Preventive Effects of Polyphenols from Insect Tea on Acetaminophen-Induced Hepatic Injury in ICR Mice
SUN Peng, FENG Xia, YI Ruokun, ZHAO Xin
(Chongqing Collaborative Innovation Center for Functional Food, Chongqing Engineering Research Center of Functional Food, Chongqing Engineering Laboratory for Research and Development of Functional Food, Department of Biological and Chemical Engineering, Chongqing University of Education, Chongqing 400067, China)
Preventive Effects of Polyphenols from Insect Tea on Acetaminophen-Induced Hepatic Injury in ICR Mice
SUN Peng, FENG Xia, YI Ruokun, ZHAO Xin*
(Chongqing Collaborative Innovation Center for Functional Food, Chongqing Engineering Research Center of Functional Food, Chongqing Engineering Laboratory for Research and Development of Functional Food, Department of Biological and Chemical Engineering, Chongqing University of Education, Chongqing 400067, China)
The purpose of this study was to investigate the preventive effects of crude polyphenols from Insect tea (CPIT) on hepatic damage induced by acetaminophen in ICR mice. CPIT could increase body weight and reduce liver weight and liver index after induction of liver injury in mice. Serum levels of alanine aminotransferase, aspartate aminotransferase, lacate dehydrogenase, triglycerides, and blood urea nitrogen were decreased, while total cholesterol and albumin were increased in mice treated with CPIT as compared with liver injury controls. CPIT also resulted in a reduction in IL (interleukin)-6, IL-12, TNF (tumor necrosis factor)-α and IFN (interferon)-γ in comparison with control mice. It could increase the mRNA expression of IκB-α, Mn-SOD, Cu/Zn-SOD, CAT and GSH-Px, and decrease the mRNA expression of NF-κB, iNOS and COX-2. From the above findings, we can conclude that CPIT has a preventive effect on hepatic injury in vivo.
Insect tea; polyphenol; acetaminophen; hepatic injury; expression
SUN Peng, FENG Xia, YI Ruokun, et al. Preventive effects of polyphenols from Insect tea on acetaminophen-induced hepatic injury in ICR mice[J]. 食品科学, 2016, 37(21): 257-264. DOI:10.7506/spkx1002-6630-201621044. http://www.spkx.net.cn
SUN Peng, FENG Xia, YI Ruokun, et al. Preventive effects of polyphenols from Insect tea on acetaminophen-induced hepatic injury in ICR mice[J]. Food Science, 2016, 37(21): 257-264. (in English with Chinese abstract) DOI:10.7506/ spkx1002-6630-201621044. http://www.spkx.net.cn
Insect tea is a natural organic tea which has the essence of insect and plant. Insect tea was used as a drink for 600 a, and its nutritional and medicinal properties were advantageous for human health. People put wild rattan and leaves of vine tea (Ampelopsis megalophylla Diels et Gilg), Kudingcha (Ilex Kuding tea C. J. Tseng), toringo (Malus sieboldii (Regel) Rehd.) and dyetree (Platycarya strobilacea Sieb. et Zucc.) together to lure the larvae of Nodaria niphona Butler, Aglossa dimidiate Haworth, Hydrillodes morose Butler, Fujimacia bicoloralis Leech and Herculia glaucinalis L. to eat them, leaving the droppings there. Then from the residue of these rattan and tea leaves out of droppings, which is named as Insect tea[1]. Insect tea contained many functional materials (amino acids, vitamins, polysaccharides, flavones, polyphenols etc.)[2]. These contents altogether fabricate the functional activities of Insect tea, such as anti-inflammatory effect of gastric ulcers[3]and anticancer effects of buccal mucosa cancer[4].
Polyphenols are the most important chemical substances in tea. Tea polyphenols have many functional effects, such as protective effects on liver. The studies showed that green tea has the hepatic damage protective effects[5-6]. Insect tea contains polyphenols[7], the polyphenol content of Insect tea is almost 10%, which is lower than common green tea. Moreover Insect tea also contains catechins, flavonoids, flavonols and anthocyanin. Phenolic acids, phenolic polymerization shrinkage, epigallocatechin gallate (EGCG), epigallocatechin (EGC), epicatechin 3-gallate (ECG) and epicatechin (EC), accounted for more than 70% of the content of tea polyphenols, and these constituents is the same as green tea[8]. The Insect tea polyphenols may also have many other protective effects, these effects need to be researched.
Acetaminophen act as a pain reliever and a fever reducer, and it could treat headache, muscle aches, arthritis, backache, toothaches, colds, and fevers. Studies showed that acetaminophen doses could also lead to liver damage[9-10]. Therefore, acetaminophen was used as chemical revulsant of inducing hepatic injury in mice[11]. In this study, the liver damage animal model was used for the research of preventive effects of crude polyphenols of Insect tea on hepatic injury. And the mechanisms of these preventive effects were determined by serum levels and molecular biology assays of serum and tissues.
1 Materials and Methods
1.1 Materials and Reagents
Insect tea was purchased in a local market (Guizhou, China).
Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), triglyceride (TG) and total cholesterol (TC), blood urea nitrogen (BUN), albumin (ALB) kits Nanjing Jiancheng Bioengineering Institute, China; serum lactate dehydrogenase (LDH) kit Cayman Chemical Co., USA; cytokines interleukin (IL)-6, IL-12, tumor necrosis factor (TNF)-α and interferon (IFN)-γ BioLegend Co., USA; acetaminophen Sigma, USA; trizol reagent Invitrogen, CA, USA; RNeasy kit Qiagen, Germany; avian myeloblastosis virus reverse transcriptase GE Healthcare, UK; primers Tiangen Biotech Co. Ltd., China.
1.2 Instruments and Equipments
N-1100 rotary evaporator Eywla, Japan; iMark microplate reader, T100 PCR instrument Bio-Rad, USA; BX41 microscope Olympus, Japan.
1.3 Methods
1.3.1 Extraction of CPIT
Insect tea were powdered after being frozen and dried. After that 500 g of the powder was mixed into 3 L distilled water and stirred at 90 ℃ for extraction for 1 h. After filtering, the filtrate was extracted for 2 h with 3 L acetic ether twice. The two organic phases were combined and dried with anhydrous sodium sulfate. Then acetic ether solvent was removed through depression and distillation. At last, CPIT were obtained as a yellow powder[4].
1.3.2 Induction of hepatic injury
The 6 weeks old of 40 male ICR mice were purchased from the Experimental Animal Center of Chongqing Medical University (Chongqing, China). The mice were divided into 4 groups (normal, control, 50 mg/kg CPIT and 100 mg/kg CPIT) for 10 mice in each group. The normal and control groups mice were administered equal amount of water and diet. The CPIT treated groups mice were received oral administration of 50 and 100 mg/kg CPIT treatment once a day for 14 days. Then the control, 50 and 100 mg/kg CPIT treated mice were received lumbar injection of acetaminophen at the treated concentration of 800 mg/kg. All the mice were killed using CO2after 16 h of acetaminophen treatment[10]. Blood from the inferior vena cava was collected in a tube and centrifuged at 3 000×g for 10 min at 4 ℃. Serum liver tissues were collected and preserved at -70 ˚C until required for the biological assays. And the liver index was analyzed by the formula as follow:
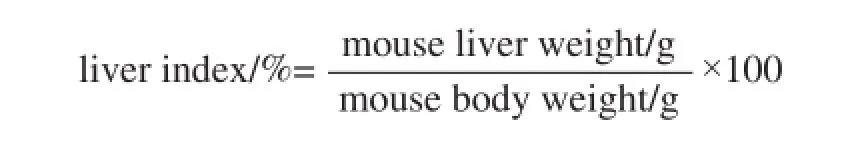
The experimental protocols were approved by the Animal Ethics Committee of Chongqing Medical University (SCXK (Yu) 2012-0001, Chongqing, China).
1.3.3 Serum levels assay
Serum levels of AST, ALT, TG, TC, BUN, ALB were determined using the experiment kits. Serum levels of LDH were also determined by a commercially available kit.
1.3.4 Serum cytokines analysis
The proinflammatory related serum cytokines of IL-6, IL-12, TNF-α and IFN-γ in mice were measured using enzyme linked immunosorbent assay (ELISA), according to the manufacturer’s instructions.
1.3.5 Histological assay of liver tissue
The liver tissue of mice were fixed in 10% (V/V) buffered formalin for 24 h, dehydrated in ethanol and embedded in paraffin. Subsequently, 4 μm thick sections were prepared and stained with hematoxylin and eosin (HE) to observe under an microscope.
1.3.6 Inflammatory related mRNA expression levels in the liver tissue by reverse transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA from mice liver tissues were isolated using trizol reagent according to the manufacturer’s recommendation. The extracted RNA was digested with RNase-free DNase (Roche, Basel, Switzerland) for 15 min at 37 ℃and purified using an RNeasy kit. cDNA was synthesized from 2 μg of total RNA by incubation at 37 ℃for l h with avian myeloblastosis virus reverse transcriptase with random hexanucleotides according to the manufacturer’s instruction. Then the sequences of primers used to specifically amplify the genes of NF-κB (forward: 5’-CAC TTA TGG ACA ACT ATG AGG TCT CTG G-3’and reverse: 5’-CTG TCT TGT GGA CAA CGC AGT GGA ATT TTA GG-3’), IκB-α (forward: 5’-GCT GAA GAA GGA GCG GCT ACT-3’ and reverse: 5’-TCG TAC TCC TCG TCT TTC ATG GA-3’), iNOS (forward: 5’-AGA GAG ATC GGG TTC ACA-3’ and reverse: 5’-CAC AGA ACT GAG GGT ACA-3’), COX-2 (forward: 5’-TTA AAA TGA GAT TGT CCG AA-3’ and reverse: 5’-AGA TCA CCT CTG CCT GAG TA-3’), Mn-SOD (forward: 5’-TTC AAT AAG GAG CAG GGA C-3’ and reverse: 5’-CAG TGT AAG GCT GAC GGT TT-3’), Cu/Zn-SOD (forward: 5’-GAA GAG AGG CAT GTT GGA GA-3’ and reverse: 5’-CCA ATT ACA CCA CGA GCC AA-3’), CAT (forward: 5’-AGA TAC TCC AAG GCG AAG GTG-3’ and reverse: 5’-AAA GCC ACG AGG GTC ACG AAC-3’) and GSH-Px (forward: 5’-GCG GGA GCA GGA CTT CTA CGA-3’ and reverse: 5’-CCC GAT AGT GCT GGT CTG TGA A-3’) were determined. And GAPDH (forward: 5’-CGG AGT CAA CGG ATT TGG TC-3’ and reverse: 5’-AGC CTT CTC CAT GGT CGT GA-3’) gene was amplified as an internal control gene. Amplification was performed in a thermal cycler and the PCR products were separated in 1.0% agarose gels with ethidium bromide staining[12].
1.4 Statistical analysis
Experimental data were presented as theDifferences between the mean values for individual groups were assessed with a one-way ANOVA with Duncan’s multiple range test. Differences were considered significant when P<0.05. SAS version 9.2 (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses.
2 Results and Analysis
2.1 Body weight, liver weight and liver index of experiment mice
The body weight of hepatic injury of control mice was lowest, but the liver weight and liver index were highest in all group mice (Table 1). CPIT could reduce liver weight and liver index, but on contrary it increase the body weight as compared with the control mice. The body weight, liver weight and liver index of high concentration of 100 mg/kg treated mice were very close to the normal mice.
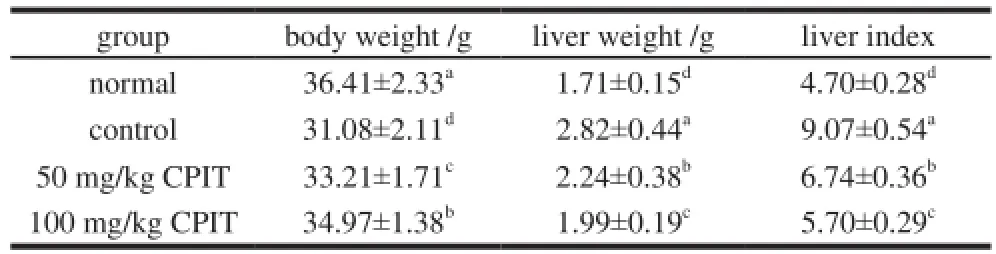
Table 1 Body weight, liver weight and liver index in mice treated with CPIT
The body weight, liver weight and liver index were important indexes of liver disease evaluation, the injured liver mice showed the higher liver weight and liver index as compared with the normal mice, and the body weight of injured liver mice was lower than normal mice[11]. In this study, the mice induced liver damage showed the same results, and CPIT could reduce this damage.
2.2 Serum levels of AST, ALT and LDH
After inducing hepatic injury of acetaminophen, nosample of CPIT treated control groups mice showed the increased serum levels of AST, ALT and LDH (Table 2). Meanwhile, the normal group mice had the lowest AST, ALT and LDH serum levels because of no hepatic injury inducing treatment. Furthermore, the CPIT groups mice were treated by acetaminophen, although the mice showed the lower levels of AST, ALT and LDH than control mice, however, higher than normal mice. And these levels of higher concentration of 100 mg/kg CPIT treated mice were lower than 50 mg/kg treatment.
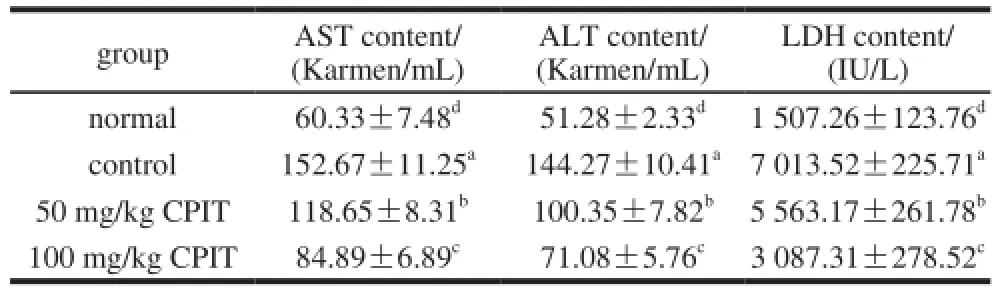
Table 2 Serum AST, ALT and LDH levels in mice treated with CPIT
After the live cell injury, AST and ALT level could release into the general circulation[13]. And LDH level raise is also an important index of liver damage, therefore the AST, ALT and LDH levels are clinically and experimentally important to check the liver damage[14]. CPIT could decrease the serum levels of AST, ALT and LDH, therefore it might decrease the liver injury.
2.3 Serum levels of TG, TC, BUN and ALB
The TC and ALB serum levels of normal mice were highest, and the normal mice showed the lowest TG and BUN levels in serum (Table 3). After inducing hepatic injury, the TC and ALB level were decreased, but the TG and BUN level were increased. CPIT could weaken these changes and the high concentration (100 mg/kg) of CPIT showed the strong interception.
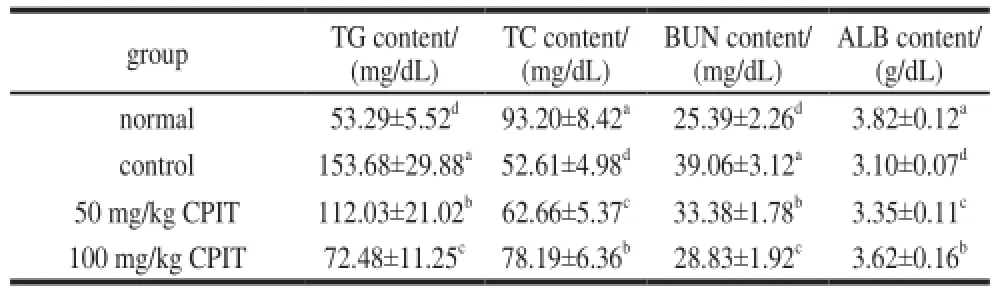
Table 3 Serum TG, TC, BUN and ALB levels in mice treated with CPIT
The liver is an important organ for human lipid metabolism, meantime TC is mainly synthetized in liver cells, the synthesis would be reduced after liver injury[15]. The liver also plays an important role in protein metabolism, it could synthesize the protein, the liver injury cause ALB synthesis hindrance, as a result ALB content would decrease[16]. The research reported that TG and BUN level could be increase after inducing damage to the liver[11]. CPIT could increase TC and ALB levels, and decrease TG, BUN levels in hepatic damaged mice. CPIT had hepatic damage preventive effects by these serum level changes.
2.4 Serum cytokine levels of IL-6, IL-12, TNF-α and IFN-γ
The serum cytokine levels of IL-6, IL-12, TNF-α and IFN-γ in the ICR mice which were treated with 50 and 100 mg/kg CPIT were significantly lower than control group mice (Table 4). However, the levels of these cytokines of 100 mg/kg CPIT treated mice were close to the levels of normal group mice. The levels of 100 mg/kg CPIT treated mice were significantly lower compared with those in the 50 mg/kg CPIT treated mice.
Patients with liver diseases exhibit elevated levels of serum cytokines of IL-6, IL-12 and TNF-α, as compared with healthy people[17]. Cytokine receptors and the cytokines of IL-6, IL-12, TNF-α and IFN-γ played the important pathogenic roles in gastric disease, the lower levels of these cytokines indicates an preventive effects on gastric injury in mice[19]. CPIT could decrease liver injury by the cytokine levels of IL-6, IL-12, TNF-α and IFN-γ reduce.
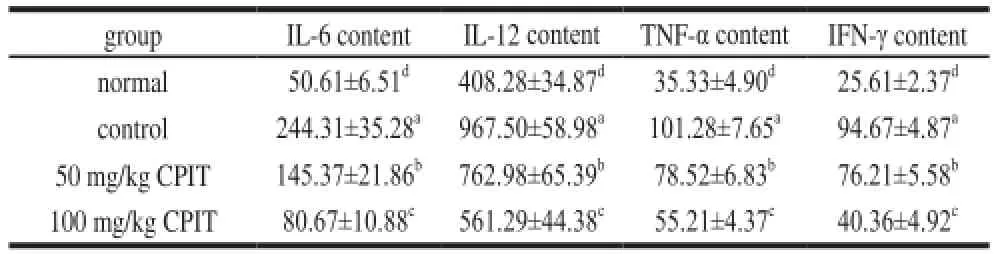
Table 4 Serum cytokine levels of IL-6, IL-12, TNF-αand IFN-γin mice treated with CPITpg/mL
2.5 Histopathology of liver
Acetaminophen could induce the liver injury (control group), this course could cause histopathological changes in the liver tissue of the mice, the centrilobular cells of liver tissue appeared to be degenerated and necrotized (Fig. 1). CPIT reduce the hepatic injury, 100 mg/kg dose could help the liver tissue to get back to normal, these were only some liver cells demonstrated a little congestion and hemorrhage in the area around the centrilobular vein.
Histopathology is an important technique for diagnosing liver injury, HE analysis of mice liver sections could evaluate the hepatoprotective activity[18]. These results showed that the higher concentration of CPIT decreased the degree of hepatic damage in mice.
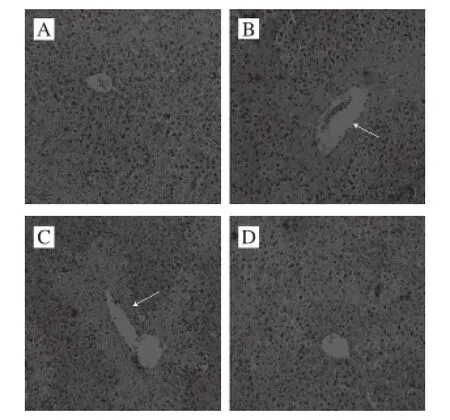
Fig. 1 Histological images of liver tissues from mice with acetaminophen-induced hepatic damage (× 200)
2.6 Gene expression of NF-κB, IκB-α, COX-2 and iNOS
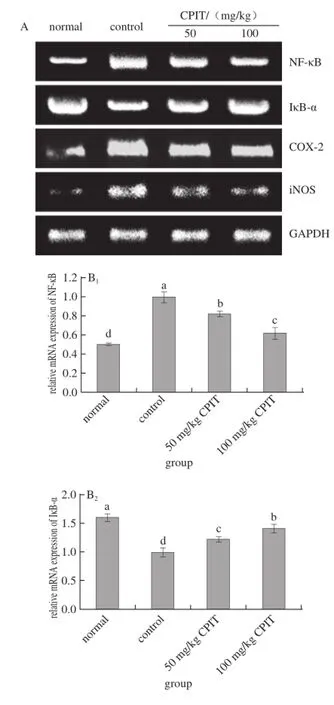
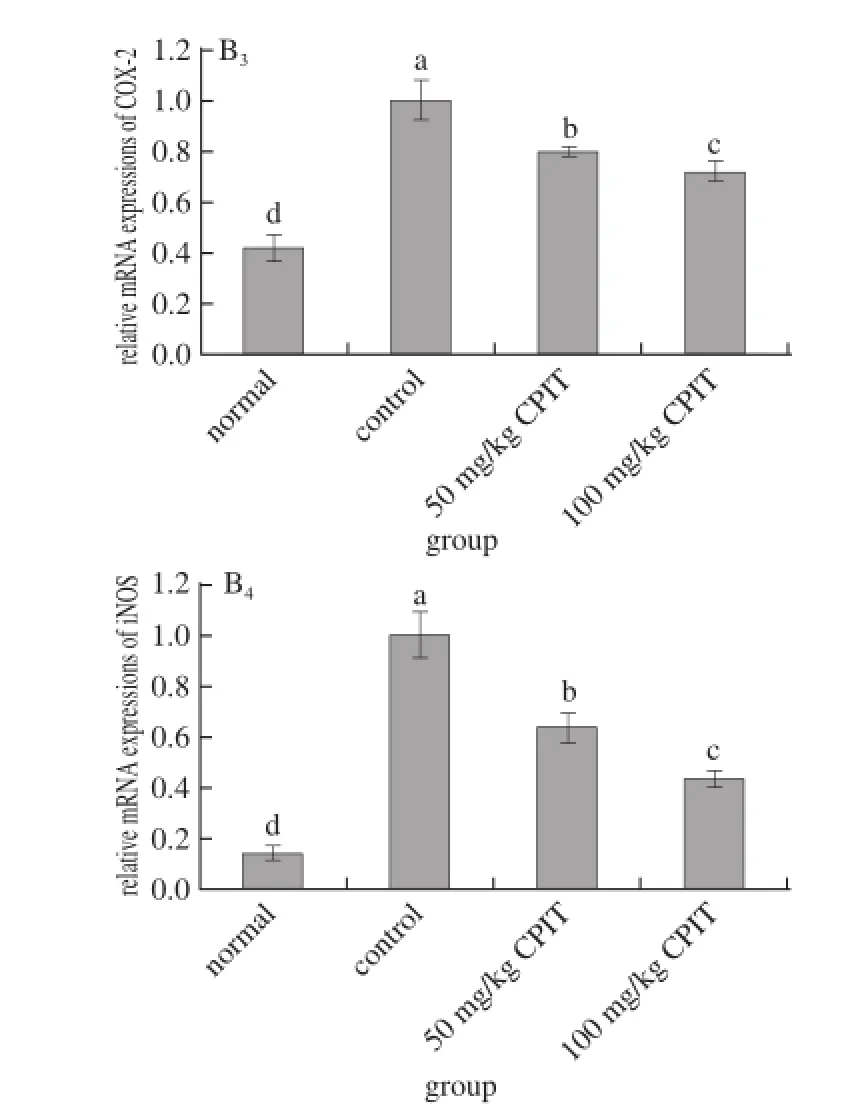
Fig. 2 Effect of CPIT on the mRNA expression levels of NF-κB, IκB-α, COX-2 and iNOS in liver tissues from mice with hepatic injury induced by acetaminophen
As indicated in Fig. 2, CPIT could decrease the mRNA expressions of NF-κB and increase the expressions of IκB-α compared with the liver tissues of mice having hepatic injury (control group). The 100 mg/kg CPIT treated mice showed the higher IκB-α (1.40 folds as control) expressions and lower NF-κB (0.61 folds of control) expressions, on the other hand 50 mg/kg CPIT treated mice (1.22 and 0.82 folds as control). NF-κB is a transcription factor, it could regulate the expression levels of genes required for inflammatory responses, such as the liver damage[20]. IκB-α could release NF-κB by reducing itself, the high NF-κB level and low IκB-α level marked the liver damage[21]. CPIT could keep the high IκB-α mRNA expression and low NF-κB expression after liver injury, which means CPIT has the preventive effects of liver injury.
The iNOS and COX-2 mRNA expression of CPIT treated mice were lower than those of control mice, these levels of 100 mg/kg CPIT treated mice were only 0.44 and 0.72 times of control mice, also slightly higher then the normal mice. The lower concentration of 50 mg/kg CPIT could decrease iNOS (0.64 folds as control group) andCOX-2 (0.80 folds as control group) mRNA expressions as compared with control mice. COX-2 and iNOS have been observed to cause deleterious effects in the liver following stimulating inflammation[22]. Where as iNOS and COX-2 enzymes could enhance inflammatory responses in the status of liver injury[23]. The liver injury mice could decrease the inflammatory responses by CPIT treatment, CPIT might control the levels of iNOS and COX-2 to prevent liver damage. Both polyphenols of Insect tea and green tea could reduce the hepatic injury through decreasing the inflammatory related genes by their polyphenols[24-25].
2.7 Gene expression of Mn-SOD, Cu/Zn-SOD, CAT and GSH-Px
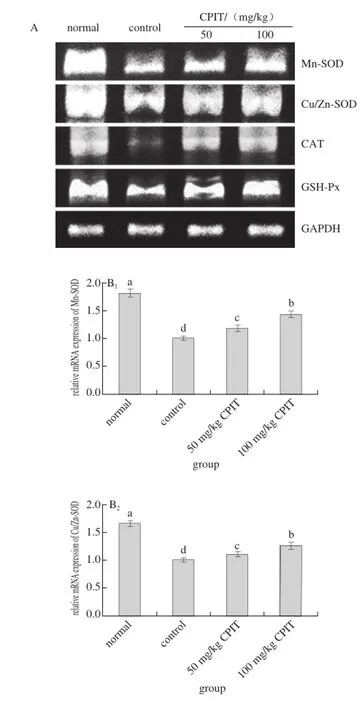
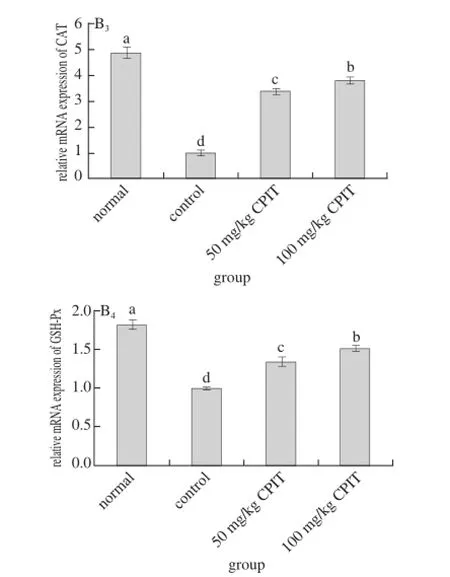
Fig. 3 Effect of CPIT on the mRNA expression levels of Mn-SOD, Cu/Zn-SOD, CAT and GSH-Px in liver tissues of mice with hepatic injury induced by acetaminophen
The mRNA expressions of Mn-SOD, Cu/Zn-SOD, CAT and GSH-Px in normal mice were highest where as the control had the lowest expressions (Fig. 3). The high concentration (100 mg/kg) of CPIT could raised the Mn-SOD, Cu/Zn-SOD, CAT and GSH-Px expressions (1.44, 1.26, 3.82 and 1.51 folds as control) as compared to the 50 mg/kg CPIT treated mice (1.18, 1.12, 3.41 and 1.35 folds as control) and control mice.
After liver damage, the liver tissues had oxidation phenomenon[26]. As important antioxidant enzymes, SOD and GSH-Px could convert peroxide caused by liver tissue oxidation to low-poisonous or harmless substance[27-28]. Mn-SOD and Cu/Zn-SOD are two kinds of SOD isozyme, they were beneficial to the recovery of liver injury[29]. CAT also could remove oxide free radicals, and it was able to protect liver injury[30]. Polyphenols of green tea could raise the oxidation related genes of Mn-SOD, Cu/Zn-SOD, CAT and GSH-Px[31-32], polyphenols of Insect tea also had antioxidation effects[33], and the anti-oxidation effects might derived from the Mn-SOD, Cu/Zn-SOD, CAT and GSH-Px genes change abilities, these changes could help Insect tea to reduce the hepatic injury.
3 Conclusions
In summary, this study demonstrated the preventive effect of crude CPIT on hepatic injury using a in vivo experiment, including serum tests of AST, ALT, LDH, TG, TC, BUN and ALB, and serum cytokine examinations of IL-6, IL-12, TNF-α and IFN-γ levels, However, the liver tissues of RT-PCR assay for determining the expressions of the genes associated with inflammation of NF-κB, IκB-α, iNOS, COX-2 and oxidation associated genes of Mn-SOD, Cu/Zn-SOD, CAT, GSH-Px. All the results showed that CPIT treatment prevented acetaminophen induced hepatic injury in ICR mice, and higher the concentration of CPIT better the treatment. CPIT one of the important components of Insect tea, these components have the functional effect on hepatic injury prevention. CPIT had a good hepatic injury preventive effect throngh its molecular biological actions like the green tea polyphenols.
[1] ZHAO X, WANG Q, LI G J, et al. In vitro antioxidant, anti-mutagenic, anti-cancer and anti-angiogenic effects of Chinese bowl tea[J]. Journal of Fucntional Foods, 2014, 7: 590-598. DOI:10.1016/j.jff.2013.12.026.
[2] FENG Xia, LUO Min, ZHAO Xin. Inbibitional effect of sandy tea on the carcinoma cells growth and tumor metastasis[J]. Modern Food Science and Technology, 2013, 29(8): 1898-1901.
[3] ZHOU Y L, WANG R, FENG X, et al. Preventive effect of insect tea against reserpine-induced gastric ulcers in mice[J]. Experimental and Therapeutic Medicine, 2014, 8(4): 1318-1324. DOI:10.3892/ etm.2014.1859.
[4] ZHAO X, WANG R, QIAN Y, et al. In vivo preventive effects of insect tea on buccal mucosa cancer in ICR mice[J]. Journal of Cancer Research and Therapeutics, 2014, 10(3): 651-657. DOI:10.4103/0973-1482.138081.
[5] ZHEN M C, WANG Q, HUANG X H, et al. Green tea polyphenol epigallocatechin-3-gallate inhibits oxidative damage and preventive effects on carbon tetrachloride-induced hepatic fibrosis[J]. Journal of Nutritional Biochemistry, 2007, 18(12): 795-805. DOI:10.1016/ j.jnutbio.2006.12.016.
[6] El-BESHBISHY H A, TORK O M, El-BAB M F, et al. Antioxidant and antiapoptotic effects of green tea polyphenols against azathioprineinduced liver injury in rats[J]. Pathophysiology, 2011, 18(2): 125-135. DOI:10.1016/j.pathophys.2010.08.002.
[7] XU G M, PENG X J, WANG C J, et al. Extraction of polyphenols in aglossa dimidiata with ions Al3+and Zn2+sedimentation[J]. Journal of Traditional Chinese Medicine University of Hunan, 2007, 27(2): 46-48. DOI:10.3969/j.issn.1674-070X.2007.02.016.
[8] HE B H. Study on extraction techniques of trilobite tea polyphenols[J]. Journal of Hunan Ecological Science, 2014, 1(4): 40-44. DOI:10.3969/ j.issn.1671-6361.2014.04.008.
[9] KRENKEL O, MOSSANEN J C, TACKE F. Immune mechanisms in acetaminophen-induced acute liver failure[J]. Hepatobiliary Surgery and Nutrition, 2014, 3(6): 331-343. DOI:10.3978/ j.issn.2304-3881.2014.11.01.
[10] OLALEYE M T, AMOBONYE A E, KOMOLAFE K, et al. Protective effects of Parinari curatellifolia flavonoids against acetaminopheninduced hepatic necrosis in rats[J]. Saudi Journal of Biological Sciences, 2014, 21(5): 486-492. DOI:10.1016/j.sjbs.2014.06.005.
[11] YI Ruokun, ZHAO Xin. Protective effects of taemyeongcheong against acetaminophen-induced hepatic injury in mice[J]. Modern Food Science and Technology, 2015, 31(1): 11-15. DOI:10.13982/ j.mfst.1673-9078.2015.1.003.
[12] ZHAO X, PANG L, LI J, et al. Apoptosis inducing effects of kuding tea polyphenols in human buccal squamous cell carcinoma cell line BcaCD885[J]. Nutrients, 2014, 6(8): 3084-3100. DOI:10.3390/ nu6083084.
[13] ZHAO X. Hawk tea (Litsea coreana Levl. var. lanuginose) attenuates CCl4-induced hepatic damage in Sprague-Dawley rats[J]. Experimental and Therapeutic Medicine, 2013, 5(2): 555-560. DOI:10.3892/ etm.2012.840.
[14] ZHAO X, SONG J L, KIL J H, et al. Bamboo salt attenuates CCl4-induced hepatic damage in Sprague-Dawley rats[J]. Nutrition Research and Practice, 2013, 7(4): 273-280. DOI:10.4162/nrp.2013.7.4.273.
[15] REŽEN T, TAMASI V, LÖVGREN-SANDBLOM A, et al. Effect of CAR activation on selected metabolic pathways in normal and hyperlipidemic mouse livers[J]. BMC Genomics, 2009, 10: 384. DOI:10.1186/1471-2164-10-384.
[16] PALIPOCH S, PUNSAWAD C. Biochemical and histological study of rat liver and kidney injury induced by cisplatin[J]. Journal of Toxicologic Pathology, 2013, 26(3): 293-299. DOI:10.1293/ tox.26.293.
[17] GRATACOS J, COLLADO A, FILELLA X, et al. Serum cytokines (IL-6, TNF-alpha, IL-1 beta and IFN-gamma) in ankylosing spondylitis: a close correlation between serum IL-6 and disease activity and severity[J]. Rheumatology, 1994, 33(10): 927-931. DOI:10.1093/ rheumatology/33.10.927.
[18] LI G J, SUN P, WANG Q, et al. Dendrobium candidum Wall. ex Lindl. attenuates CCl4-induced hepatic damage in imprinting control region mice[J]. Experimental and Therapeutic Medicine, 2014, 8(3): 1015-1021. DOI:10.3892/etm.2014.1834.
[19] GIRISH C, KONER B C, JAYANTHI S, et al. Hepatoprotective activity of six polyherbal formulations in CCl4-induced liver toxicity in mice[J]. Indian Journal of Experimental Biology, 2009, 47(4): 257-263.
[20] BAEUERLE P A. κB-NF-kappaB structures: at the interface of inflammation control[J]. Cell, 1998, 95(6): 729-731.
[21] SÁNCHEZ-PÉREZ I, BENITAH S A, MARTÍNEZ-GOMARIZ M, et al. Cell stress and MEKK1-mediated c-Jun activation modulate NFkappaB activity and cell viability[J]. Molecular Biology of the Cell, 2002, 13(8): 2933-2945. DOI:10.1091/mbc.E02-01-0022.
[22] HUANG G J, DENG J S, CHIU C S, et al. Hispolon protects against acute liver damage in the rat by inhibiting lipid peroxidation, proinflammatory cytokine, and oxidative stress and downregulating the expressions of iNOS, COX-2, and MMP-9[J]. Evidence-Based Complementary and Alternative Medicine, 2012: 480714. DOI:10.1155/2012/480714.
[23] KIM D H, CHUNG J H, YOON J S, et al. Ginsenoside Rd inhibits the expressions of iNOS and COX-2 by suppressing NF-κB in LPS-stimulated RAW264.7 cells and mouse liver[J]. Journal of Ginseng Research, 2013, 37(1): 54-63. DOI:10.5142/jgr.2013.37.54.
[24] WANG R, SUN P, ZHAO X. Protective effects of crude polysaccharide in Insect tea against carbon tetrachloride-induced hepatic damage in mice[J]. Modern Food Science and Technology, 2015, 31(5): 6-11. DOI:10.13982/j.mfst.1673-9078.2015.5.002.
[25] YI R K, WANG R, SUN P, et al. Antioxidant-mediated preventative effect of Dragon-pearl tea crude polyphenol extract on reserpineinduced gastric ulcers[J]. Experimental and Therapeutic Medicine, 2015, 10(1): 338-344. DOI:10.3892/etm.2015.2473.
[26] CHENG N, WU L, ZHENG J, et al. Buckwheat honey attenuates carbon tetrachloride-induced liver and DNA damage in mice[J]. Evidence-Based Complementary and Alternative Medicine, 2015: 987385. DOI:10.1155/2015/987385.
[27] CHEN J H, YU G F, JIN S Y, et al. Activation of α2 adrenoceptor attenuates lipopolysaccharide-induced hepatic injury[J]. International Journal of Clinical and Experimental Pathology, 2015, 8(9): 10752-10759.
[28] ZHAO P, QI C, WANG G, et al. Enrichment and purification of total flavonoids from Cortex Juglandis Mandshuricae extracts and their suppressive effect on carbon tetrachloride-induced hepatic injury in mice[J]. Journal of Chromatography B Analytical Technologies in the Biomedical and Life Sciences, 2015, 1007: 8-17. DOI:10.1016/ j.jchromb.2015.10.019.
[29] YANG Y Y, LEE P C, HUANG Y T, et al. Involvement of the HIF-1α and Wnt/β-catenin pathways in the protective effects of losartan on fatty liver graft with ischaemia/reperfusion injury[J]. Clinical Science, 2014, 126(2): 163-174. DOI:10.1042/CS20130025.
[30] YANG B Y, ZHANG X Y, GUAN S W, et al. Protective effect of procyanidin B2 against CCl4-induced acute liver injury in mice[J]. Molecules, 2015, 20(7): 12250-12265. DOI:10.3390/ molecules200712250.
[31] LI S, TAN H Y, WANG N, et al. The role of oxidative stress and antioxidants in liver diseases[J]. International Journal of Molecular Sciences, 2015, 16(11): 26087-26124. DOI:10.3390/ijms161125942.
[32] SKRZYDLEWSKA E, OSTROWSKA J, FARBISZEWSKI R, et al. Protective effect of green tea against lipid peroxidation in the rat liver, blood serum and the brain[J]. Phytomedicine, 2002, 9(3): 232-238. DOI:10.1078/0944-7113-00119.
[33] ZHAO X, LI G J. Comparison of antioxidant effects of Insect Tea and its raw tea of Kuding tea[J]. The Food Industry, 2015, 36(6): 235-238.
虫茶多酚对乙酰氨基酚致小鼠肝损伤的预防效果
孙 鹏,冯 霞,易若琨,赵 欣*
(重庆第二师范学院生物与化学工程系,重庆市功能性食品协同创新中心,重庆市功能性食品工程技术研究中心,功能性食品研发重庆市工程实验室,重庆 400067)
研究虫茶粗多酚(crude polyphenols of Insect tea,CPIT)对乙酰氨基酚致小鼠急性肝损伤的预防效果。在诱导肝损伤后,CPIT可以增加小鼠的体质量和减小肝脏质量,降低肝指数。经过CPIT处理后的小鼠较对照组小鼠(肝损伤)血清谷丙转氨酶、谷草转氨酶、乳酸脱氢酶、甘油三酯、血尿素氮含量均下降,而总胆固醇,白蛋白水平则上升。同时经过CPIT处理后,白细胞介素(interleukin,IL)-6、IL-12、肿瘤坏死因子-α和干扰素-γ的水平也较对照组小鼠降低。CPIT还可以上调肿瘤坏死因子-α、锰超氧化物歧化酶、铜锌超氧化物歧化酶、过氧化氢酶、谷胱甘肽过氧化物酶mRNA的表达和下调核转录因子(nuclear factor-κB, NF-κB)、一氧化氮合酶异型、环氧化酶的表达。通过结果可以看出,CPIT具有预防动物体内肝损伤效果。
虫茶;多酚;对乙酰氨基酚;肝损伤;表达
TS272.5
A
1002-6630(2016)21-0257-08
2015-12-06
重庆高校创新团队建设计划资助项目(CXTDX201601040);重庆市工程技术研究中心建设项目(cstc2015yfpt_gcjsyjzx0027)
孙鹏(1983—),男,讲师,硕士,研究方向为食品化学与营养学。E-mail:lazhuyu2@163.com
10.7506/spkx1002-6630-201621044
*通信作者:赵欣(1981—),男,教授,博士,研究方向为食品化学与营养学。E-mail:foods@live.cn

