基于2-甲基-8-羟基喹啉的镝单分子磁体的晶体结构及磁性
王慧娜 刘颖昕 李荣 周琦 付文升
(重庆师范大学,重庆市绿色合成与应用重点实验室,重庆401331)
基于2-甲基-8-羟基喹啉的镝单分子磁体的晶体结构及磁性
王慧娜刘颖昕李荣周琦*付文升*
(重庆师范大学,重庆市绿色合成与应用重点实验室,重庆401331)
以2-甲基-8-羟基喹啉(HL)为配体合成了2个含有镝离子的配位化合物[Dy2L4(HL)4(H2O)2](ClO4)2·2H2O(1)和[Dy2L6(C2H5OH)] ·H2O(2)。虽然在这两个配位化合物中配体都是2-甲基-8-羟基喹啉,但其参与配位的方式不同。这导致2个化合物中镝离子所处的配位环境不同,进而对化合物的磁性产生了影响。
配位化合物;分子磁体;镧系金属;磁弛豫作用
Over the past decade,single-molecule magnet (SMM)has received considerable attention due to potential applications in high density data storage and quantum computer[1-5].The early research of singlemolecule magnet mainly focused on 3d transition metalclusters,especiallyon Mn element.A considerable amount of Mn clusters with various structures has been synthesized,such as[Mn32],[Mn84][6-10].Besides, their magnetic properties have been studied extensively.Although 3d transition metal clusters have high ground state spins,the feeblish magnetic anisotropy limits further increase energy barrier of SMM[11-12]. Thus,4f lanthanide ion with strong magnetic anisotropy is introduced into 3d transition metal clusters in order to form 3d-4f heteronuclear metal clusters that maycombinethelargemagneticanisotropyof lanthanide ions with the high spin-states of transition ions[13-17].In recent years,design and synthesis of 4f compounds which only contain lanthanide metal have become the topic on single-molecule magnet research, and a number of lanthanide metal clusters have been synthesized,such as[Tb2],[Dy2],[Dy3],[Dy4],[Dy5],[Dy6][18-23].These compounds could play a significant role in SMM area owing to their large magnetic moments and huge magnetic anisotropy.This combination may lead to a high barrier for their spin reversal.
Ithasbeendemonstratedthattheoverall electronic structure of lanthanide ion is very sensitive to its coordination environment.Even subtle ligand changescandrasticallyinfluenceontheoverall physical properties of the lanthanide compounds,and the SMM behaviour of lanthanide ions is highly dependent on the ligands[24-31].We also investigated the effect of different ligands on the magnetic exchange interactions and the relaxation dynamics[32-33].As we noticed that the same ligand could lead to different coordinationenvironment,wewonderwhatwill happen in the compounds contain the same ligands. For example,2-methyl-8-hydroxylquinolinate(HL)can coordinate to metal ions with two modes,chelating or not.Itwillcertainlyleadtoaquitedifferent coordination environment(coordination numbers and geometries),and in turn,it may make a difference in the magnetic behaviours.
In this paper,we synthesized two dysprosium coordination compounds with binuclear structure using HL as ligand,namely[Dy2L4(HL)4(H2O)2](ClO4)2·2H2O (1)and[Dy2L6(C2H5OH)]·H2O(2).As we anticipated, the coordination environment of the Dy3+ions was significantly different.The Dy3+ion in both of the compounds is eight-coordinated.In compound 1,the dinuclear core is bridged by two O ions,giving rise to a[Dy2O2]core.In compound 2,the two Dy ions are bridged by three O ions,which form a[Dy2O3]core. Magnetic measurements demonstrate that the compounds exhibit weak intra-binuclear antiferromagnetic interaction.Compound 1 show frequency-dependent ac-susceptibility indicative of slow magnetic relaxation.On the contrary,no out-of-phase ac susceptibility (χ″)signal was observed in 2.
1 Experimental
1.1Reagents and physical peasurements
All reagents and solvents were commercially available and were used without further purification. Elemental analyses of carbon and hydrogen were carried out on a Perkin-Elmer 240C elemental analyzer. IR spectra as KBr pellets were recorded with a Magna 750 FT-IR spectrophotometer using reflectance technique over the range of 4 000~400 cm-1.X-ray powder diffraction(XRPD)patterns were taken on a Rigaku D/max 2550 X-ray Powder Diffractometer with Cu Kα radiation(λ=0.154 18 nm,U=30 kV,I=40 mA).All magnetization were obtained with a Quantum Design MPMS SQUID VSM magnetometer.The variabletemperaturemagneticsusceptibilitywasmeasured with an external magnetic field of 1 000 Oe.Samples were restrained in eicosane to prevent torqueing. Pascal′sconstantswereusedtoestimatethe diamagnetic corrections,which were subtracted from the experimental susceptibilities to give the molar paramagnetic susceptibilities(χM).
1.2Synthesis
[Dy2L4(HL)4(H2O)2](ClO4)2·2H2O(1):A mixture of Dy(ClO4)3·6H2O(0.25 mmol,0.14 g)and HL(1.0 mmol, 0.159 g)in a mixture of ethanol(8 mL)and acetonitrile(2 mL)was stirred for 30 min,then the resulting mixture were filtered.The filtrate was heated at 60℃for 7 days in a Teflon-lined steel autoclave(20 mL). Yellow block-shaped crystals formed and were collected in 40%yield.IR(KBr,cm-1):3 233(m),3 178(m),3 061 (w),1 645(m),1 586(s),1 454(s),1 432(m),1 348(s), 1 121(m),1 001(w),935(m),855(m),813(s),716(m), 576(m).Anal.Calcd.for C80H76N8Cl2Dy2O20(%):C 51.51, H 4.11,N 6.01.Found(%):C,51.28,H 4.09,N 5.91.
[Dy2L6(C2H5OH)]·H2O(2):A mixture of Dy(ClO4)3· 6H2O(0.25 mmol,0.14 g)and HL(0.75 mmol,0.119 g)in ethanol(10 mL)was stirred for 30 min,then the resulting mixture were filtered.The filtrate was heated at 80℃for 3 days in a Teflon-lined steel autoclave (20 mL).Yellow block-shaped crystals formed were collected in 37%yield.IR(KBr,cm-1):3 245(m), 3 089(w),3 061(w),1 606(m),1 569(s),1 494(s),1 465 (s),1 381(s),1 118(s),1 024(m),911(w),826m),816 (s),733(m),489(m).Anal.Calcd.for C62H56N6Dy2O8(%): C 55.65,H 4.22,N 6.28.Found(%):C,55.38,H 4.15,N 6.22.
1.3X-ray crystallography
The data collection and structural analysis of crystals 1 and 2 were performed on a Rigaku RAXISRAPID equipped with a narrow-focus,5.4 kW sealed tube X-ray source(graphite-monochromated Mo Kα radiation,λ=0.071 073 nm).The data processing was accomplished with the PROCESS-AUTO processing program.The data were collected at a temperature of 293(2)K.Direct methods were used to solve the structure using the SHELXTL crystallographic software package[34].All non-hydrogen atoms were easily found from the difference Fourier map.All non-hydrogen atoms wererefinedanisotropically.Thehydrogen atoms were set in calculated positions.Crystal data for compounds are listed in Table 1,and selected bond lengths and angles for compounds are listed in Table 2 and Table 3.
CCDC:1054513,1;1052697,2.

Table 1Crystallographic data for the compounds 1 and 2
aR1=
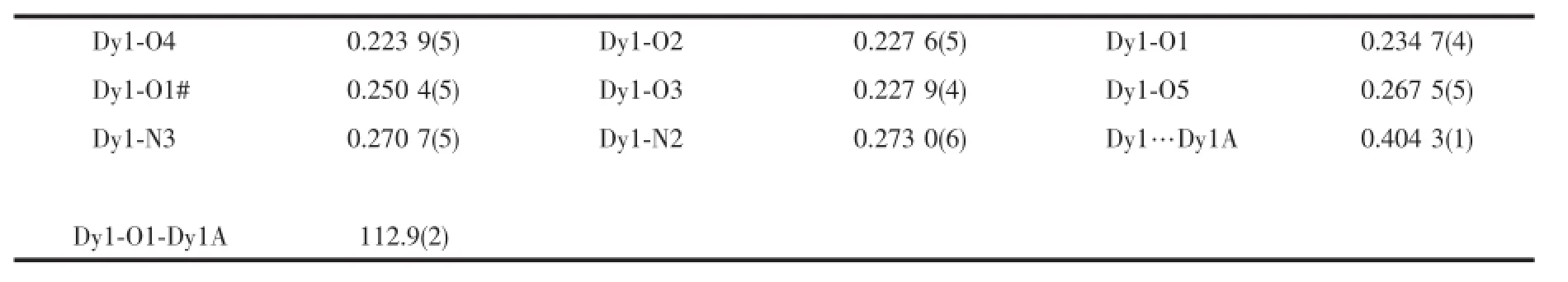
Table 2Main bond lengths(nm)and angles(°)of compound 1

Table 3Main bond lengths(nm)and angles(°)of compound 2
2 Results and discussion
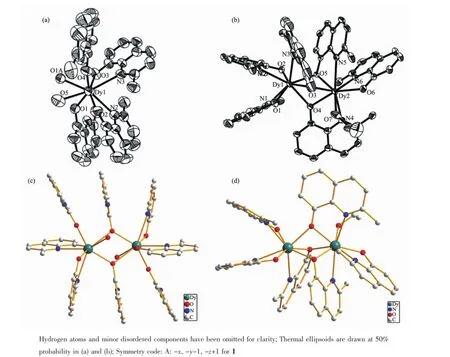
Fig.1(a)Coordination environment of Dyion in compound 1;(b)Coordination environments of Dyions in compound 2; (c)Molecular structure of compound 1;(d)Molecular structure of compound 2
2.1Crystal structure
Compound 1 crystallizes in the monoclinic space group P21/c and the structure is shown in Fig.1a.The dysprosium ion is coordinated by two bridging ligand (O1,O1A),two chelating ligand(O2,N2,O3,N3), one terminal ligand(O4)and one water molecules (O5).The eight-coordinated Dy ions are characterized by distorted biaugmented trigonal prism geometry,as calculated using the SHAPE software.Besides,there is one isolated water molecule and one perchlorate which is the counterion in the asymmetric structure unit.The centrosymmetric dinuclear core is composedof two eight coordinate dysprosium ions bridged by two ions bridged by two oxygen ions,giving rise to a Dy2O2core with a Dy-Dy distance of 0.404 3(1)nm and a Dy-O-Dy angle of 113.9(2)°.Similar Ln2O2structures havebeenalsoreported,such as[Dy2(hmi)2(NO3)2(MeOH)2],[Dy2(ovph)2(NO3)2(H2O)2],[Tb2(valdien)2(NO3)2]and[Gd2(Hsabhea)2(NO3)2][26,31,37].The shortest intermol-ecularDy-Dyseparationdistance is 1.139 7(5)nm.Only two HL molecules coordinate to metal ions with a chelating mode,and the rest were not.
Compound 2 crystallizes in the triclinic space group P1 and the structure is shown in Fig.1b.The Dy3+ions are eight-coordinated and characterized by a triangular dodecahedron environment(calculated by means of SHAPE software).Dy1 is coordinated by three chelating ligands(O1,O2,O3,N1,N2 and N3) and two bridging oxygen atoms(O4 and O5).Dy2 is coordinated by three chelating ligands(O4,O5,O6, N4,N5 and N6),one bridging oxygen atoms(O3),and one terminal ethanol(O7).Two eight coordinate dysprosium ions are bridged by three oxygen atoms, giving rise to a Dy2O3core with a Dy-Dy distance of 0.351 2(1)nm.The Dy-O-Dy angles are 96.84(15)°, 95.58(13)°and 97.33(13)°,respectively.The shortest intermolecular Dy-Dy separation distance is 0.866 9(5) nm.In contrast with compound 1,all the ligands coordinating to Dy3+ions have a chelating mode.
Although both of 1 and 2 contain two eight coordinate dysprosium ions,the coordination environments of Dy3+ions are different.In compound 1,the Dy3+ions are characterized by a distorted dodecahedral environment,and the Dy2O2core is centrosymmetric. On the other hand,in compound 2,the Dy3+ions are characterized by a square antiprism environment,and the Dy2O3core is non-centrosymmetric.We expected thatthedifferencesofcoordinationenvironments could influence on the magnetic properties of the compounds.
2.2Magnetic properties
Thesolid-statevariable-temperaturedirectcurrent(dc)magnetic susceptibility measured for the compounds have been carried out in an applied magnetic field of 1 000 Oe in the temperature range of 2~300 K.The plots of χMT vs T are shown in Fig.2. For compounds 1 and 2,the room temperature χMT values are 27.98 and 27.46 cm3·K·mol-1,respectively, ingoodagreementwiththatexpectedfortwo uncoupled Dy ions(6H15/2,S=5/2,L=5,J=15/2,g=4/3, χMT=14.17 cm3·K·mol-1).Upon decreasing the temperature,the χMT slightly decreases between 300 K and 25 K,then further decreases sharply until reaches values of 11.28 and 13.45 cm3·K·mol-1at 2 K, respectively.The overall behaviours of χMT are caused by thermal depopulation of the Stark sublevels and significant magnetic anisotropy of the dysprosium ions. The weak antiferromagnetic interactions between the metal centres may also make some contribution[35].
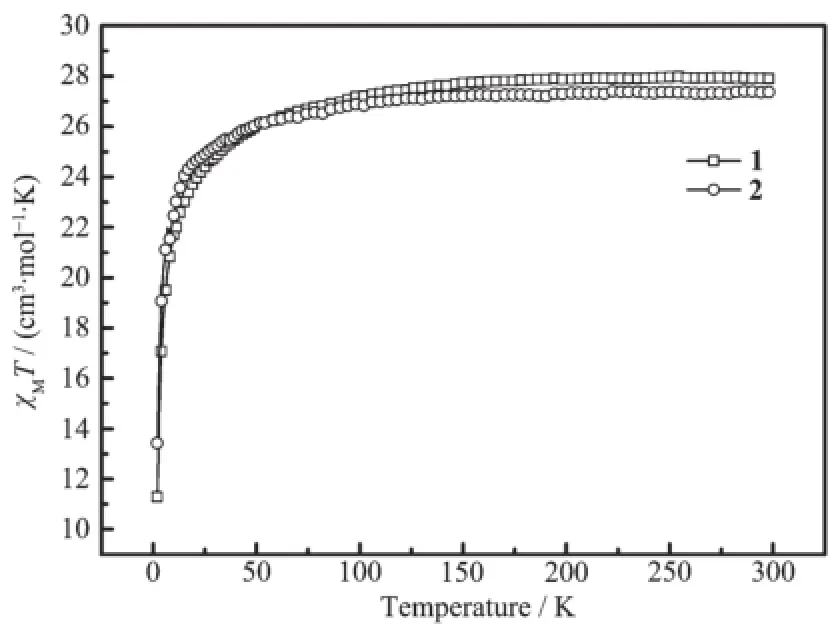
Fig.2Temperature dependence of χMT for compounds 1 and 2 at 1 000 Oe
Field-dependence measurements of the magnetization up to 5 T were performed at 2 K.For compounds 1 and 2,the values of the magnetization at 5 T are 10.59μBand 10.22μB,respectively,lower than the expected saturation value of 20μBfor two Dy ions.The spin orbit coupling and crystal-field effect may make the contributions[36].The lack of saturation on the M vs H data confirms low lying excited states.
To further investigate magnetization dynamics of the compounds,alternating-current(ac)susceptibility measurements have been carried out(dc field 0 Oe, ac field 3.0 Oe,frequency 10~800 Hz).As shown in Fig.3,frequency-dependentonalternating-current magnetic susceptibilities are observed in compound 1. Thisindicatesthepresenceofslowmagnetic relaxation at low temperature,which reveals thesingle-molecule magnet behavior.
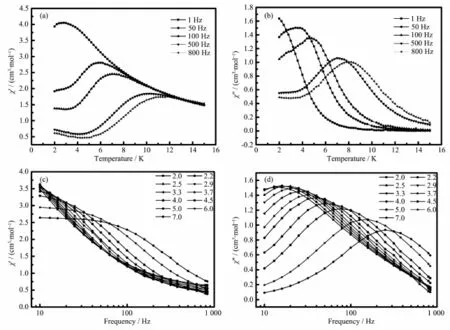
Fig.3Temperature dependence of the in-phase(a)and out-of-phase(b)ac susceptibility and frequency dependence of in-phase(c)and out-of-phase(d)ac susceptibilities for compound 1 under zero dc field
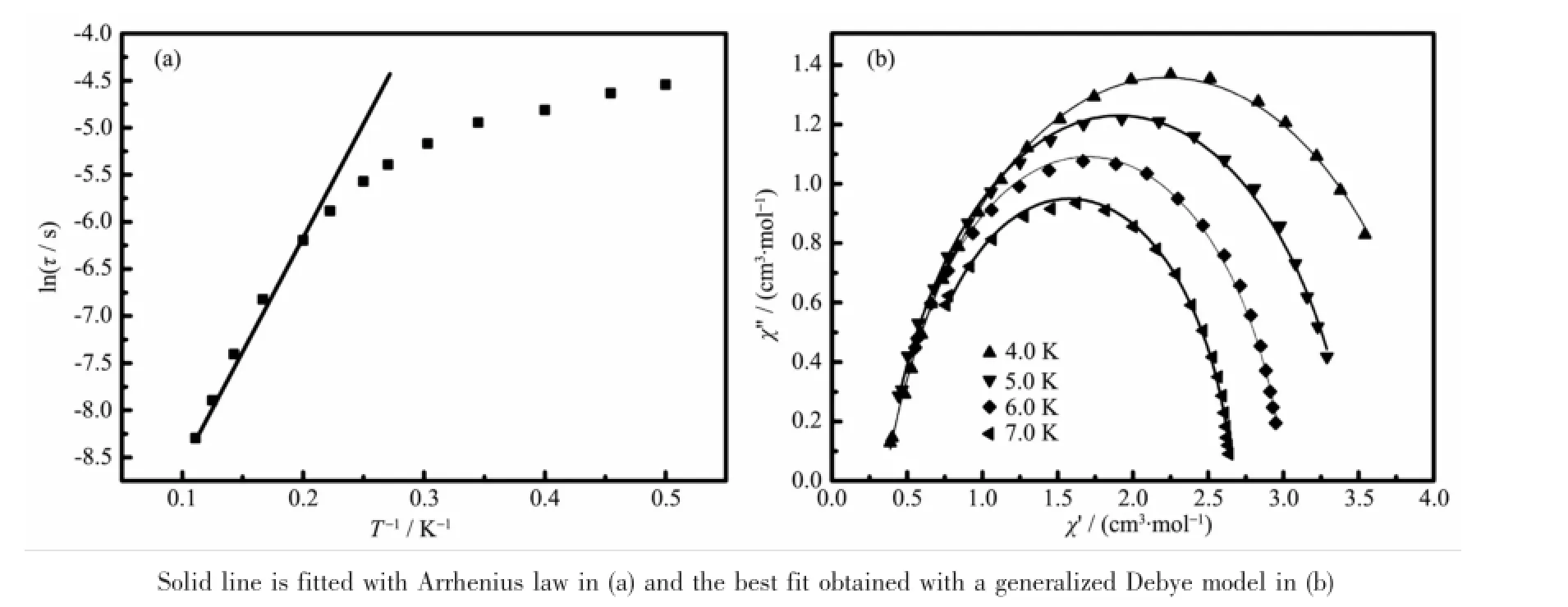
Fig.4 (a)ln(τ)versus T-1plot for compound 1 under zero dc field;(b)Cole-Cole plots measured for compound 1
Therelaxationtimewasextractedfromthe frequency-dependent data,and the Arrhenius plot obtained from these data is showed in Fig.4a The relaxation follows a thermally activated mechanism withanenergybarrierof17.2Kandapreexponential factor of τ0=5.91×10-5s,in agreement with that for DyⅢsingle-molecule magnet with binuclear structure(ΔE/kB17~198 K,τ010-8~10-5s)[37-38].The data plotted as Cole-Cole plots can be fitted to the generalized Debye model with α parameters below 0.20(Fig.3b),indicating the presence of a single relaxation process[39-40].On the contrary,no out-ofphase signals were observed in compound 2(Fig.5). Thus,it may be inferred that the different coordination modes of the ligand can drastically influence on thedynamicmagneticbehaviorsofthelanthanide compounds.
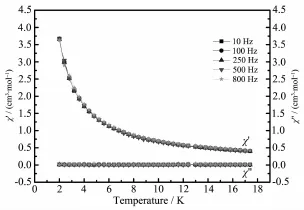
Fig.5Temperature dependence of the in-phase and outof-phase ac susceptibility for 2 under zero dc field
3Conclusions
In summary,we have synthesized two dysprosium coordination compounds,[Dy2L4(HL)4(H2O)2](ClO4)2· 2H2O(1)and[Dy2L6(C2H5OH)]·H2O(2)with the same ligandsHL.Magneticmeasurementsrevealthat compound 1 does show a single-molecule magnet behavior with the energy barrier ΔE/kB=17.1 K and the pre-exponential factor τ0=5.95×10-5s,while no out-of-phase signals are observed in compound 2.It may be inferred that the different coordination modes of the ligand lead to a difference in the dynamic magnetic behaviors of the lanthanide coordination compounds.
Supporting information is available at http://www.wjhxxb.cn
References:
[1]Leuenberger M N,Loss D.Nature,2001,410:789-791
[2]Hill S,Edwards R S,Aliaga-Alcalde N,et al.Science,2003, 302:1015-1018
[3]Yamanouchi M,Chiba D,Matsukura F,et al.Nature,2004, 428:539-542
[4]Saitoh E,Miyajima H,Yamaoka T,et al.Nature,2004,432: 203-206
[5]Bogani L,Wernsdorfer W.Nat.Mater,2008,7:179-186
[6]Maheswaran S,Chastanet G,Teat S J,et al.Angew.Chem. Int.Ed.,2004,43:2117-2121
[7]Scott R T W,Milios C J,Vinslava A,et al.Dalton Trans, 2006:3161-3163
[8]Wang W G,Zhou A J,Zhang W X,et al.J.Am.Chem.Soc., 2007,129:1014-1015
[9]Stamatatos T C,Foguet-Albiol D,Wernsdorfer W,et al.Chem. Commu.,2011,47:274-276
[10]Ako A M,Hewitt I J,Mereacre V,et al.Angew.Chem.Int. Ed.,2006,45:4926-4929
[11]Milios C J,Vinslava A,Wood P A,et al.J.Am.Chem.Soc., 2007,129:8-9
[12]Milios C J,Vinslava A,Wernsdorfer W,et al.J.Am.Chem. Soc.,2007,129:2754-2755
[13]Benelli C,Gatteschi C.Chem.Rev.,2002,102:2369-2387
[14]Osa S,Kido T,Matsumoto N,et al.J.Am.Chem.Soc., 2004,126:420-421
[15]Kong X J,Ren Y P,Long L S,et al.J.Am.Chem.Soc., 2007,129:7016-7017
[16]Mereacre V M,Ako A M,Clérac R,et al.J.Am.Chem. Soc.,2007,129:9248-9249
[17]Ako A M,Mereacre V,Clérac R,et al.Chem.Commun., 2009,45:544-546
[18]Yue Y,Sun J,Yan P,et al.Inorg.Chem.Commun.,2015, 51:42-45
[19]Hewitt I J,Lan Y,Anson C E,et al.Chem.Commun.,2009, 45:6765-6767
[20]Lin P H,Burchell T J,Ungur L,et al.Angew.Chem.Int. Ed.,2009,48:9489-9492
[21]Blagg R J,Muryn C A,McInnes E J,et al.Angew.Chem. Int.Ed.,2011,50:6530-6533
[22]Rinehart J D,Fang M,Evans W J,et al.Nat.Chem.,2011, 3:538-542
[23]Rinehart J D,Fang M,Evans W J,et al.J.Am.Chem.Soc., 2011,133:14236-14269
[24]Sessoli R,Powell A K.Coord.Chem.Rev.,2009,253:2328-2341
[25]Long J,Habib F,Lin P H,et al.J.Am.Chem.Soc.,2011, 133:5319-5328
[26]Ke H.,Xu G F,Guo Y N,et al.Chem.Commun.,2010,46: 6057-6059
[27]Tian H,Wang M,Zhao L,et al.Chem.Eur.J.,2012,18: 442-445
[28]Ma Y,Xu G F,Yang X,et al.Chem.Commun.,2010,46: 8264-8266
[29]Guo Y N,Xu G F,Wernsdorfer W,et al.J.Am.Chem.Soc., 2011,133:11948-11951
[30]Xu G F,Wang Q L,Gamez P,et al.Chem.Commun.,2010, 46:1506-1508
[31]Pointillart F,Klementieva S,Kuropatov V,et al.Chem. Commun.,2012,48:714-716
[32]Yang F,Zhou Q,Zeng G,et al.Dalton Trans.,2014,43:1238-1245
[33]Zhou Q,Yang F,Liu D,et al.Inorg.Chem.,2012,51:7529-7536
[34]Sheldrick G M.SADABS,Siemens Area Detector Absorption Correction,University of Göttingen,Germany,2005.
[35]Kahn M L,Ballou R,Porcher P,et al.Chem.Eur.J..2002, 8:525-531
[36]Osa S,Kido T,Matsumoto N,et al.J.Am.Chem.Soc., 2004,126:420-421
[37]Song Y M,Luo F,Luo M B,et al.Chem.Commun.,2012, 48:1006-1008
[38]Zou L,Zhao L,Chen P,et al.Dalton Trans.,2012,41:2966-2971
[39]Aubin S M J,Sun Z,Pardi L,et al.Inorg.Chem.,1999,38: 5329-5340
[40]Cole K S,Cole R H.J.Chem.Phys.,1941,9:341-351
Structures and Magnetic Properties of Single-Molecule Magnet Based on Dyand 2-Methyl-8-quinolinol Ligand
WANG Hui-NaLIU Ying-XinLI RongZHOU Qi*FU Wen-Sheng*
(Chongqing Key Laboratory of Green Synthesis and Applications,Chongqing Normal University,Chongqing 401331,China)
Two dysprosium coordination compounds,[Dy2L4(HL)4(H2O)2](ClO4)2·2H2O(1)and[Dy2L6(C2H5OH)]·H2O (2),have been synthesized and characterized.Both compounds contain the same ligands 2-methyl-8-hydroxylquinolinate(HL),while the different coordination modes lead to quite different coordination environments. Magnetic measurements reveal that the different coordination modes of the ligand lead to a difference in the dynamic magnetic behaviors.Compound 1 does show a single-molecule magnet behavior,while no out-of-phase signals are observed in compound 2.CCDC:1054513,1;1052697,2.
coordination compound;molecule magnet;lanthanide metal;magnetic relaxation
O614.342
A
1001-4861(2016)02-0343-08
10.11862/CJIC.2016.047
2015-09-09。收修改稿日期:2015-12-30。
国家自然科学基金(No.21271192,21501017)、重庆市教委科学技术研究项目(No.KJ1500304)和重庆市科委国际合作项目(No.cstc2014gjhz0030)资助。
*通信联系人。E-mail:fuwensheng@hotmail.com,lnwoq172@163.com;会员登记号:S06N0386S1202。

