两个Schiff碱铜配合物的合成、晶体结构、光谱性质及取代基效应
孙银霞 李春宇 杨成娟 赵亚元 郭建强 俞彬
(兰州交通大学化学与生物工程学院,兰州730070)
两个Schiff碱铜配合物的合成、晶体结构、光谱性质及取代基效应
孙银霞*李春宇杨成娟赵亚元郭建强俞彬
(兰州交通大学化学与生物工程学院,兰州730070)
合成了2个含肟基Schiff碱Cu配合物[Cu(L1)2](1)和[Cu(L2)2](2)(HL1=1-(3-((2-hydroxybenzylidene)amino)phenyl)ethanone O-benzyl oxime;HL2=1-(3-((2-Hydroxy-naphthalen-1-ylmethylene)-amino)-phenyl)-ethanone oxime),并通过元素分析、红外光谱、紫外光谱,荧光光谱及X射线单晶衍射分析进行了表征和分析。结果表明,配合物1和2具有相似的结构,均由1个中心Cu离子和2个双齿配体单元组成,且Cu离子的配位数为4,具有平面四边形结构。配合物1和2均形成了二维层状超分子结构,不同的是配合物1通过分子间π…π堆积作用相连接,而配合物2通过分子间O-H…N和C-H…π氢键作用而形成了超分子结构。进一步讨论了配合物1的荧光性质及两个配合物间配体的取代基效应。
铜配合物;晶体结构;Schiff碱配体;取代基效应
0Introduction
Schiff base compounds have been playing an important part in the development of coordination chemistry because of their potential application in catalysis[1-2],bioscience[3-4],new materials[5],magnetic properties[6-9]and constructing supramolecular structu
res[10-11].Also,the Schiff base ligands are strong donors and therefore the oxime-containing ligands were found to efficiently stabilize high oxidation states of metal ions like Cuand Ni.The presence of additional donor groups together with the oxime group in the ligand molecule may result in significant increase of chelating efficiency and ability to form polynuclear complexes.Herein,two new Cucomplexes with the Schiffbaseligand,[Cu(L1)2](1)(HL1=1-(3-((2- hydroxybenzylidene)amino)phenyl)ethanone O-benzyl oxime)and[Cu(L2)2](2)(HL2=1-(3-((2-Hydroxy naphthalen-1-ylmethylene)-amino)-phenyl)-ethanone
oxime)have been synthesized and characterized by elementalanalyses,IRspectra,UV-Visspectra, fluorescence measurement and X-ray single crystal diffraction.X-ray crystallographic analyses reveal that the structures of the complexes 1 and 2 are very similar.They are both mononuclear structures and all the Cuions are four-coordinated.
1 Experimental
1.1Materials
3-Aminoacetophenone,O-benzylhydroxylamine, salicylaldehyde and 2-hydroxy naphthaldehyde were purchased from Alfa Aesar and used without further purification.The other reagents and solvent were analyticalgradefromTianjinChemicalReagent Factory.
1.2Methods
C,H and N analyses were carried out with a GmbH VariuoEL V3.00 automatic elemental analyzer. Elemental analysis for Cu was detected by an IRIS ER/S·WP-1 ICP atomic emission spectrometer.FTIR spectrawererecordedonaVERTEX70FTIR spectrophotometer,with samples prepared as KBr pellet(400~4000 cm-1).UV-Vis absorption spectra were recorded on a Shimadzu UV-2550 spectrometer. X-Ray single crystal structure was determined on a Bruker Smart 1000 CCD area detector.Melting points were measured by the use of a microscopic melting point apparatus made in Beijing Taike Instrument LimitedCompanyandthethermometerwas uncorrected.
1.3Syntheses of HL1and HL2
HL1(1-(3-((2-hydroxybenzylidene)amino)phenyl) ethanone O-benzyl oxime)and HL2(1-(3-((2-Hydroxynaphthalen-1-ylmethylene)-amino)-phenyl)-ethanone
oxime)were synthesized according to the reported method(Scheme 1)[12-13].
1.4Synthesis of[Cu(L1)2](1)
A solution of copperacetate monohydrate(1.3 mg,0.007 mmol)in methanol(4 mL)was added dropwise to a solution of HL1(1.7 mg,0.005 mmol)in dichloromethane(5 mL)at room temperature.The mixing solution turned to yellow immediately.Then it was filtered and the filtrate was allowed to stand at room temperature for about one week.Brown red block single crystals which is suitable for X-ray structural determination were obtained.Anal.Calcd. for C44H38CuN4O4(%):C,70.43;H,5.10;N,7.47;Cu, 8.47.Found(%):C,70.38;H,5.17;N,7.45;Cu,8.50. 1.5Synthesis of[Cu(L2)2](2)
A solution of copperacetate monohydrate(2.3mg,0.01 mmol)in methanol(3 mL)was added dropwise to a solution of HL2(1.5 mg,0.005 mmol)in dichloromethane(5 mL)at room temperature.The colorofthemixingsolutionturnedyellow immediately,and it was allowed to stand at room temperature for three weeks.Then the solvent partially evaporated and red-brown prismatic single crystals suitableforX-raycrystallographicanalysiswere obtained.Anal.Calcd.for C38H30CuN4O4(%):C,68.10; H,4.51;N,8.36;Cu,9.48.Found(%):C,67.98;H, 4.57;N,8.29;Cu,9.52.
1.6Crystal structure determination
Thesinglecrystalsofcomplexeswith approximate dimensions of 0.21×0.11×0.05 mm(1)and 0.18×0.14×0.09 mm(2)were placed on a Bruker Smart 1000 CCD area detector.The diffraction data were collected using a graphite monochromated Mo Kα radiation(λ=0.071 073 nm)at 298(2)K.Empirical absorption correction was applied to the data using SADABS program[14].The structure was solved by direct methods and refined by full-matrix leastsquares method on F2using the SHELXTL program[15].All nonhydrogen atoms were refined anisotropically.All the hydrogen atoms were generated geometrically and refined isotropically using the riding model.Details of the crystal parameters,data collection and refinements for complexes 1 and 2 are summarized in Table 1.
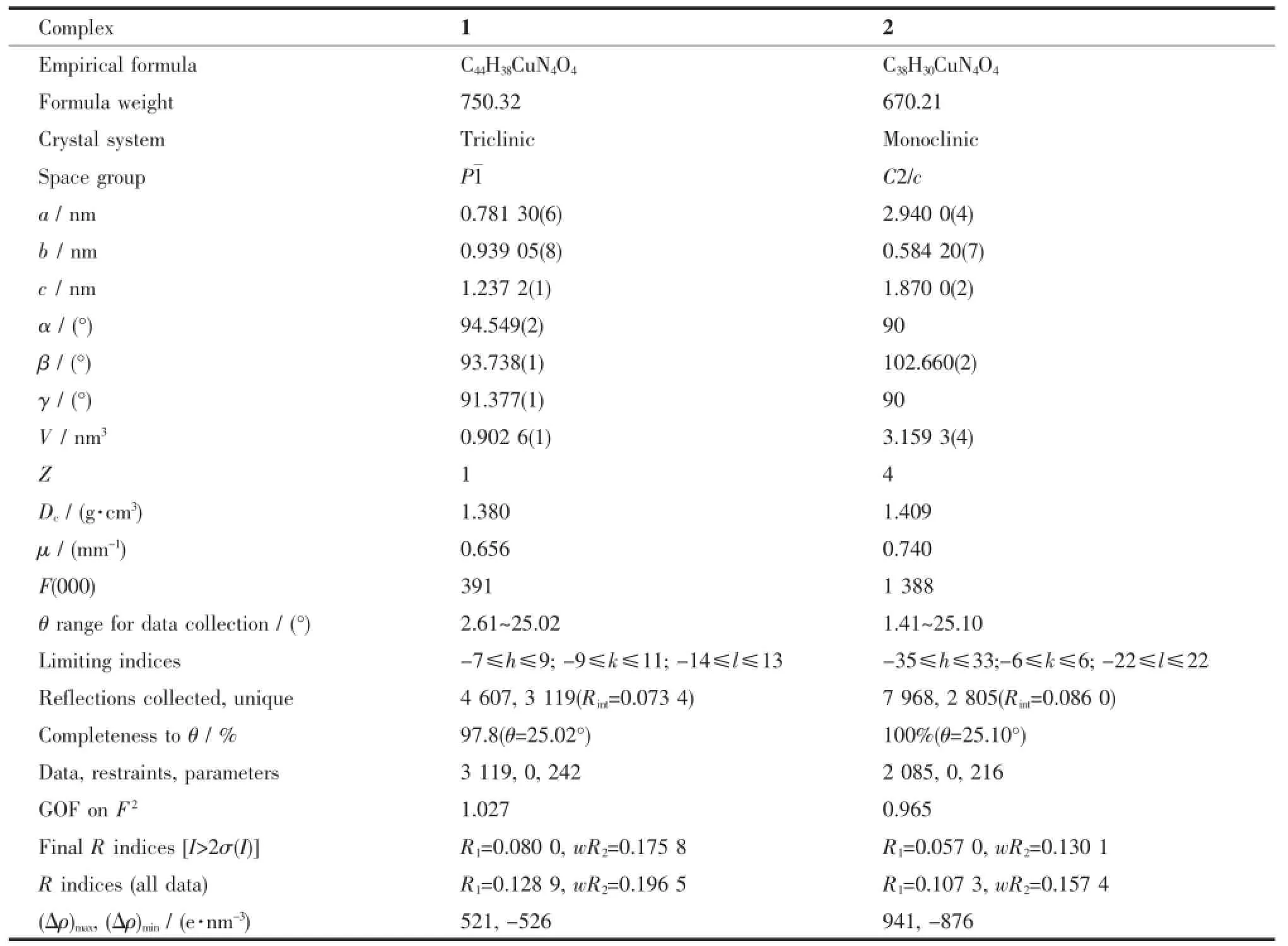
Table 1Crystal data and structure refinement for complexes 1 and 2
2 Results and discussion
2.1IR spectra analyses
TheFTIRspectraofHL1,HL2andtheir corresponding Cucomplexes 1 and 2 exhibit various bands in the 400~4 000 cm-1region.The most important FTIR bands for HL1,HL2and complex 1~2 are given in Table 2.The free ligand HL1and HL2exhibit characteristic C=N stretching bands at 1 617and 1 620 cm-1,while that of complex 1 and complex 2 were observed in the 1 600 cm-1and 1 602 cm-1, respectively.The C=N stretching frequencies are all shifted to lower frequencies by ca.17 and 18 cm-1upon complexation respectively,indicating a decrease in the C=N bond order due to the coordinated bond of the Cuatom with the imino nitrogen lone pair[16].In the 1 445~1 564 cm-1region,the observed bands were attributedtoaromaticC=Cvibrations.Upon coordination these bands shift to lower frequencies for the Cucomplexes[17].The Ar-O stretching frequency appears as a strong band within the 1 178~1 144 cm-1as reported for similar ligands[18].This band occurs at 1 178 cm-1for the ligand HL1,and at 1 144 cm-1for complex 1,the band occurs at 1 182 cm-1for the ligand HL2,and at 1 137 cm-1for complex 2.The Ar-O stretching frequency is shifted to a lower frequency, indicating that the Cu-O bond was formed between the Cuion and phenolic oxygen atom of phenolic hydroxylgroup[13].Thefar-infraredspectraof complexes 1 and 2 was also obtained in the region 500~100 cm-1in order to identify frequencies due to the Cu-O and Cu-N bonds.The FTIR spectrum of complex 1 showed ν(Cu-N)and ν(Cu-O)vibration absorption frequencies at 540 cm-1and 503 cm-1(or 519 and 463 cm-1for complex 2),respectively.These assignmentsareconsistentwiththeliterature frequency values[19].
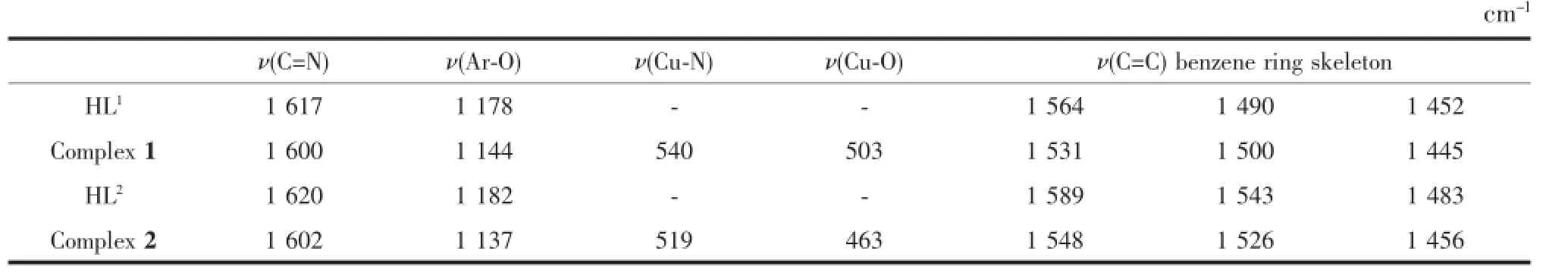
Table 2 Main bands in IR spectra of the ligand HL1,HL2and their Cucomplexes
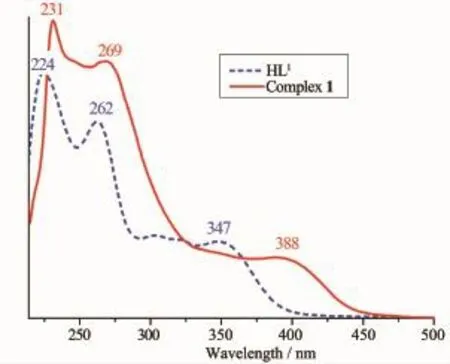
Fig.1 UV-Vis absorption spectra of HL1and the complex 1 in diluted CH2Cl2solution at room temperature (c=5×10-5mol·L-1)
2.2UV-Vis absorption spectra analyses
The absorption spectra of ligand HL1and its corresponding Cucomplex 1 were determined in diluted CH2Cl2solution,and ligand HL2and its corresponding Cucomplex 2 were determined in diluted DMF solution as shown in Fig.1 and Fig.2.
UV-Vis spectrum of the free ligand HL1exhibits three absorption peaks at ca.224,262 and 347 nm (Fig.1).The absorption peak of HL1at 224 nm assigned to the π-π*transition of the benzene rings shifts to low energy region by ca.7 nm in complex 1, indicating Cuion coordinated with the O and N atoms of ligand units.The absorption peak of HL1at 262 nm attributed to the intra-ligand π-π*transition of the C=N bonds is shifted to 269 nm in complex 1 upon complexation.The absorption peak of HL1at 347 nm is absent in complex 1,however,a new absorption peak is observed at 388 nm assigned to an L→M charge-transfer transition which is characteristic of a transitionmetalcomplexwithN2O2coordination sphere[20].It can be seen that the absorption peaks of complex 2 are obviously different from those of the HL1uponcomplexation(Fig.2).Comparedwith complex 2,an important feature of the absorption spectrum of HL2is shown that three absorption peaks are observed at 243,382 and 437 nm,which are absent in the spectrum of complex 2.The other feature is that the absorption peak at 267 and 320 nm in HL2is shifted to 269 and 311 nm in complex 2, respectively,indicating that the coordination of Cuatom with HL2.
2.3Fluorescence spectra
The emission spectrum of the complex 1 in diluted CH2Cl2solution at room temperature is shown in Fig.3.An intense excitation peak is observed at 311 nm.The bluish violet emission for complex 1 can be observed,where the maximum emission wavelength wasat430nm.TheStokesshiftbetweenthe maximum wavelength of the fluorescence emission and the fluorescence excitation is 119 nm.This red-shift might be related to the coordination of the Cuatom to the ligand and increase of the rigidity of the ligands,which can diminish the loss of energy via vibrationalmotionsandincreasetheemission efficiency.
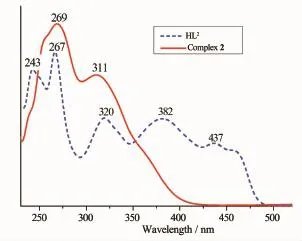
Fig.2 UV-Vis absorption spectra of HL2and the complex 2 in diluted DMF solution at room temperature (c=5×10-5mol·L-1)
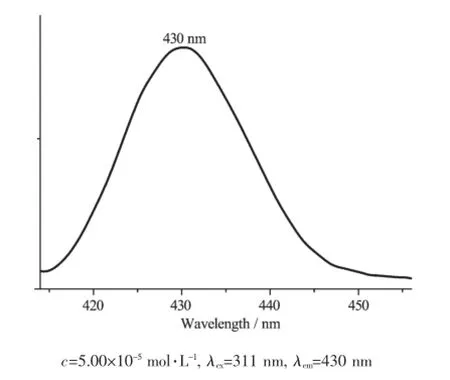
Fig.3 Emission spectrum of the complex 1 in diluted CH2Cl2at room temperature

Fig.4 Molecular structure of complex 1 showing 30%probability displacement ellipsoids
2.4StructuralDescriptionofthecomplexes1and2
The single crystal structures of complexes 1 and 2 were confirmed by X-ray crystallography(Fig.4 and 5). The selected bond lengths and angles of complexes 1 and 2 are listed in Table 3.The complex 1 crystallizes in the triclinic system and P1 space group,whereas the complex 2 crystallizes in the monoclinic system and C2/c space group.Both of the complexes can be described as centrosymmetric mononuclear Cucomplexes.TheCuion lying on the inversion centre, is four-coordinated in a trans-CuN2O2square-planar geometry,with two phenolic O and two imino N atoms from two N,O-bidentate oxime-type ligands(HL1and HL2).
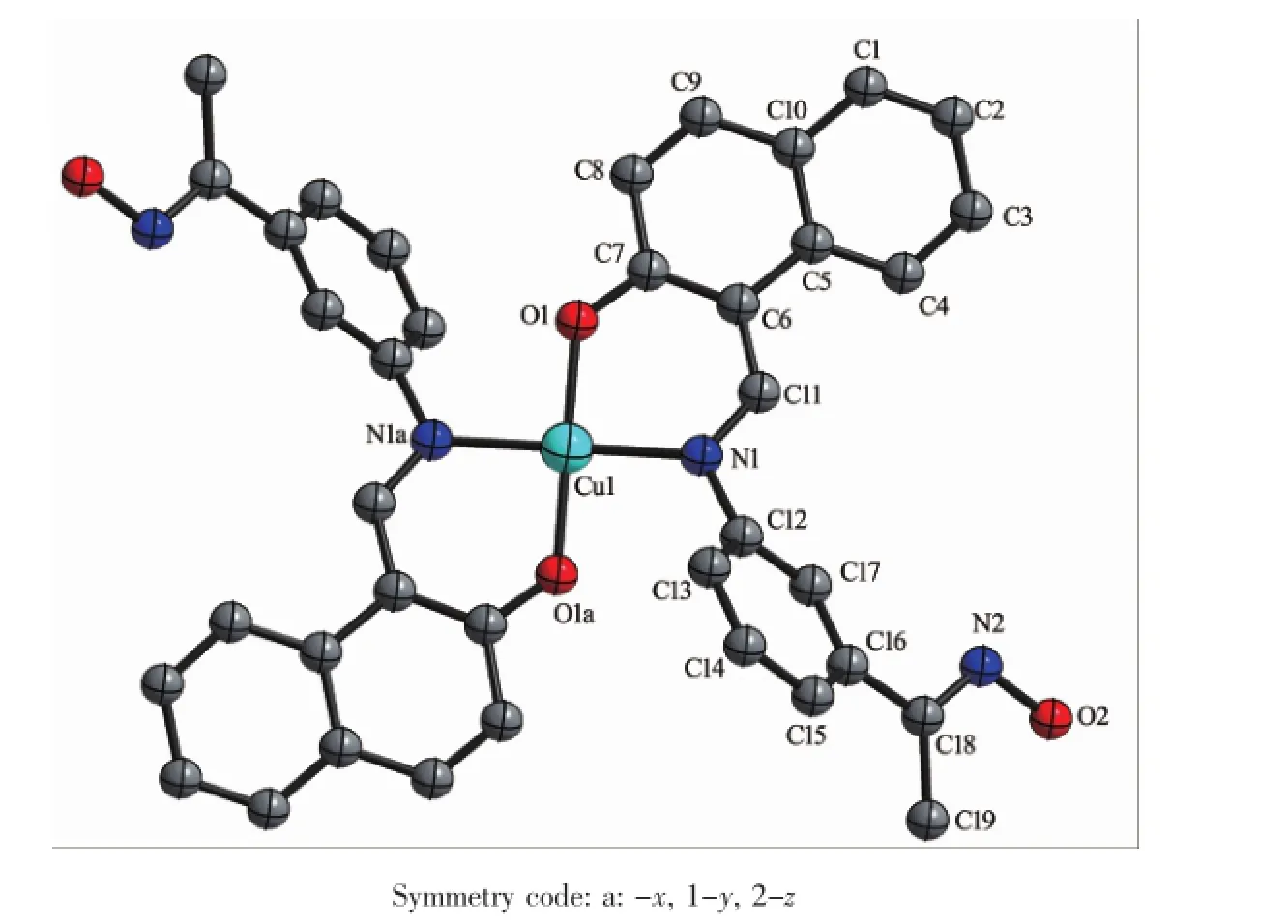
Fig.5 Molecular structure of complex 2 showing 30%probability displacement ellipsoids
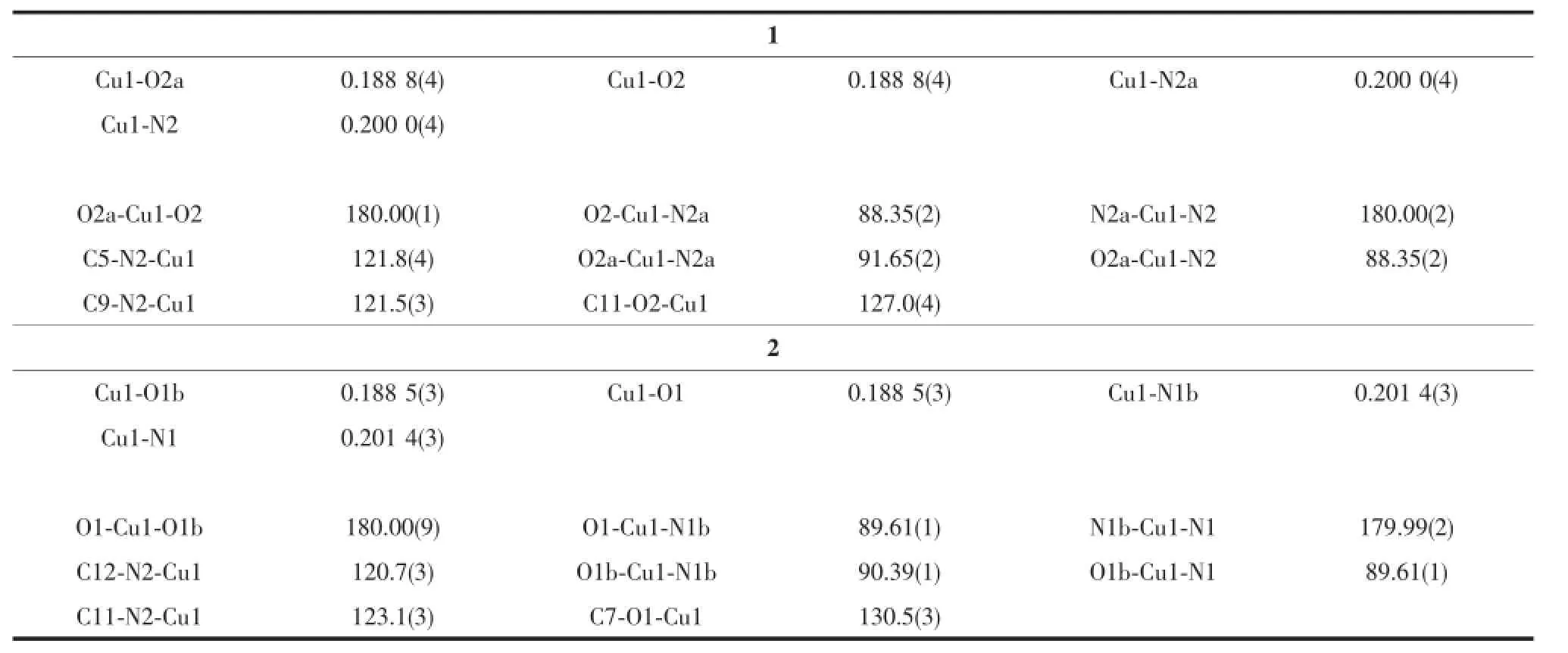
Table 3Selected bond lengths(nm)and bond angles(°)for complexes 1 and 2
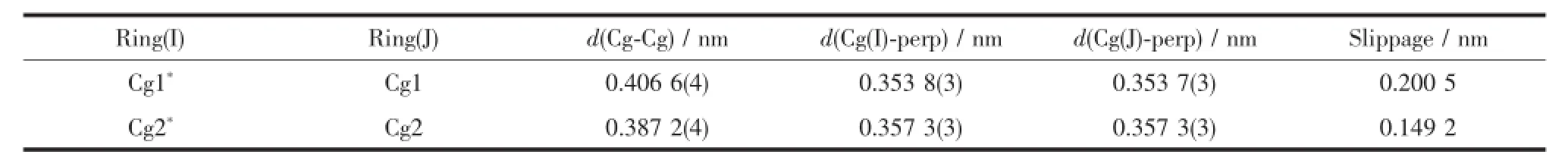
Table 4 Putative π-π stacking interactions for complex 1
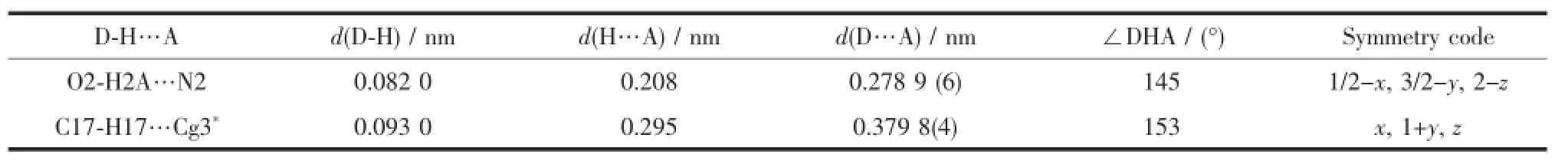
Table 5 Hydrogen-bonding for complex 2
As shown in Fig.6,complex 1 is stabilized by the πcentroid(C10-C15)-πcentroid(C10-C15)stackinginteractions of benzene rings linking the neighboring molecules into a 1D infinite chain parallel to the a axis(Table 4).In addition,this adjacent chains along a axis are furtherheld together by the other intermolecular πcentroid(C17-C22)-πcentroid(C17-C22)stackinginteractions(Fig.7)to form 2D supramolecular structure parallel to the ac planes (Fig.8).Whereas in complex 2,as illustrated in Fig.9, each oxime nitrogen atom N2 of the two ligand molecules is hydrogen-bonded to the-O2H2A group of the oxime group from the other neighboring molecule, which resulted in the formation of a classical sixmember ring and a 1D infinite chain synchronously. Furthermore,this linkage is further stabilized by four C17-H17…π(Cg3)interactions to form the 2D infinite supramolecular planar(Fig.10 and Table 5).

Fig.6 View of the 1D single chain motif within complex 1 along the a axis
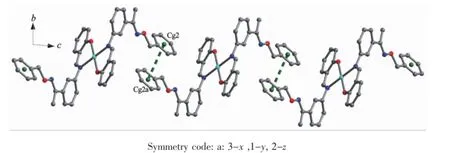
Fig.7 View of the 1D single chain motif within complex 1 along the c axis
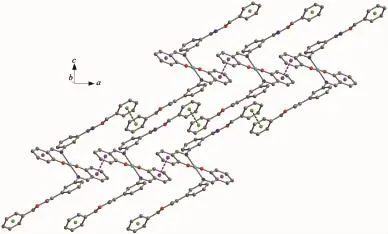
Fig.8 Part of 2D supramolecular structure of the complex 1 showing the formation the two π…π stacking interaction
2.5Substituent effects
The overall structures of the two complexes are found to be identical except that the oxime portion and aromatic ring differs in the ligands.The bond lengths of complex 1 are different from those of complex 2 due to the different substituent group inthe bidentate ligands.In both bases,twoimino nitrogen and two phenolic oxygen atoms form the square base with the Cu-N bonds being slightly longer than the corresponding Cu-O bonds.The elongation of the Cu-N bonds probably due to the weaker coordination ability of the nitrogen atom than that of phenolic oxygen atom.Similar elongation has been observed in Cucomplexes with Schiff base ligands[21-22].However, the coordination bond Cu-N(0.200 0 nm)of complex 1 is shorter than the Cu-N(0.201 4 nm)of complex 2, but Cu-O(0.188 8 nm)of complex 1 is almost equal to the Cu-O(0.188 5 nm)of complex 2.Meanwhile, the angle of N2-Cu1-O2(88.35°)in complex 1 is smaller with the angle ofN1-Cu1-O1(89.61°)in complex 2.In addition,the supramolecular structures of the two complexes 1 and 2 are also similar twodimensionalsupramolecularstructurelinkingby different intermolecular interaction,in which complex 1 is stabilized by two π…π interaction,while that of complex 2 by intermolecular O-H…N and C-H…π hydrogen bond interactions.The substituent effects also give rise to the variations in IR and UV-Vis spectra in complexes 1 and 2.
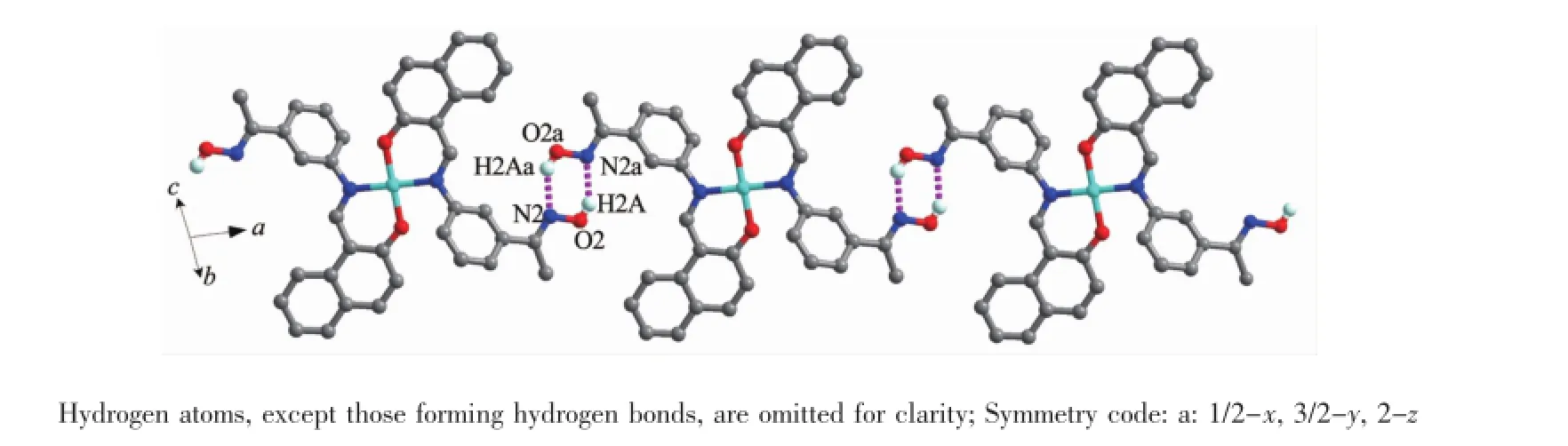
Fig.9 View of a 1D single chain motif containing hydrogen bonds for complex 2
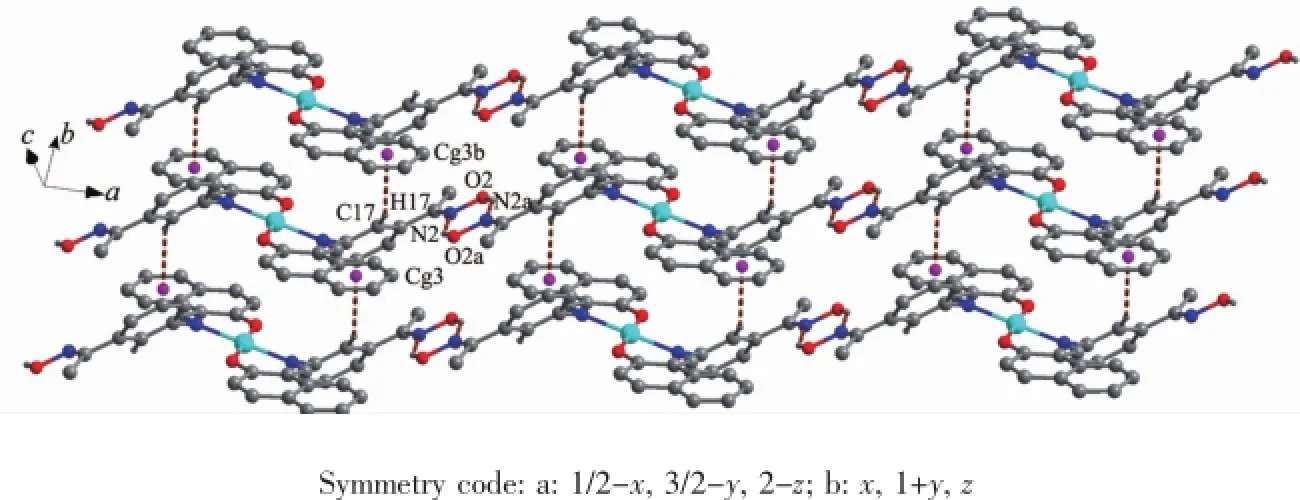
Fig.10 Part of 2D supramolecular structure of the complex 2 showing the formation of C17-H17…Cg3 and O2-H2A…N2 hydrogen bond
3 Conclusions
According to the data and discussion above,two Schiff base mononuclear Cucomplexes 1 and 2 have been synthesized and characterized structurally.The Cucomplexes are tetra-coordinated by two nitrogen atoms and two oxygen atoms of two deprotonated L-units definingtheN2O2basalplane.Thecoordination environment around Cuatom is best regarded as the square-planar geometry.Complexes 1 and 2 also formed a 2D-layer supramolecular structure by different intermolecular interaction,π…π interaction and O-H…N,C-H…π hydrogen bond interactions,respectively. Consequently,theintermolecularnon-classical hydrogen-bonding plays a very important role in the construction ofsupramolecularnetworksstructure. Furthermore,the substituted groups on the ligands could play a small impact on the coordination geometries and supramolecularstructureof complexes 1and2.
References:
[1]Pini D,Mandoli A,Orlandi S,et al.Tetrahedron:Asymmetry, 1999,10(20):3883-3886
[2]Yang H Q,Zhang L,Zhong L,et al.Angew.Chem.Int.Ed., 2007,46(36):6861-6865
[3]Brewer C,Brewer G,Scheidt W R,et al.Inorg.Chim.Acta., 2001,313(1):65-70
[4]Brayshaw P A,Bünzli J-C G,Froidevaux P,et al.Inorg. Chem.,1995,34:2068-2076
[5]Miyasaka H,Matsumoto N,Okawa H,et al.J.Am.Chem. Soc.,1996,118(5):981-994
[6]Bencini A,Benelli C,Caneschi A,et al.J.Am.Chem.Soc., 1985,107(26):8128-8136
[7]Sasaki M,Manseki K,Horiuchi H,et al.J.Chem.Soc. Dalton Trans.,2000,3:259-263
[8]Edder C,Piguet C,Bünzli J-C G,et al.Chem.Eur.J.,2001, 7(14):3014-3024
[9]Bünzli J C G,Piguet C.Chem.Rev.,2002,102(6):1897-1928
[10]Kohawole G A,Patel K S J.Chem.Soc.,Dalton Trans., 1981,1241-1245
[11]Sun Y X,Wang L,Dong X Y,et al.Synth.React.Inorg. Met.-Org.Nano-Met.Chem.,2013,43(5):599-603
[12]Sun Y X,Xu L,Zhao T H,et al.Synth.React.Inorg.Met-Org.Nano-Met.Chem.,2013,43(4):509-513
[13]DONG Wen-Kui(董文魁),TANG Xiao-Lu(唐晓璐),HE Xue-Ni(何雪妮),et al.Chinese.J.Inorg.Chem.(无机化学学报),2009,25(3):528-532
[14]Sheldrick G M.SADABS,University of Göttingen,Germany, 1996.
[15]Sheldrick G M.SHELXS 97,Program for Refinement of Crystal Structures,University of Göttingen,Germany,1997.
[16]Asadi M,Jamshid K A,Kyanfar A H.Inorg.Chim.Acta, 2007,360:1725-1730
[17]Tümer M,Koksal H,Sener M K,et al.Transition Met.Chem., 1999,24(4):414-420
[18]Majumder A,Rosair G M,Mallick A,et al.Polyhedron, 2006,25:1753-1762
[19]Akine S,Taniguchi T,Nabeshima T.Inorg.Chem.,2004,43: 6142-6144
[20]Gomes L,Pereira E,Castro D B.J.Chem.Soc.Dalton Trans., 2000,8:1373-1379
[21]Dong W K,He X N,Yan H B,et al.Polyhedron,2009,28: 1419-1428
[22]Nathan L C,Koehne J E,Gilmore J M,et al.Polyhedron, 2003,22(6):887-894
Two CuComplexes with Schiff Base Ligands:Syntheses,Crystal Structures, Spectroscopic Properties,and Substituent Effect
SUN Yin-Xia*LI Chun-YuYANG Cheng-JuanZHAO Ya-YuanGUO Jian-QiangYU Bin
(School of Chemical and Biological Engineering,Lanzhou Jiaotong University,Lanzhou 730070,China)
Two new Cucomplexes[Cu(L1)2](1)(HL1=1-(3-((2-hydroxybenzylidene)amino)phenyl)ethanone O-benzyl oxime)and[Cu(L2)2](2)(HL2=1-(3-((2-Hydroxy-naphthalen-1-ylmethylene)-amino)-phenyl)-ethanone oxime) have been synthesized and characterized by elemental analyses,IR spectra,UV-Vis spectra,fluorescence measurement and X-ray single crystal diffraction method.X-ray crystallographic analyses have indicated that complexes 1 and 2 have the similar structure,consisting of one Cuion,two ligandunits.In the complexes,the Cuion lying on an inversion centre,is four-coordinated in a trans-CuN2O2square-planar geometry by two hydroxy O and two imine N atoms from two symmetry-related N,O-bidentate Schiff base ligands.The supramolecular structures of the two complexes 1 and 2 are also similar two-dimensional supramolecular structure linking by different intermolecular interaction.Complex 1 forms an infinite two-dimensional supramolecular structure by two π…π interaction,while that of complex 2 by intermolecular O-H…N and C-H…π hydrogen bond interactions.The fluorescence property of complex 1 and substituent effects of the two complexes are discussed.CCDC:1003363,1; 1043506,2.
Cucomplex;crystal structure;Schiff base ligand;substituent effect
O614.121
A
1001-4861(2016)02-0327-09
10.11862/CJIC.2016.034
2015-09-08。收修改稿日期:2015-11-25。
兰州交通大学青年科学基金(No.2011007)、兰州交通大学研究生教改项目(No.160012)和甘肃省科技支撑计划项目(No.1204GKCA052)资助。
*通信联系人。E-mail:sun_yinxia@163.com

