尼古丁减轻高脂高果糖诱导的非酒精性脂肪性肝炎小鼠的肝脏炎症
陈小梅,李富强,严 速,吴小翠,唐翠兰△
(1. 浙江中医药大学附属第二医院肝病科, 杭州 310005; 2. 温州医科大学附属第一医院腔镜外科, 浙江温州 325000)
·论著·
尼古丁减轻高脂高果糖诱导的非酒精性脂肪性肝炎小鼠的肝脏炎症
陈小梅1,李富强1,严 速2,吴小翠1,唐翠兰1△
(1. 浙江中医药大学附属第二医院肝病科, 杭州 310005; 2. 温州医科大学附属第一医院腔镜外科, 浙江温州 325000)
目的:探讨活化胆碱能抗炎通路对非酒精性脂肪性肝炎(non-alcoholic steatohepatitis,NASH)模型小鼠肝脏炎症的抑制作用及其分子机制。方法:60只雄性6周龄的无特定病原体(specific pathogen free,SPF)级C57BL/6J小鼠被随机分为4组:正常饮食小鼠生理盐水注射组、正常饮食小鼠尼古丁注射组、NASH模型小鼠生理盐水注射组和NASH模型小鼠尼古丁注射组,分别给予普通饮食及高脂饮食加高果糖饮水,喂养17周后建立NASH小鼠模型,然后予各组小鼠生理盐水或尼古丁腹腔注射,每天1次,注射量为400 μg/kg,注射3周。3周后处死动物进行肝组织病理检查,取小鼠血清行酶联免疫吸附测定(enzyme linked immunosorbent assay,ELISA)检测炎症因子白细胞介素-6(interleukin-6,IL-6)和肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α),同时原代分离培养肝巨噬细胞,使用Western blot和荧光共聚焦显微镜检测α7尼古丁型乙酰胆碱能受体(alpha 7 nicotinic acetylcholine receptors,α7nAChR)、Toll样受体-4(Toll-like receptors-4,TLR-4)和磷酸化核转录因子-κB(nuclear factor κB of phosphorylation,p-NF-κB)的蛋白水平。结果:成功建立了NASH小鼠模型。给予小鼠尼古丁治疗后,小鼠肝组织病理结果显示,小鼠肝脏炎症和脂肪变性明显减轻;ELISA结果显示,小鼠血清中炎症因子IL-6、TNF-α水平下降;Western blot和荧光共聚焦显微镜结果显示,尼古丁治疗组小鼠α7nAChR蛋白水平上调,p-NF-κB水平下调。结论:活化胆碱能抗炎通路可以通过抑制NF-κB通路减轻NASH小鼠的肝脏炎症。
尼古丁;非酒精性脂肪性肝病;受体,胆碱能;炎症介导素类;小鼠
慢性炎症反应在非酒精性脂肪性肝炎(non-alcoholic steatohepatitis,NASH)的发生、发展中起重要作用。NASH的炎症反应由多种细胞因子参与,如果能找到一种同时抑制多种炎症介质的治疗靶位,有效抑制炎症反应的发生、发展,将会在治疗NASH中发挥重要作用,有着巨大的潜在临床应用价值。胆碱能抗炎通路可以同时抑制多种炎症介质的产生,前期研究已经发现,体内外活化胆碱能抗炎通路可以减轻肥胖诱导的炎症和胰岛素抵抗[1],如果能证实活化胆碱能抗炎通路能抑制NASH的肝脏炎症,将有重要的临床意义。因此,本研究通过模拟NASH患者高脂肪、高果糖的饮食习惯建立小鼠NASH模型[2],进一步行尼古丁腹腔注射,系统地研究活化胆碱能抗炎通路对NASH小鼠肝脏炎症的抑制作用及其分子机制。
1 材料与方法
1.1 材料与试剂
果糖购于美国Amerisco公司,尼古丁和Ⅳ型胶原酶购于美国Sigma公司,Percoll液购于上海季美生物公司,异硫氰酸荧光素标记的α-银环蛇毒(fluorescein isothiocyanate α-bungarotoxin,FITC-αBGT)购于美国Sigma公司,小鼠肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)、白细胞介素-6(interleukin-6,IL-6)酶联免疫吸附测定(enzyme linked immunosorbent assay,ELISA)试剂盒购于深圳达科为公司。Toll样受体-4(Toll-like receptors-4,TLR-4)抗体购于美国Bioworld公司,α7尼古丁型乙酰胆碱能受体(alpha 7 nicotinic acetylcholine receptors,α7n-AChR)抗体购于美国Proentech公司,甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphate dehydrogenase,GAPDH)抗体和磷酸化核转录因子-κB(nuclear factor κB of phosphorylation,p-NF-κB)抗体购自美国Cell Signal公司。
1.2 动物模型制备
无特定病原体(specific pathogen free,SPF)级雄性4周龄C57BL/6J小鼠共60只(购于上海西普尔-必凯实验动物有限公司),SPF级空调室内饲养,室温22~25 ℃,自由摄水和食物,适应饲养2周后,随机分为4组(随机数字表法):正常饮食小鼠生理盐水注射组(n=15)、正常饮食小鼠尼古丁注射组(n=15)、NASH模型小鼠生理盐水注射组(n=15)和NASH模型小鼠尼古丁注射组(n=15),分别予以普通饲料饮食加普通纯净水饮水和高脂饮食(高脂饲料:胆固醇1.5%、胆酸钠0.5%、奶粉5%、猪油10%、蛋黄粉5%、普通饲料78%)加高果糖饮水(30%果糖)。造模开始后每周测体重,观察动物的食欲、行为习惯、毛色等的改变,于7、11、17周分别在正常组和高脂组中取9只小鼠,剪尾采血检测谷草转氨酶(aspertate aminotransferase,AST)、谷丙转氨酶(alanine aminotranferease,ALT), 同时于7和11周每组取2只小鼠、17周每组取6只小鼠处死,取肝组织做病理染色,评价造模情况。第17周小鼠造模成功后,分别予各组小鼠行生理盐水或尼古丁腹腔注射,尼古丁注射量为400 μg/kg,生理盐水注射量为对应尼古丁注射量的体积,每天1次,注射3周后,每组小鼠眼睛取血检测IL-6、TNF-α水平,然后全部处死(正常小鼠生理盐水注射组9只、正常小鼠尼古丁注射组8只、NASH小鼠生理盐水注射组9只和NASH小鼠尼古丁注射组10只),称取肝脏湿重,统一取肝左叶做肝组织病理检测和免疫荧光,余肝组织裂解后提取蛋白做Western blot检测α7nAChR、TLR-4和p-NF-κB的蛋白水平。
1.3 组织学和血清学检测
分别于第7、11、17、20周予禁食16 h后处死小鼠,统一取肝左叶,采用冰冻切片进行油红O染色,观察肝细胞脂肪变性采用石蜡切片进行HE染色,观察肝组织炎症活动度。请两位以上不了解本研究的病理学专家参照NASH临床研究网络(NASH clinical research network,NASH-CRN)病理委员会提供的NASH活性评分系统(NAS)进行评分,包括脂肪变性(0~3)、小叶炎症(0~2)、肝细胞气球样变(0~2),NAS评分总和(0~8)。同时于第7、11、17周每组取9只小鼠,剪尾采血于离心管中,室温静置30 min后离心取上清,使用日立7180生化分析仪检测小鼠血清AST、ALT。第20周所有小鼠完成腹腔注射后眼球取血,室温静置30 min后离心取上清,使用ELISA试剂盒检测各组小鼠血清中IL-6、TNF-α水平。
1.4 Western blot
将小鼠肝组织用磷酸盐缓冲液(phosphate buf-fer saline,PBS)洗净,置于离心管中,用干净的剪刀将组织块尽量剪碎,每100 mg肝组织加入500 μL含苯甲基磺酰氟化物(phenylmethyl sulfonylfluoride,PMSF)的放射免疫沉淀测定(radio immunoprecipitation assay,RIPA)蛋白裂解液,于手动匀浆器上进行匀浆,1 min后置于冰上,3 min后再次匀浆并置于冰上,如此重复,裂解30 min后,在4 ℃下12 000×g离心15 min,取得的上清即为蛋白,将组织中提取的蛋白用二喹啉甲酸(bicinchoninic acid,BCA)法测定细胞蛋白浓度后,加上样缓冲液后沸水煮8 min,迅速水浴冷却,取等量蛋白样品以10%(质量分数)SDS聚丙烯胺凝胶电泳分离,余下蛋白分装保存于-80 ℃冰箱待用。分离后的蛋白用电转移法转移到聚偏氟乙稀(polyvinylidene fluoride,PVDF)膜上,转膜完成后用5%(质量分数)脱脂牛奶室温孵育封闭1 h,加入一抗于4 ℃孵育过夜,TBST缓冲液清洗3遍后加入二抗,室温孵育1 h,TBST清洗3遍,将PVDF膜平铺于暗盒中,取适量化学发光试剂(electrochemiluminescence,ECL)均匀滴于PVDF膜上,迅速盖上暗盒,在暗室内压片曝光显影,压片结果拍照分析保存。每份蛋白样品单独分析,每份蛋白样品重复3次试验。
1.5 荧光共聚焦显微镜检测小鼠肝组织中α7nAChR和p-NF-κB的表达
统一取小鼠肝右叶组织固定在甲醛溶液中24 h,脱水、透明、包埋、切片、贴片,制作石蜡切片,然后恒温箱中60 ℃烤片2 h,二甲苯、酒精脱蜡、水化,0.3%(体积分数)过氧化氢封闭内源性过氧化物酶,柠檬酸钠(0.01 mol/L,pH 6.0)隔水煮沸10 min修复抗原,3%(体积分数)牛血清白蛋白(bovine serum albumin,BSA)室温孵育封闭15 min,然后加入不带荧光的p-NF-κB抗体(1 ∶100)和带荧光的FITC-αBGT抗体(1 ∶100)4 ℃孵育过夜(α-BGT对α7nAChR具有高亲和力,能与α7nAChR形成专一性的、饱和的和不可逆性的结合,因此本实验通过FITC-αBGT检测α7nAChR,FITC-αBGT需避光孵育)。PBS缓冲液洗3次,孵育p-NF-κB抗体的切片需再加带荧光的二抗(1 ∶100)室温孵育1 h,PBS缓冲液洗3次,然后将所有切片加7-氨基放线菌素D(7-amino-actinomycin D,7-AAD,1 ∶100)染核45 min,PBS缓冲液洗3次,抗荧光猝灭剂封片,尽快在荧光共聚焦显微镜下观察。
1.6 统计学分析
2 结果
2.1 高脂高果糖饮食成功制备NASH小鼠模型
随着喂养时间的增加,普通饮食小鼠和高脂高果糖饮食小鼠体重均有增加,但高脂高果糖组小鼠体重增加趋势较普通饮食组小鼠明显(图1A),于造模第17周,高脂高果糖组小鼠与正常组小鼠体重差异有统计学意义(P<0.01)。临床生物化学分析显示,高脂高果糖饮食的小鼠血清ALT水平在第7、11周时升高不明显,但在第17周时明显升高且与普通饮食小鼠差异有统计学意义(P<0.01,图1B);高脂高果糖饮食小鼠血清AST水平与普通饮食小鼠在第7、11、17周时差异均有统计学意义(P<0.01,图1C)。肉眼观察小鼠肝组织发现,高脂高果糖饮食小鼠肝脏膨胀,肝脏湿重明显高于普通饮食小鼠;HE染色和油红O染色显示,高脂高果糖饮食小鼠在第7周开始出现轻微炎症和脂肪变性,脂肪变性以小泡脂滴为主,到11、17周,炎症和脂肪变性逐渐加重,脂肪变性以中、大脂滴为主(图2)。NASH-CRN显示,高脂高果糖饮食小鼠得分随着喂养时间逐渐增高。
2.2 尼古丁治疗减轻NASH小鼠肝脏炎症
正常小鼠和NASH小鼠行3周腹腔注射后发现,生理盐水注射后的小鼠肝脏湿重没有明显变化,而尼古丁注射后的小鼠肝脏湿重与注射前相比明显减轻(图3A)。ELISA结果显示,与生理盐水注射相比,尼古丁注射能明显降低小鼠血清中IL-6和TNF-α水平(图3B、3C)。肝组织病理染色显示,尼古丁注射组肝脏炎症和脂肪变性与生理盐水注射组相比明显减轻(图4)。
2.3 尼古丁治疗减轻NASH小鼠肝脏炎症的分子机制
Western blot结果显示,尼古丁注射组较生理盐水注射组肝组织中α7nAChR蛋白表达水平上调,p-NF-κB蛋白表达水平下调,TLR-4表达水平无明显变化(图5)。另外,免疫荧光结果显示,加入FITC-αBGT抗体后,尼古丁注射组的荧光强度和密度高于生理盐水注射组,加入p-NF-κB抗体后,尼古丁注射组的荧光强度和密度低于生理盐水注射组(图6)。
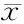
ALT, amino transferase; AST, aspartic amino transferase; SC, standard chow; HFHF, high-fat and high-fructose. Data are expressed as ±s. *P<0.01, vs. SC.图1 高脂高果糖饮食对小鼠体重(A,n=30)、血清ALT(B,n=9)及AST(C,n=9)水平的影响Figure 1 Effects on body weight (A, n=30), serum levels of ALT (B, n=9) and AST (C, n=9) in mice with high-fat and high-fructose diet
SC, standard chow; HFHF, high-fat and high-fructose.
图2 高脂高果糖饮食诱导的NASH模型小鼠的病理表现(×20)
Figure 2 Pathologic manifestation of mice model of NASH induced by high-fat and high-fructose diet (×20)
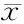
TNF-α, tumour necrosis factor-α; IL-6, interleukin-6; SC, standard chow; NASH, non-alcoholic steatohepatitis; NT, nicotine; NS, saline. Data are expressed as ±s; *P<0.01, vs. before injection.图3 腹腔注射尼古丁3周后小鼠肝脏湿重(A)和血清中TNF-α(B)、IL-6(C)水平变化Figure 3 The change of liver weight (A), TNF-α (B) and IL-6(C) in mice after intraperitoneal injection for 3 weeks
图4 NASH模型小鼠腹腔注射后肝组织病理变化(×20)
Figure 4 The change of liver histopathological conditions after intraperitoneal injection for 3 weeks in NASH mice (×20)
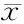
NT, nicotine; NS, saline; NASH, non-alcoholic steatohepatitis; α7nAChR, alpha 7 nicotinic acetylcholine receptors; TLR-4, Toll-like receptors-4; p-NF-κB, nuclear factor κB of phosphorylation. Data are expressed as ±s.*P<0.01, vs. NASH+NS.图5 腹腔注射后NASH小鼠肝组织中α7nAChR、TLR-4和p-NF-κB的蛋白表达水平Figure 5 The expression of α7nAChR, TLR-4, p-NF-κB protein after intraperitoneal injection in NASH mice
Abbreviations as in Figure 5.
图6 腹腔注射后NASH小鼠肝组织中α7nAChR、
p-NF-κB的免疫荧光图片
Figure 6 Immunohistochemistry of α7nAChR, p-NF-κB protein after intraperitoneal injection for 3 weeks in NASH mice
3 讨论
近来研究发现,在NASH的发病机制中存在肝的天然免疫功能紊乱,来源于肝巨噬细胞的炎症因子对NASH的发生、发展起重要作用[3],巨噬细胞失活后可以阻断NASH大鼠肝脂肪变性和炎症的发展[4]。巨噬细胞的活化主要由肠道来源的内毒素脂多糖(lipopolysaccharides,LPS)启动[5],LPS通过与巨噬细胞TLR-4结合,活化丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)信号通路和NF-κB[6],导致细胞因子IL-6、TNF-α等产生[7]。IL-6、TNF-α在NASH发病机制中起重要作用,其中TNF-α不但可以引起肝细胞损伤,还可以导致胰岛素抵抗[8]。同时巨噬细胞也是参与胆碱能抗炎通路的主要靶细胞[9],胆碱能抗炎通路主要通过活化巨噬细胞表面的α7nAChR发挥抗炎作用[10]。
本研究首先通过模拟NASH患者高脂肪、高果糖的饮食习惯建立小鼠NASH模型[2]。虽然目前还没有明确定义的NASH小鼠模型,但我们通过在实验过程中定期监测实验小鼠的体重,并对实验小鼠进行血清生化分析和肝组织病理检查,观察到随着高脂高果糖饮食喂养时间的延长,小鼠肝组织从单纯脂肪变性逐渐发展到脂肪性肝炎,血清AST和ALT水平逐渐增高,较为完整的呈现了人类NASH疾病的发生、发展过程。因此,此模型对于进一步研究NASH疾病发生、发展的分子机制研究具有重要意义。
其次,我们给予造模成功的C57BL NASH小鼠尼古丁腹腔注射3周后,发现NASH小鼠整体体重和肝脏湿重明显减轻,显微镜下肝组织病理染色发现NASH小鼠肝脏炎症程度下降,ELISA检测血清中TNF-α和IL-6水平下降,与前期实验中尼古丁减轻BALB/cNASH小鼠肝组织炎症程度,下调血清TNF-α水平,以及体外实验中尼古丁能抑制RAW264.7细胞产生TNF-α的结果一致[11],并进一步观察到尼古丁对炎症因子IL-6水平的影响。
NF-κB是NASH中关键的前炎症信号途径,是免疫和炎症的主要调节因子,前期发现体外给予RAW264.7细胞尼古丁处理对NF-κB通路有抑制作用[11]。本研究进一步从体内实验观察到腹腔注射尼古丁能明显抑制小鼠肝Kupffer细胞上NF-κB的磷酸化,抑制NF-κB的转核,同时小鼠肝Kupffer细胞上的α7nAChR蛋白水平上调,但对模式受体TLR-4的表达影响不明显。另外,荧光共聚焦显微镜结果亦证实了尼古丁治疗可以上调α7nAChR蛋白水平,抑制NF-κB的转核。
本研究通过高脂饮食加高果糖饮水成功建立NASH小鼠模型,并通过体内实验观察到尼古丁通过上调α7nAChR激活胆碱能抗炎通路,抑制NF-κB通路的激活,减少炎症因子IL-6、TNF-α的释放,从而减轻小鼠的肝脏炎症,说明α7nAChR介导的抗炎信号传导通路与TLR-4介导的致炎信号传导通路均通过NF-κB信号通路调节,TLR-4介导的巨噬细胞活化在NASH的炎症反应中起重要作用[12],而α7nAChR体内活化胆碱能抗炎通路对NASH炎症具有抗炎作用。
[1]Wang X, Yang Z, Xue B, et al. Activation of the cholinergic antiinflammatory pathway ameliorates obesity-induced inflammation and insulin resistance[J]. Endocrinology, 2011, 152(3): 836-846.
[2]Kohli R, Kirby M, Xanthakos SA, et al. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis[J]. Hepatology, 2010, 52(3): 934-944.
[3]Zhan YT, An W. Roles of liver innate immune cells in nonalcoholic fatty liver disease[J]. World J Gastroentero, 2010, 16(37): 4652-4660.
[4]Rivera CA, Adegboyega P, van Rooijen N, et al. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the patho-genesis of nonalcoholic steatohepatitis[J]. J Hepatol, 2007, 47(4): 571-579.
[5]Chung MY, Yeung SF, Park HJ, et al. Dietary α-and γ-toco-pherol supplementation attenuates lipopolysaccharide-induced oxidative stress and inflammatory-related responses in an obese mouse model of nonalcoholic steatohepatitis[J]. J Nutr Biochem, 2010, 21(12): 1200-1206.
[6]O’Neill LA, Bowie AG. The family of five: 11R-domain-con-taining adaptors in Toll-like receptor signaling[J]. Nat Rev lmmunol, 2007, 7(5): 353-364.
[7]Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view[J]. J Hepatol, 2009, 51(1): 212-223.
[8]Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis[J]. Hepatology, 2010, 52(5): 1836-1846.
[9]Pohanka M, Snopkova S, Havlickova K, et al. Macrophage-assisted inflammation and pharmacological regulation of the cholinergic anti-inflammatory pathway[J]. Curr Med Chem, 2011, 18(4): 539-551.
[10]Bencherif M, Lippiello PM, Lucas R, et al. Alpha7 nicotinic receptors as novel therapeutic targets for inflammation-based diseases[J]. Cell Mol Life Sci, 2011, 68(6): 931-949.
[11]周舟, 陈小梅, 李富强, 等. 活化胆碱能抗炎通路对非酒精性脂肪性肝炎炎症反应的抑制作用及其机制[J]. 中华肝脏病杂志, 2015, 23(1): 64-68.
[12]Liu J, Zhuang ZJ, Bian DX, et al. Toll-like receptor-4 signaling in the progression of non-alcoholic fatty liver disease induced by high-fat and high-fructose diet in mice[J]. Clin Exp Pharmacol Physiol, 2014, 41(7): 482-488.
(2014-12-29收稿)
(本文编辑:赵 波)
Nicotine alleviates the liver inflammation of non-alcoholic steatohepatitis induced by high-fat and high-fructose in mice
CHEN Xiao-mei1, LI Fu-qiang1, YAN Su2, WU Xiao-cui1, TANG Cui-lan1△
(1. Department of Liver Disease, The Second Affiliated Hospital, Zhejiang Chinese Medical University, Hangzhou 310005, China; 2. Department of Endoscopic Surgery, The First Affiliated Hospital of Whenzhou Medical University, Wenzhou 325000, Zhejiang, China)
Objective:To investigate the anti-inflammation effects by activation of the cholinergic anti-inflammatory pathway and its mechanisms in non-alcoholic steatohepatitis (NASH) model mice. Me-thods: 6-week-old male C57BL/6J (B6) mice were randomly divided into four groups: the first group was normal mice, injected with saline; the second group was normal mice, injected with nicotine; the third group was NASH model mice, injected with saline; the fourth group was NASH model mice, injected with nicotine. The experimental mice were fed with either standard chow (SC) or high-fat and high-fructose (HFHF) for 17 weeks to generate an NASH model mice. The mice received injection once daily for 3 weeks [nicotine dose, 400 μg/kg]. Then, their pathological characteristics and function of the liver were assessed. The expressions of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) in serum were analyzed by enzyme linked immunosorbent assay (ELISA). The expressions of alpha 7 nicotinic acetylcholine receptors (α7nAChR), Toll-like receptors-4 (TLR-4) and nuclear factor κB of phosphory-lation (p-NF-κB) in Kupffer cells were determined by Western blot and immunofluorescence assays. Results: We successfully generated NASH model mice by imitating the high-fat and high-fructose dietary style of NASH patients. The results of our investigation demonstrated that nicotine could reduce significantly the levels of IL-6, and TNF-α in serum (P<0.05). The expression of p-NF-κB protein in the group which was NASH model mice injected with nicotine declined significantly as compared with the group which was NASH model mice injected with saline (P<0.05). And the expression of α7nAChR protein elevated significantly conversely (P<0.05). Conclusion: Activation of the cholinergic anti-inflammatory pathway could inhibit the release of inflammatory factors as TNF-α and IL-6 in NASH model mice, and the mechanism for the inhibition of inflammatory was mediated by NF-κB pathway.
Nicotine; Non-alcoholic fatty liver disease; Receptors, cholinergic; Inflammation mediators; Mice
国家自然科学基金(81100279)和浙江省新苗人才计划项目(2014R410058)资助Supported by the National Natural Science Foundation of China (81100279) and Xin-miao Talent Program of Zhejiang Province (2014R410058)
时间:2016-6-29 14:22:18
http://www.cnki.net/kcms/detail/11.4691.R.20160629.1422.024.html
R575.1
A
1671-167X(2016)05-0777-06
10.3969/j.issn.1671-167X.2016.05.005
△Corresponding author’s e-mail, 1747603542@qq.com

