由V-型双咪唑和芳香羧酸根配体共同构筑的Zn配位聚合物的合成,结构与性能
李霞 孙淑香 黄爱萍 李文杰 赵红 吕路路 吴本来*,
(1河南城建学院化学与材料工程学院,平顶山467044) (2河南化工职业学院,郑州450042) (3郑州大学化学与分子工程学院,郑州450001)
由V-型双咪唑和芳香羧酸根配体共同构筑的Zn配位聚合物的合成,结构与性能
李霞*,1孙淑香2黄爱萍2李文杰3赵红3吕路路3吴本来*,3
(1河南城建学院化学与材料工程学院,平顶山467044) (2河南化工职业学院,郑州450042) (3郑州大学化学与分子工程学院,郑州450001)
在溶剂热条件下,将一个V-型双咪唑配体1,1′-(5-甲基-1,3-亚苯基)二(1H-咪唑)(Bim)与其它V-型辅助配体(具有不同的对称性和功能基团的芳香羧酸)一起与金属盐ZnSO4·6H2O进行反应,分别得到2个新的配位聚合物{[Zn(Bim)(Bra)2]·CH3OH}n(1)和{[Zn(Bim)(Mpa)]·H2O}n(2)(Bra-=5-溴烟酸根,Mpa2-=5-甲基间苯二甲酸根)。采用红外光谱、元素分析、X射线粉末衍射、X射线单晶衍射和热重分析对配合物进行了表征。配合物1是由配体Bim桥联形成的一维链状结构并进一步通过链间的π…π、C-H…π和Br…π作用堆积成一个三维超分子。在2中,Zn离子通过Mpa2-桥联形成左手和右手螺旋链,这些具有不同手性的螺旋链进一步通过Bim联接形成具有(4,4)拓扑结构的内消旋的二维层。相邻的二维层之间的交错对插、层间配体分子Bim的咪唑环和苯环的双重π…π作用最终形成了三维超分子。固态荧光测试结果表明:在室温条件下,配体Bim和配合物1~2均出现了有趣的多重发射峰,而且在配合物1中四配位的Zn离子中心能显著地敏化配体Bim的高强度发射峰。
配位聚合物;晶体结构;热稳定性;荧光;双咪唑配体
0 Introduction

Scheme 1Schematic representation of the selected ligands
Since coordination polymers(CPs)have structural diversitiesandexcellentpropertiesforpotential applications in various areas,they are attracting great interest of chemists and materials scientists[1-3].In the design and synthesis of CPs there are several key factors,suchastemperature,solvent,reactant concentration,and pH value,as well as some internal factors involving in the coordination number and coordination geometry of central metals,and the bite number,configuration and rigidity or flexibility of organic ligands can influence the architectures and properties of the assemblies[4-8].
However,the effective and facile approach for synthesizing these target CPs is still the proper choice (or ingenious design)of organic ligands as bridges and the suitable metal ions or metal clusters(building blocks with metal ions)as nodes[1-5].Zincion is one of currently selected metal ions for the construction of functional CPs,because its coordination chemistry properties,such as variable coordination number from 4 to 6,various coordination geometry and d10electron configuration,usually result in the zinc-based CPs with unique structures and remarkable performances in adsorption,exchange,and optics materials[9-15].In our attempt to obtain functional CPs materials of zinc,abis-imidazoleligand1,1′-(5-methyl-1,3-phenylene)bis(1H-imidazole)(Bim),whose coordinationchemistryhasnotbeenexploredyet,was deliberately selected as the main ligand in our work. As shown in Scheme 1,this ligand has a V-shaped rigid skeleton with two electron-deficient imidazoles beingboundtotheelectron-richcentral methylbenzene through the covalent C-N bonds,and allows other two Nimidazoleatoms for binding sites and supramolecular assembly through π…π interactions. And thus it presents a typically angular ditopic bridge with tunable configurations owing to the relative rotation of the aromatic rings along the joint C-N bonds.In particular,the large conjugated system and push-pull electronic effect in Bim may provide the resulting CPs with outstanding optical properties. Additionally,two V-shaped aromatic carboxylic acids 5-bromonicotinic acid(HBra)and 5-methylisophthalic acid(H2Mpa)with different symmetry and functional groups were selected as auxiliary ligands(Scheme 1), considering that they can not only act as counter anions but also extend the structural dimensions throughtheirversatilecoordinationmodesandintriguingly supramolecular interactions(Br-bonding, H-bonding and aromatic stacking)as observed in literatures[16-19].
By using V-shaped ligand Bim in combination with auxiliary ligands Bra-and Mpa2-to react with ZnSO4·6H2O under solvothermal condition,two new zincCPs,{[Zn(Bim)(Bra)2]·CH3OH}n(1)and{[Zn (Bim)(Mpa)]·H2O}n(2),were obtained.
1 Experimental
1.1Materials and physical measurements
All of the reagent were obtained from commercial sources and used without further purification unless otherwise stated.The ligands Bim,HBra and H2Mpa were obtained from Jinan Henghua Sci.&Technol. Co.,Ltd.The IR spectra were recorded using Nicolet IR-470 with KBr disks in the range of 4 000~400 cm-1. Element analyses were performed with a Carlo-Erba 1 106 elemental analyzer.The solid-state fluorescence was measured using Hitachi-4500 with spectral slit width of 5 nm in the range of 200~900 nm.Powder X-ray diffraction(PXRD)patterns of the samples were recordedbyaRIGAKU-DMAX2500X-ray diffractometer with Cu Kα radiation(λ=0.154 18 nm). Thermogravimetric analyses(TGA)were conducted on a STA 409 PC thermoanalyzer under air atmosphere.
1.2Syntheses
1.2.1Synthesis of{[Zn(Bim)(Bra)2]·CH3OH}n(1)
A mixture of ZnSO4·6H2O(0.05 mmol,0.018 6 g),Bim(0.05 mmol,0.011 2 g),HBra(0.1 mmol, 0.020 2 g),NaOH(0.1 mmol,0.004 g),CH3OH(4 mL) and H2O(4 mL)was stirred at room temperature for 30 min,and then sealed into a 25 mL teflon-lined stainless steel autoclave.The autoclave was heated in an oven at 120℃for 4 d and then gradually cooled to room temperature at a rate of 5℃·h-1.After having been filtered,colorless needle crystals of 1 were obtained in a yield of 42%.IR(KBr,cm-1):3 450(s), 1 616(s),1 513(s),1 322(m),1 216(m),1 049(m),951 (m),820(m),650(m).Anal.Calcd.for C26H22Br2N6O5Zn (%):C,43.15;H,3.06;N,11.61.Found(%):C, 43.41;H,3.04;N,11.61.
1.2.2Synthesis of{[Zn(Bim)(Mpa)]·H2O}n(2)
A mixture of ZnSO4·6H2O(0.05 mmol,0.018 6 g), Bim(0.05 mmol,0.011 2 g),H2Mpa(0.05 mmol, 0.009 g),NaOH(0.1 mmol,0.004 g),CH3OH(6 mL) and H2O(2 mL)was stirred at room temperature for 30 min,and then sealed into a 25 mL Teflon-lined stainless steel autoclave.The autoclave was heated in an oven at 90℃for 4 d and then gradually cooled to room temperature at a rate of 5℃·h-1.After having been filtered,colorless block crystals of 2 were obtained in a yield of 36%.IR(KBr,cm-1):3 430(s), 1 618(s),1 570(m),1 511(m),1 414(m),1 355(s), 1 241(m),1 107(s),1 067(s),940(m),851(m),761(m), 734(m),653(w).Anal.Calcd.for C22H20N4O5Zn(%):C, 54.39;H,4.15;N,11.53.Found(%):C,54.47;H, 4.18;N,11.50.
1.3Single-crystal structure determination
On an Oxford diffractometer equipped with a CCD detector equipped with a graphite crystal and incident beam monochromator,crystallographic data of crystals 1 and 2 were collected using Mo Kα radiation (λ=0.071 073 nm)and Cu Kα radiation(λ=0.154 178 nm)at 293(2)K,respectively.Diffraction data collection and reduction were done using CrysAlisPro software[20].Empiricalabsorptioncorrectionusing sphericalharmonics,implementedinSCALE3 ABSPACK scaling algorithm.Structures were solved by direct method and refined by full-matrix leastsquares on F2using SHELXTL[21].
Non-hydrogenatomswererefinedwith anisotropic displacement parameters during the final cycles.Organichydrogenatomswereplacedin calculatedpositionswithisotropicdisplacement parameters setting to 1.2×Ueqof the attached atoms.H atoms of water molecules were located in difference mapsandtheirpositionswerefixedduringthe refinement such that they remained in chemically meaningful positions.Crystal data are summarized in Table 1.Selected bond lengths and bond angles as well as hydrogen bond data for 1 and 2 are listed in Table 2 and 3.The parameters of π…π,C-H…π and Br…π interactions for 1 and 2 are summarized in Table 4.
CCDC:1061914,1;1061915,2.
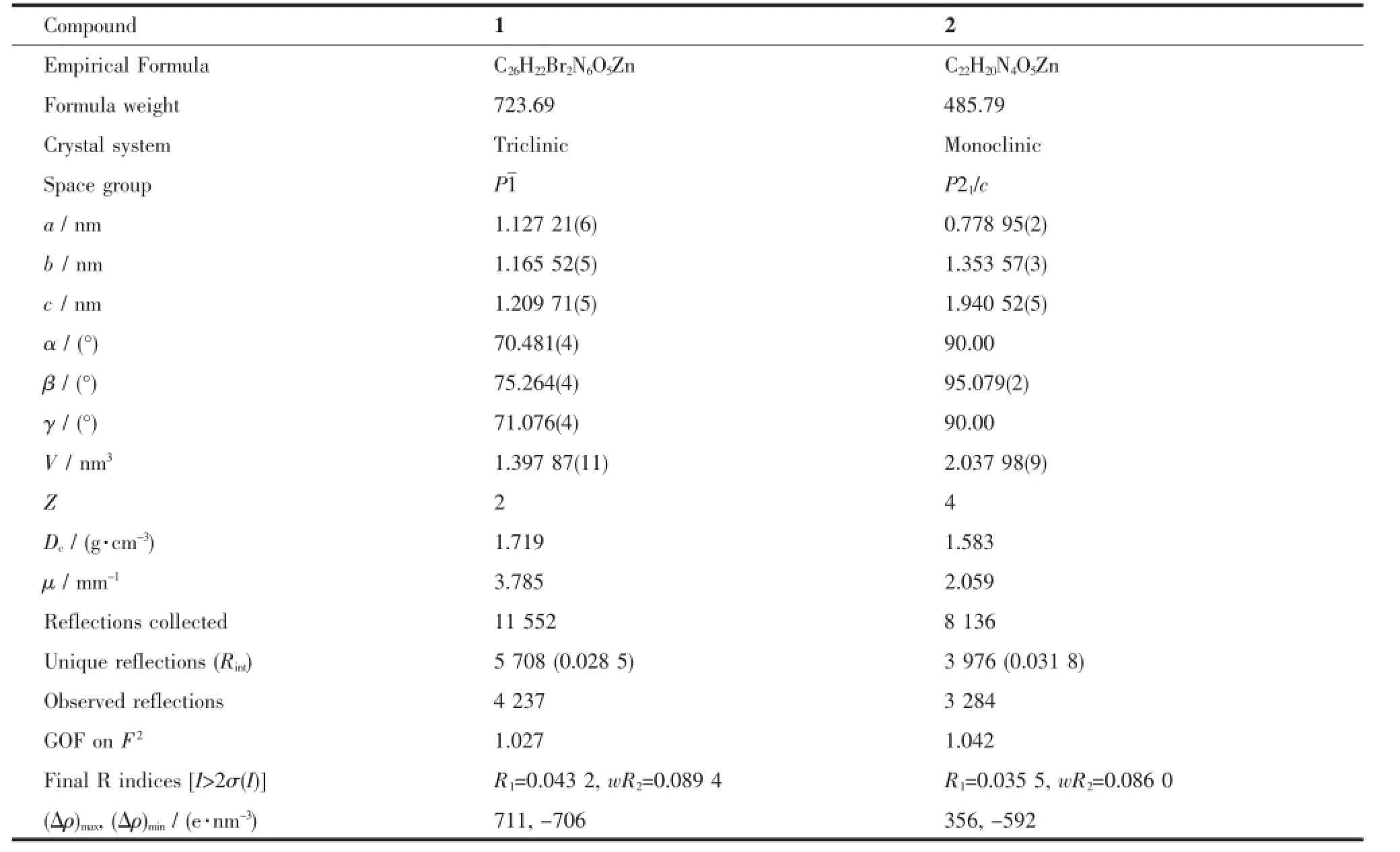
Table 1 Crystal data and structure refinement for 1 and 2
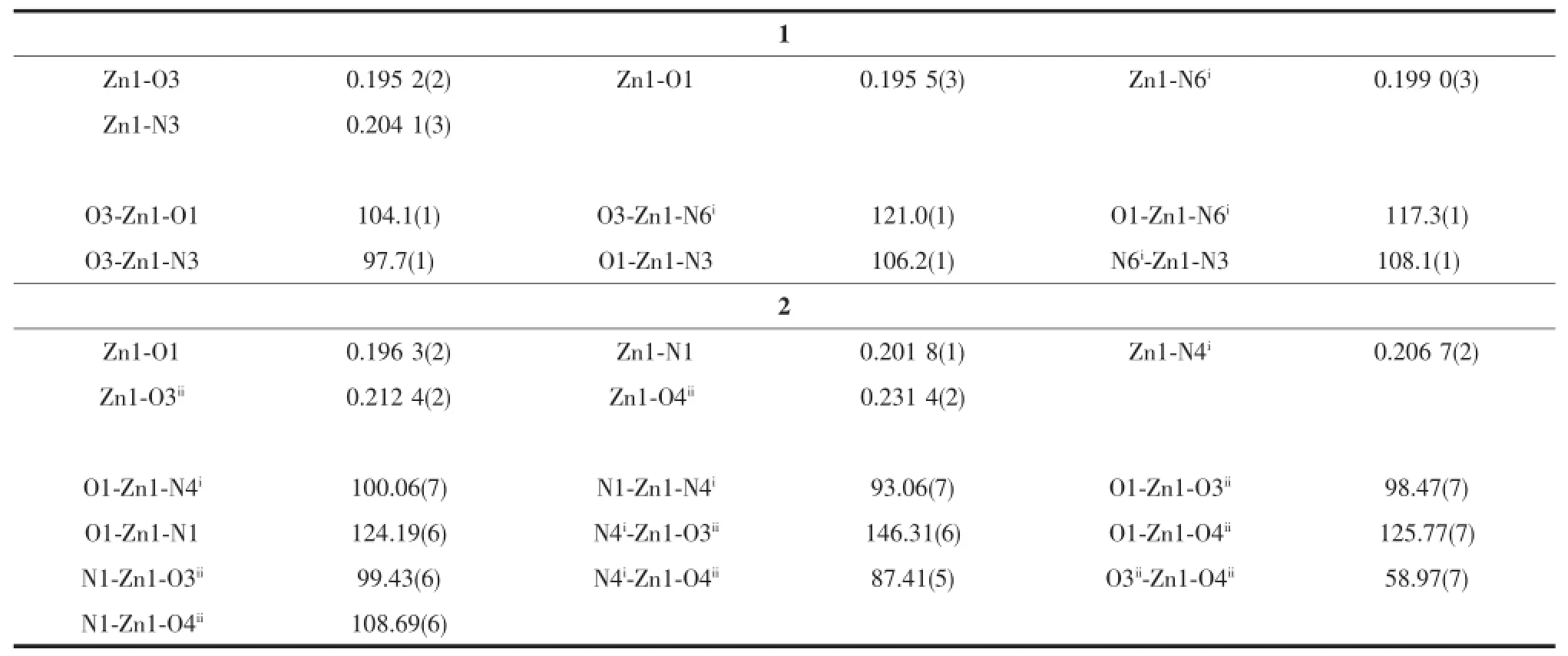
Table 2Selected bond distances(nm)and bond angles(°)for 1 and 2
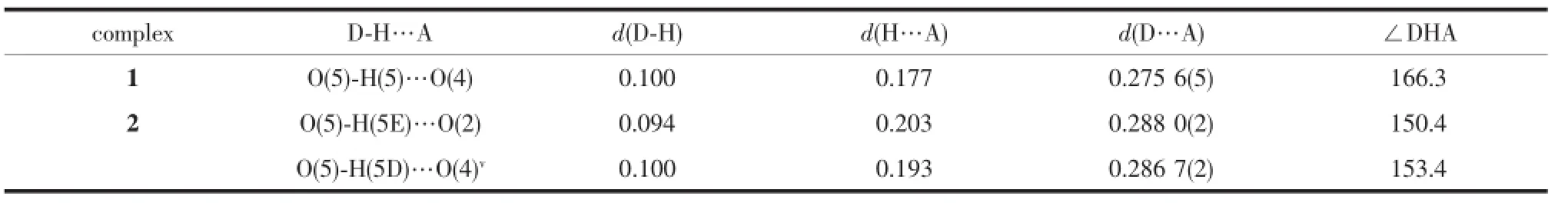
Table 3 Hydrogen bond distances(nm)and bond angles(°)for 1 and 2
2 Results and discussion
2.1Crystal structures of 1 and 2
Compound 1 crystallizes in the triclinic P1 space group,and its asymmetric unit consists of one Zn, one Bim molecule,two Bra-anions and one solvent molecule CH3OH.As shown in Fig.1,every Znis four-coordinated by two nitrogen atoms(N3 and N6i; Symmetry code:ix,y+1,z)from two Bim and two carboxylate oxygen atoms(O1 and O3)from two Bra-, and thus generate a distorted tetrahedral coordination geometry(Zn-N and Zn-O bonds cover the ranges of 0.199 0(3)~0.204 1(3)and 0.195 2(2)~0.195 5(3)nm, respectively).The bond lengths of Zn-N and Zn-O in 1 are normal values[22-23].

Fig.1 Coordination environment of Znin 1
In 1 the deprotonated aromatic carboxylates Brajust act as terminal ligands whereas Bim molecules bridge Znions to form a 1D chain structure extending along b-axis with a separation distance of Zn…Zn being 1.165 52(8)nm(Fig.2a).In Bim the two imidazole rings slightly distort from the central benzene ring,with dihedral angles being 11.4(2)°and 29.3(2)°,respectively.
AspresentedinFig.2b,severalinterchain supramolecular interactions,such as π…π,C-H…π and Br…π contribute to the formation of a 3D supramolecular network of 1,with the lattice methanol moleculesbeingboundtothepolymericchains through hydrogen-bonds(O5…O4 0.275 5(6)nm,O(5) -H(5)…O(4)166.3°)(Table 3).As listed in detail in Table 4,the interchain supramolecular interactions involve in π…π interactions between the pyridine rings(C2C3N1C4C5C6 and C2iiiC3iiiN1iiiC4iiiC5iiiC6iii,or C8C9N2C10C11C12andC8ivC9ivN2ivC10ivC11ivC12iv; Symmetry codes:iii-x+1,-y+1,-z+2;iv-x+1,-y+2,-z+1)of Bra-,π…π interactions between the pyridine ring(C8C9N2C10C11C12)of Bra-and the central benzenering(C16vC17vC18vC19vC20vC21vC22v; Symmetry code:v-x+2,-y+1,-z+1)of Bim,π…π interactionsbetweenthecentralbenzenerings (C16C17C18C19C20C21C22 and C16viC17viC18viC19viC20viC21viC22vi;Symmetry code:vi-x+2,-y,-z+2)of Bim,C-H…π interactions originating from the C4iii-H4iiiin the pyridine ring of Bra-to the imidazole ring (C13N3C14C15N4)of Bim,and Br…π interactions originating from the Br2 in one crystallographicallyindependent Bra-to the pyridine ring(C2viiC3viiN1viiC4viiC5viiC6vii;Symmetry code:viix,y,z-1)of the other crystallographically-independent Bra-.
Comparatively,compound 2 crystallizes in the monoclinic P21/c space group,and its asymmetric unit consists of one Zn,one Bim molecule,one Mpa2-anion and one guest molecule H2O.In 2 every Znis five-coordinated by two nitrogen atoms(N1 and N4i; Symmetry code:ix,-y+1/2,z+1/2)from two different Bim and three carboxylate oxygen atoms(O1,O3iiand O4ii;Symmetry code:ii-x+1,y+1/2,-z+3/2)from twoMpa2-,and thereby forming a sharply distorted trigonal bipyramid coordination geometry with N1,O1 and O4iiin the trigonal plane and N4iand O3iiat apical sites (Fig.3).The Zn-N and Zn-O bond distances range from 0.201 8(1)~0.206 7(2)and 0.196 3(2)~0.231 4(2) nm,respectively,and the bond angles cover a range of 58.97(7)°~146.31(6)°.Those parameters can be compared with those of the five-coordinated Znwith trigonal bipyramid coordination geometry in reported complexes[24-25].
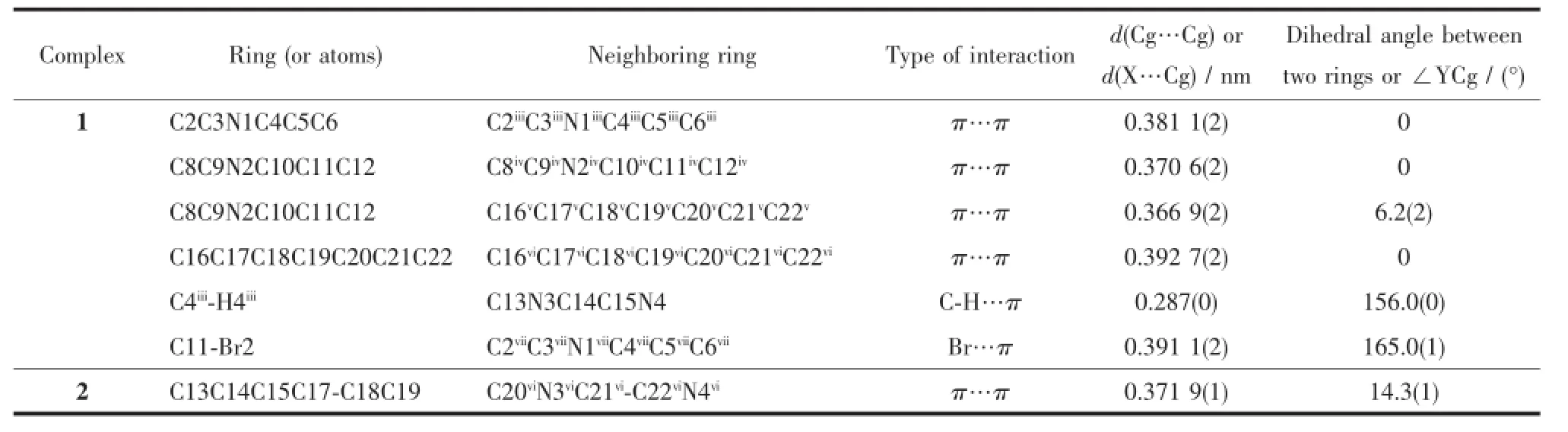
Table 4 Parameters of π…π,C-H…π and Br…π interactions in 1 and 2
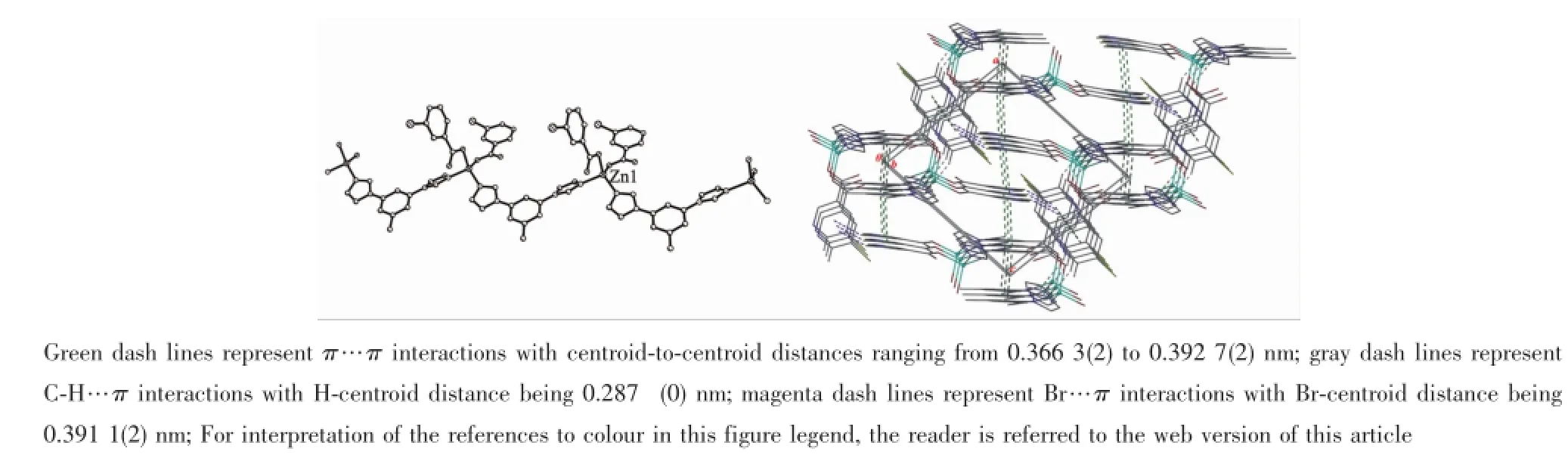
Fig.2 (a)Chain structure of 1;(b)View of 3D supramolecular network of 1 formed through interchain π…π,C-H…π and Br…π interactions

Fig.3 Coordination environment of Znin 2
In 2 the doubly deprotonated carboxylates Mpa2-adopt μ-κO,O′:κO″coordination mode,and bridge metal centers Zninto b-axially extended metallohelicates with the 21helical pitches being 1.353 6(5) nm.ThoseMpa2--bridgedleft-andright-handed metallohelicates are alternately linked together by the bridging ligands Bim to give an undulating meso layer of(4,4)topology(Fig.4),with two lattice water molecules being inhabited in every net through doubly hydrogen-bonding interactions originating from the water molecules to the monodentate and chelating carboxylates of Mpa2-(O5…O2 0.288 0(2)nm,and O5…O4v0.286 7(2)nm;Symmetry code:v-x+1,-y,-z+ 1).In 2,ligand Bim separates from Zncenters in a distance of 0.971 08(5)nm being obviously shorter than that found in 1,and its two imidazole rings distort from the central benzene ring with dihedral angles being 14.3(1)°and 47.1(1)°,respectively. Finally,a 3D supramolecular framework of 2 forms throughtheinterdigitationanddoubleπ…π interactionsbetweenthephenyl(C13C14C15 C17C18C19)and imidazole(C20viN3viC21viC22viN4vi; Symmetry code:vi-x+2,-y+1,-z+1)of Bim molecules in adjacent layers as depicted in Fig.5(centroid-tocentroid distance=0.371 9(1)nm,Table 4).
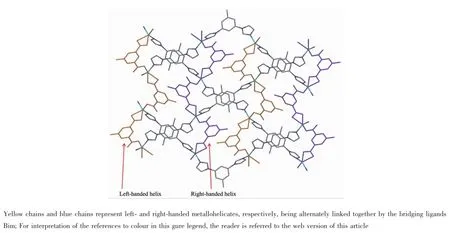
Fig.4 View of meso layer of(4,4)topology in 2,showing Mpa2--bridged left-and right-handed metallohelicates

Fig.5 View of 3D supramolecular framework of 2 formed through the interdigitation and double π…π interactions between the imidazole and phenyl of Bim molecules in adjacent layers(centroid-to-centroid distance 0.371 9(1)nm)
2.2PowderX-raydiffraction(PXRD)and thermogravimetric analysis(TGA)
The experimental and simulated PXRD patterns of compounds 1 and 2 are shown in Fig.S1 and Fig. S2,in which the main peaks in the experimental spectra of 1 and 2 are well consistent with simulated ones,indicating thegoodphasepurityofthose synthesized crystalline products.
Toinvestigatetheirthermalstability,the crystalline samples of 1 and 2 were heated under air condition from room temperature to 850℃at a heating rate of 10℃·min-1.As shown in Fig.6,an initial weight loss of 3.23%for 1 occurred between 94 and 180℃,it may be attributed to the loss of CH3OH molecules(Calcd.4.42%).On further heating,a consecutive decomposition was observed between 290 and 700℃with a weight loss of 80.76%,implying the completedecompositionoftheframework.The remaining residual of 15.19%for 1 is supposed to be ZnO(Calcd.11.25%)and deposition carbon.The decomposition of compound 2 underwent obviously two-stepweightlosses(Fig.6).Thedehydration process occurred between 120 and 240℃,and then suffered a broad platform of no loss of weight.Up to 390℃,a sharp weight loss occurred until to 550℃, suggestingthecompletedecompositionofthe framework.The final residual of 18.90%may be ZnO (Calcd.16.67%)and deposition carbon.
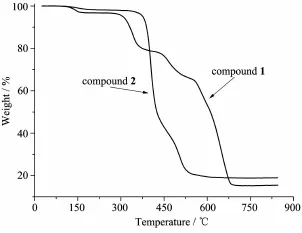
Fig.6 TGA curves of compounds 1 and 2
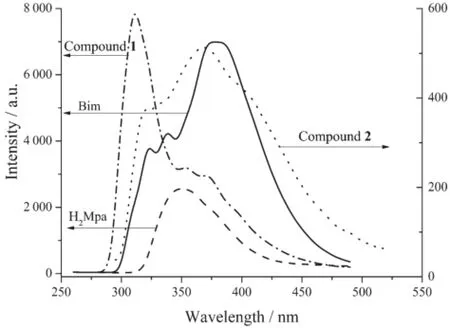
Fig.7 Solid-state photoluminescent spectra of compounds 1~2,and free ligands Bim and H2Mpa at room temperature
2.3Photoluminescence properties
The zinccomplexes of conjugated aromatic ligandsalwayshaveinterestingfluorescent properties[26-29].So the solid state photoluminescent properties of 1~2,and free ligands Bim,H2Mpa and HBra were measured at room temperature,and the results were shown in Fig.7.As excited at 250 nm, free ligand Bim displays multiple emissions with a broad stronger peak centered at 377 nm and two weakerpeakscenteredat339and324nm, respectively.The multiple fluorescent nature of Bim perhaps originates from intraligand π*→n or π*→π charge-transfer transitions.Upon exciting at the same wavelength,freeligandH2Mpaemitsweaker fluorescence centered at 350 nm but free ligand HBra has no emission.As excited at the same wavelength, both 1 and 2 also display multiple emissions with fluorescent nature similar to those of Bim but with obvious difference in intensities.Compound 1 exhibits a very intense high-energy emission centered at 311 nm and two weaker low-energy emissions centered at 353 and 370 nm.In comparison with the emissions of free ligand Bim,it is notable that the high-energy emission centered at 311 nm obviously enhances whereas the low-energy emissions centered at 353 and 370 nm quench drastically.It perhaps hints that the four-coordinated Zncenters in 1 can sensitize the high-energy emission of Bim.As for 2,its emissions very resemble the emissions of free ligand Bim in shape and position,with a broad stronger peak centeredat369nmandtwoweakershoulders centered at 333 and 321 nm,respectively.However,its emission intensities decrease greatly as compared with those of free ligand Bim and 1.
3 Conclusions
Two new structurally characterized Zncoordination polymers,{[Zn(Bim)(Bra)2]·CH3OH}n(1)and{[Zn (Bim)(Mpa)]·H2O}n(2),were successfully synthesized through solvothermal reactions.Compound 1 has 1D chainstructureswhilecompound2areameso metallohelicate structure of(4,4)topology.Notably, complicated supramolecular interactions,such as π…π,C-H…π and Br…π,play an important role in the formation of 3D metallosupermolecules 1 and 2.Solid free ligand Bim and complexes 1~2 display interesting multiple emissions.Interestingly,the four-coordinated Zncenters in 1 can obviously sensitize the highenergy emission of Bim.
Supporting information is available at http://www.wjhxxb.cn
References:
[1]Sumida K,Rogow D L,Mason J A,et al.Chem.Rev., 2012,112:724-781
[2]Zhu Y Y,Zhu M S,Yin T T,et al.Inorg.Chem.,2015,54: 3716-3718
[3]Wang S,Ding X H,Zuo J L,et al.Coord.Chem.Rev., 2011,255:1713-1732
[4]Li J X,Du Z X,Wang J G,et al.Inorg.Chem.Commun., 2012,15:243-247
[5]Li X,Wu B L,Wang R Y,et al.Inorg.Chem.,2010,49: 2600-2613
[6]Fang R Q,Zhang X M.Inorg.Chem.,2006,45:4801-4810
[7]Broker G A,Tiekink E R T.CrystEngComm,2007,9:1096-1109
[8]Xie L H,Liu S X,Gao C Y,et al.Inorg.Chem.,2007,46: 7782-7788
[9]Ji G P,Yang Z Z,Zhao Y F,et al.Chem.Commun.,2015, 51:7352-7355
[10]Li H Q,Wang P,Ma Y S,et al.Inorg.Chim.Acta, 2015,429:252-256
[11]Masoomi M Y,Morsali A.Coord.Chem.Rev.,2012,256: 2921-2943
[12]Elsaidi S K,Mohamed M H,Wojtas L,et al.J.Am.Chem. Soc.,2014,136:5072-5077
[13]Liu B.J.Coord.Chem.,2015,68:1251-1260
[14]Zhao N,Deng Y E,Liu P,et al.Polyhedron,2015,85:607-614
[15]Tahmasebi E,Masoomi M Y,Yamini Y,et al.Inorg.Chem., 2015,54:425-433
[16]Li C P,Wu J M,Du M.Chem.Eur.J.,2012,18:12437 -12445
[17]Li W J,Li G T,Lü L L,et al.J.Solid State Chem., 2015,225:297-304
[18]Li F F,Shi Z Z,Ma L F,et al.Inorg.Chim.Acta,2013,407: 153-159
[19]Han M L,Chang X H,Feng X,et al.CrystEngComm, 2014,16:1687-1695
[20]CrysAlisPro Software,1.171.36.28,Agilent Technologies, 2013.
[21]Sheldrick G M.SHELXTL Ver6.14,Structure Determination Software Suite,Bruker AXS,Madison,WI,2003.
[22]Yu C X,Ma F J,Liu L L,et al.Acta Crystallogr.Sect.C: Cryst.Struct.Commun.,2014,C70:1178-1180
[23]YIN Wei-Dong(尹卫东),LI Gui-Lian(李桂连),LIU Guang-Zhen(刘广臻),et al.Chinese J.Inorg.Chem.(无机化学学报),2015,31(7):1439-1446
[24]Xue X,Li G T,Peng Y H,et al.J.Coord.Chem.,2011,64: 1953-1962
[25]SHI Pei(石沛),SHEN Wei(沈伟),YU Yu-Ye(余玉叶), et al.Chinese J.Inorg.Chem.(无机化学学报),2015,31(1): 45-53
[26]Zhang L Y,Rong L L,Hu G L,et al.Dalton Trans., 2015,44:6731-6739
[27]Li Y W,Liu S J,Hu T L,et al.Dalton Trans.,2014,43: 11470-11473
[28]Wang H,Yang X Y,Ma Y Q,et al.Inorg.Chim.Acta,2014, 416:63-68
[29]Wang F M,Liu J Q,Jun W,et al.Inorg.Chem.Commun., 2013,35:169-171
Syntheses,Structures and Properties of ZincCoordination Polymers Constructed by V-Shaped Bis-imidazole and Aromatic Carboxylate Ligands
LI Xia*,1SUN Shu-Xiang2HUANG Ai-Ping2LI Wen-Jie3ZHAO Hong3LÜ Lu-Lu3WU Ben-Lai*,3
(1College of Chemistry and Materials Engineering,Henan University of Urban Construction,Pingdingshan,Henan 467044,China) (2Henan Vocational College of Chemical Technology,Zhengzhou 450042,China) (3College of Chemistry and Molecular Engineering,Zhengzhou University,Zhengzhou 450052,China)
A V-shaped bis-imidazole ligand 1,1′-(5-methyl-1,3-phenylene)-bis(1H-imidazole)(Bim)in combination with auxiliary V-shaped ligands aromatic carboxylic acids of different symmetry and functional groups was selected to react with ZnSO4·6H2O under solvothermal condition,and two new coordination polymers,{[Zn(Bim) (Bra)2]·CH3OH}n(1)and{[Zn(Bim)(Mpa)]·H2O}n(2),were obtained(Bra-=5-bromonicotinate and Mpa2-=5-methylisophthalate).The two compounds were characterized by IR spectra,element analysis,powder X-ray diffraction,single-crystal X-ray diffraction,and thermogravimetric analysis.Compound 1 features Bim-bridged one-dimensional chain structures which stack up through interchain π…π,C-H…π and Br…π interactions toform a three-dimensional supramolecule.In 2,zincions are bridged by Mpa2-ligands to form left-and righthanded helical chains which are further linked into(4,4)meso layers through Bim molecules.Finally,a 3D supramolecular framework forms through the interdigitation and double π…π interactions between the imidazole and phenyl of Bim molecules in adjacent layers.Solid state fluorescence measurements confirm that at room temperature both free ligand Bim and complexes 1~2 displays interesting multiple emissions,and the fourcoordinated Zncenter in 1 can notably sensitize the high-energy emission of Bim.CCDC:1061914,1; 1061915,2.
coordination polymers;crystal structure;thermal stability;fluorescence;bis-imidazole ligand
O614.24+1
A
1001-4861(2016)02-0360-09
10.11862/CJIC.2016.048
2015-09-20。收修改稿日期:2015-12-01。
国家自然科学基金(No.21271157)、河南省基础与前沿技术研究计划项目(No.122300410092)和河南省科技攻关计划项目(No. 122102210415)资助。
*通信联系人。E-mail:lixia@hncj.edu.cn;wbl@zzu.edu.cn

