疫组织化学染色SP法,操作严格按照试剂盒说明书进行,EPO及EPO-R抗体均按1∶100稀释,CD31抗体按1∶200稀释,用PBS代替一抗作为阴性对照,用试剂商提供的阳性片作为阳性对照。所有免疫组化染色结果均由2位病理医生盲法阅片判断,对每张切片至少在高倍镜(400×)下观察10个不重复视野来判断结果。EPO、EPO-R表达根据染色强度进行评分(不着色计0分、黄色计1分、棕黄色计2分、黄褐色计3分),同时将阳性细胞的比例也分为4级进行评分(阳性细胞数<10%计0分、10%~<40%计1分、40%~<70%计2分、≥70%计3分),将上述两种评分相加,0~1分为-、2分为+、3~4分为++、5~6分为+++,其中-为阴性、+、++、+++为阳性。参照Weidner评判标准[5]进行MVD检测,即肿瘤区凡染成棕黄色的单个内皮细胞或内皮细胞簇就作为1条微血管,管壁有明显肌层、纤维硬化、存在炎症及坏死的微血管均不计入总数。随机计数5个高倍视野内微血管数,取其平均值作为MVD值。

2 结果
2.1 四组宫颈组织EPO、EPO-R蛋白表达及MVD比较 EPO、EPO-R的阳性表达部位主要在细胞质和细胞膜,表现为弥漫性分布的棕黄色颗粒。观察组、HSIL组、LSIL组、正常组EPO阳性表达率分别为76.32%(58/76)、52.00%(13/25)、25.00%(5/20)、10.00%(2/20),EPO-R阳性表达率分别为82.89%(63/76)、60.00%(15/25)、30.00%(6/20)、15.00%(3/20),观察组与HSIL组、LSIL组、正常组相比,HSIL组与正常组相比,P均<0.05。
肿瘤组织区CD31染色的微血管形态不规则,可有或无管腔,主要分布在肿瘤组织边缘。观察组、HSIL组、LSIL组、正常组MVD分别为(45.46±5.62)、(25.64±3.80)、(12.35±2.70)、(6.90±1.62)条/5 HP,随着病变程度逐渐加重,MVD值逐渐增高,各组两两相比,P均<0.05。
2.2 EPO、EPO-R表达及MVD与宫颈鳞癌临床病理参数的关系 观察组EPO、EPO-R蛋白的表达与患者年龄、肿瘤直径、组织学分级、FIGO分期、脉管腔侵犯、淋巴结转移无关(P均>0.05);MVD与患者肿瘤直径、组织学分级、脉管腔侵犯、淋巴结转移有关(P均<0.05),与年龄、FIGO分期无关(P均>0.05)。见表1。
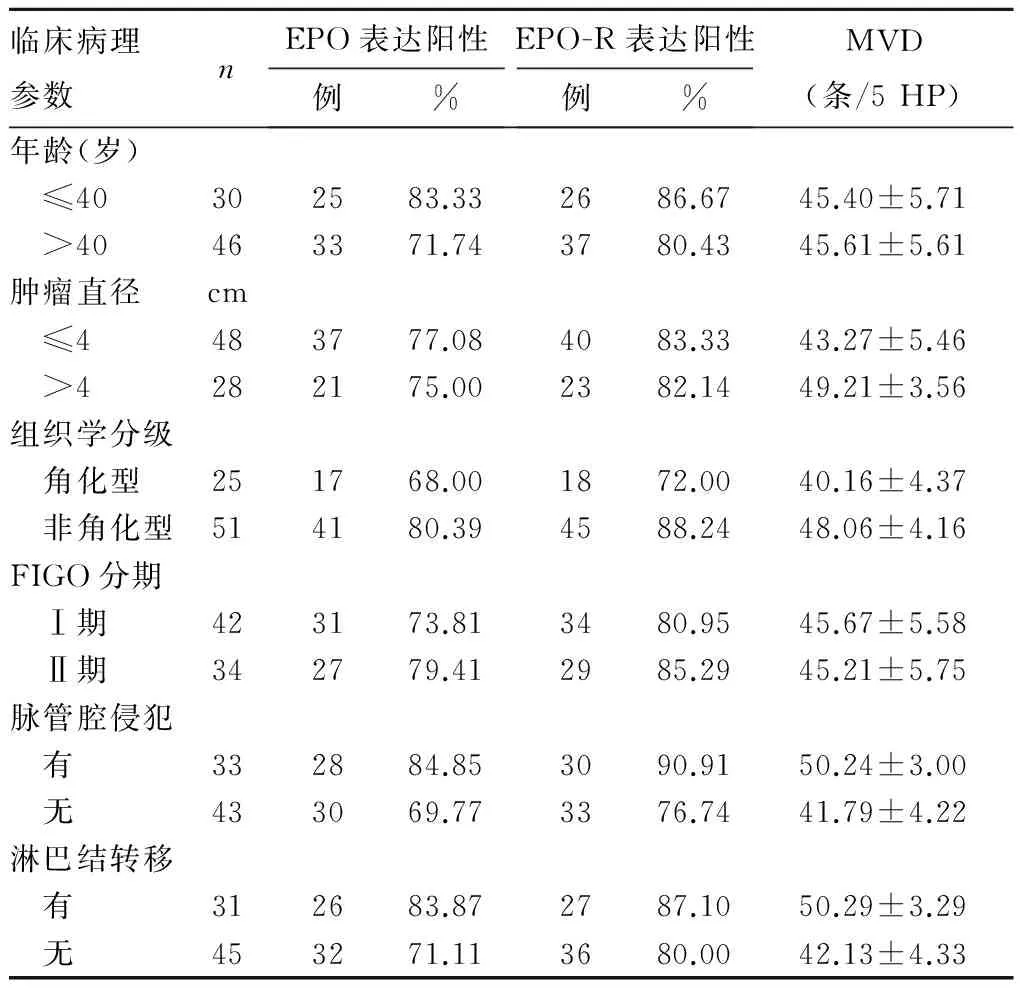
表1 EPO、EPO-R表达及MVD与宫颈鳞癌临床病理参数的关系
2.3 观察组EPO、EPO-R表达与MVD的相关性 观察组EPO阳性、阴性患者MVD分别为(47.48±4.58)、(39.61±3.35)条/5 HP,两者比较,差异有统计学意义(t=8.427,P<0.05)。EPO-R阳性、阴性患者MVD分别为(47.05±4.59)、(38.08±3.57)条/5 HP,两者比较,差异有统计学意义(t=6.630,P<0.05)。观察组MVD与EPO、EPO-R表达均呈正相关(r分别为0.623、0.575,P均<0.05)。
3 讨论
癌性贫血是晚期恶性肿瘤患者的常见并发症之一,严重影响着患者的生活质量和治疗效果。对于宫颈癌患者,肿瘤本身所引起的出血、癌细胞的骨髓转移以及接受放化疗等均可导致其贫血的发生。临床试验[6,7]发现重组人EPO(rhuEPO)能有效提高肿瘤患者的血红蛋白水平,减少输血,在癌性贫血的预防和治疗上有着十分广泛的应用。然而,后来多项研究[8]报道,肿瘤患者经rhuEPO治疗后贫血状况改善,但无进展生存期并未延长,甚至增加了患者的病死率。同时,分子生物学实验[9,10]表明EPO会促进肿瘤增殖,抑制细胞凋亡,并使肿瘤细胞对某些放化疗方案产生抵抗。鉴于rhuEPO这种利弊兼备的特点,rhuEPO的临床应用颇受争议[11]。因此,EPO、EPO-R与肿瘤关系一直都是国内外研究的热点。鳞癌是宫颈癌最常见的组织学类型,宫颈鳞癌是LSIL、HSIL等癌前病变逐步发展的结果。本研究结果显示,从正常宫颈、LSIL、HSIL到宫颈鳞癌组织,EPO、EPO-R阳性表达率随病变程度的加重而逐渐增加,这表明EPO、EPO-R的表达与宫颈鳞癌的发生发展有关,表达逐渐增强的EPO、EPO-R可能会促进宫颈鳞癌病变的进展,这与Acs等[12]的研究结果相符合。
同许多恶性实体肿瘤一样,宫颈鳞癌的发生发展依赖于持续性的氧气供应,但由于氧气在瘤体中的弥散范围有限,局部肿瘤组织常常处于缺氧的环境。缺氧随即激活多种分子通路促进肿瘤血管形成,以缓解肿瘤的缺氧状态。Smith-McCune等[13]研究发现,血管形成在宫颈癌前病变时即已发生,至宫颈鳞癌时肿瘤组织内的新生微血管已相当密集。本研究结果显示,随着宫颈鳞癌病变的进展,MVD逐渐增高。MVD与宫颈鳞癌患者的肿瘤直径、组织学分级、脉管腔侵犯、淋巴结转移有关,提示增多的肿瘤微血管可以为瘤体的生长和浸润转移提供更有利的条件。观察组EPO、EPO-R阳性者的MVD均明显高于对应的阴性者,并且观察组宫颈鳞癌组织MVD与EPO、EPO-R表达均呈正相关,表明EPO、EPO-R可能具有促进肿瘤血管形成的作用。其生物学过程可能为,宫颈鳞癌的快速生长使得肿瘤处于缺氧状态,缺氧激活EPO、EPO-R等缺氧反应基因,高表达的EPO与EPO-R结合后激活JAK-2/STAT-5、Ras/MAPK等信号转导通路[14],通过诱导大量新生微血管的形成和长入,增加肿瘤的局部氧供,便于癌细胞的浸润转移,从而继续维持或促进了宫颈癌的进展。这也可能是癌性贫血患者应用rhuEPO后肿瘤控制情况及患者预后较差的机制之一。
综上可见,宫颈鳞癌组织EPO、EPO-R蛋白表达及MVD升高,EPO/EPO-R信号系统在宫颈鳞癌的病理进程中发挥着潜在的效应,EPO、EPO-R可能通过促进肿瘤血管形成而促进宫颈鳞癌的进展。
[1] Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012[J]. CA Cancer J Clin, 2015,65(2):87-108.
[2] Randall LM, Monk BJ, Darcy KM, et al. Markers of angiogenesis in high-risk, early-stage cervical cancer: A Gynecologic Oncology Group study[J]. Gynecol Oncol, 2009,112(3):583-589.
[3] Hardee ME, Arcasoy MO, Blackwell KL, et al. Erythropoietin biology in cancer [J]. Clin Cancer Res, 2006,12(2):332-339.
[4] Randall LM, Monk BJ, Darcy KM, et al. Markers of angiogenesis in high-risk, early-stage cervical cancer: A Gynecologic Oncology Group study[J]. Gynecol Oncol, 2009,112(3):583-589.
[5] Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors [J]. Breast Cancer Res Treat, 1995,36(2):169-180.
[6] Aapro M, Jelkmann W, Constantinescu SN, et al. Effects of erythropoietin receptors and erythropoiesis-stimulating agents on disease progression in cancer[J]. Br J Cancer, 2012,106(7):1249-1258.
[7] Blohmer JU, Paepke S, Sehouli J, et al. Randomized phase III trial of sequential adjuvant chemoradiotherapy with or without erythropoietin Alfa in patients with high-risk cervical cancer: results of the NOGGO-AGO intergroup study[J]. J Clin Oncol, 2011,29(28):3791-3797.
[8] Debeljak N, Solar P, Sytkowski AJ, et al. Erythropoietin and cancer: the unintended consequences of anemia correction[J]. Front Immunol, 2014,5:563.
[9] Liang K, Esteva FJ, Albarracin C, et al. Recombinant human erythropoietin antagonizes trastuzumab treatment of breast cancer cells via Jak2-mediated Src activation and PTEN inactivation[J]. Cancer Cell, 2010,18(5):423-435.
[10] Wu P, Zhang N, Wang X, et al. The erythropoietin/erythropoietin receptor signaling pathway promotes growth and invasion abilities in human renal carcinoma cells[J]. PLoS One, 2012,7(9):e45122.
[11] Cao Y. Erythropoietin in cancer: a dilemma in risk therapy[J]. Trends Endocrinol Metab, 2013,24(4):190-199.
[12] Acs G, Zhang PJ, McGrath CM, et al. Hypoxia-inducible erythropoietin signaling in squamous dysplasia and squamous cell carcinoma of the uterine cervix and its potential role in cervical carcinogenesis and tumor progression[J]. Am J Pathol, 2003,162(6):1789-1806.
[13] Smith-McCune KK, Weidner N. Demonstration and characterization of the angiogenic properties of cervical dysplasia[J]. Cancer Res, 1994,54(3):800-804.
[14] Aguilera TA, Giaccia AJ. The end of the hypoxic EPOch[J]. In J Radiat Oncol Biol Phys, 2015,91(5):895-897.
Expression of erythropoietin/erythropoietin-receptor and microvessel density changes in cervical squamous cell carcinoma
HUXianglin1,ZHOUTiejun
(1SouthwestMedicalUniversity,Luzhou646000,China)
Objective To observe the expression of erythropoietin (EPO)/erythropoietin-receptor (EPO-R) and measure microvessel density (MVD) in cervical squamous cell carcinoma and to investigate the relationships of them with clinicopathological parameters.Methods Seventy-six cases of patients with cervical squamous cell carcinoma (observation group), 25 cases of high-grade squamous intraepithelial lesion (HSIL group), 20 cases of low-grade squamous intraepithelial lesion (LSIL group) and 20 cases of normal cervical epithelia (normal group) were collected. Immunohistochemical SP method was performed to detect the expression of EPO/EPO-R and CD31 expression and then we measured MVD in these specimens. The expression differences of EPO/EPO-R and MVD in these specimens were compared. In the observation group, correlations of EPO/EPO-R and MVD with patients' clinicopathological parameters such as age, tumor size, histological grading, FIGO staging, lymphovascular invasion and lymph node metastasis were analyzed, and correlations of EPO/EPO-R expression with MVD were also analyzed in cervical squamous cell carcinoma. Results The positive expression rates of EPO in the observation group, HSIL group, LSIL group and normal group were 76.32%, 52.00%, 25.00% and 10.00%, respectively; the positive expression rates of EPO-R were 82.89%, 60.00%, 30.00% and 15.00%, respectively. The positive expression rates of EPO and EPO-R were gradually increased from normal cervix to LSIL to HSIL and then to cervical squamous cell carcinoma, and the difference was statistically significant (allP<0.05). The MVD of the observation group, HSIL group, LSIL group and normal group was (45.46±5.62), (25.64±3.80), (12.35±2.70) and (6.90±1.62)/5HP, and significant difference was found among these groups (allP<0.05). In the observation group, EPO and EPO-R expression was not significantly correlated with age, tumor size, histological grading, FIGO staging, lymphovascular invasion and lymph node metastasis (allP<0.05). MVD was significantly correlated with tumor size, histological differentiation, lymphovascular invasion and lymph node metastasis (allP<0.05). In the observation group, MVD was positively correlated with EPO expression (r=0.623, allP<0.05) as well as EPO-R expression (r=0.575,P<0.05). Conclusions EPO/EPO-R expression and MDV increase in the cervical squamous cell carcinoma tissues, and EPO and EPO-R may promote the progression of cervical squamous cell carcinoma by enhancing the cervical angiogenesis.
cervical squamous cell carcinoma; erythropoietin; erythropoietin-receptor; microvessel density
西南医科大学附属医院科研基金资助项目[(2014)71号]。
周铁军(E-mail: tiejunzh502@163.com)
10.3969/j.issn.1002-266X.2016.35.016
R737.3
B
1002-266X(2016)35-0051-04
2016-03-01)

