转化生长因子-β1和血管内皮生长因子在浓缩生长因子各层中的分布及含量特点
陈 飞,潘韶霞,冯海兰
(1. 北京大学口腔医学院·口腔医院,修复科 口腔数字化医疗技术和材料国家工程实验室 口腔数字医学北京市重点实验室,北京 100081; 2. 中国人民解放军总医院口腔科,北京 100853)
·论著·
转化生长因子-β1和血管内皮生长因子在浓缩生长因子各层中的分布及含量特点
陈 飞1,2,潘韶霞1△,冯海兰1
(1. 北京大学口腔医学院·口腔医院,修复科 口腔数字化医疗技术和材料国家工程实验室 口腔数字医学北京市重点实验室,北京 100081; 2. 中国人民解放军总医院口腔科,北京 100853)
目的:测定浓缩生长因子(concentrated growth factors,CGF)凝胶层与红细胞(red blood cells,RBC)层中转化生长因子-β1(transforming growth factor-β1,TGF-β1)、血管内皮生长因子(vascular endothelial growth factor,VEGF)分布及含量,探究TGF-β1、VEGF在CGF凝胶层内的分布及含量与其促成骨效应的相关性并指导临床应用。方法:招募6名健康志愿者,采集静脉血制备CGF样品。免疫组织化学法观察CGF凝胶层与RBC层中TGF-β1、VEGF的分布并测定制备即刻的累积光密度值(integrated optical density,IOD)和平均光密度值(average optical density,AOD)。酶联免疫吸附(enzyme-linked immunosorbent assays,ELISA)测定制备即刻上清层与制备1 d后CGF凝胶层析出液中TGF-β1、VEGF的浓度。结果:TGF-β1与VEGF大量聚集于CGF凝胶层中,在RBC层中仅位于多核细胞胞浆和散在的血小板内;CGF凝胶层与RBC层的交界区域有大量血小板与白细胞聚集,并有较高的TGF-β1表达;CGF凝胶层析出液中TGF-β1和VEGF的浓度分别为(55 236.78±3 686.34) ng/L和(610.99±148.81)ng/L,均显著高于上清层(20 710.20±4 523.14) ng/L和(335.20±51.69) ng/L(P<0.001)。结论:CGF凝胶层内含有大量TGF-β1、VEGF,可发挥促进成骨与加速组织愈合的作用。在临床应用中建议应用时间宜早为佳,除整个CGF层外,可将上清层、CGF层与RBC层交界区域一并使用。
浓缩生长因子;转化生长因子β1;血管内皮生长因子;骨生成
浓缩生长因子(concentrated growth factors,CGF)是继富血小板血浆(platelet-rich plasma,PRP)、富血小板纤维蛋白(platelet-rich fibrin,PRF)后的新一代血小板浓缩产物,由Saccol于2006年首先提出[1],其包含了发挥骨诱导作用的自体血小板生长因子和发挥骨引导作用的纤维蛋白基质,具有改善并增强组织再生的独特性质[2-3]。由于其特殊的变速离心程序,其产物更大、更致密,生长因子含量更高[1]。
CGF能够发挥促成骨作用主要基于制备过程中血小板脱颗粒释放了大量的生长因子,其中转化生长因子-β1(transforming growth factor-β1,TGF-β1)的主要功能是促进前体成骨细胞趋化和有丝分裂,促使胶原基质中的成骨细胞聚集,同时抑制破骨细胞形成和骨吸收,使得骨形成大于骨吸收[4-5]。而血管内皮生长因子(vascular endothelial growth factor,VEGF)能促进微血管周围内皮细胞的增殖、迁移,增强血管通透性,促进血浆蛋白的渗出,利于新生血管形成[6],在术区低氧状态下,也能促进成骨细胞的分化[7-8]。目前虽有CGF应用于修复骨缺损的动物实验和应用于上颌窦提升术的临床报道[2, 9-13],但对于CGF中生长因子成分研究的报道较少[1],尚未见揭示CGF中生长因子分布及含量的相关研究。本研究旨在测定CGF凝胶层与红细胞(red blood cells,RBC)层中TGF-β1、VEGF的分布及含量,探究TGF-β1、VEGF在CGF凝胶层内的分布及含量与其促成骨效应的相关性并指导临床应用。
1 材料与方法
1.1 CGF的制备
招募6名健康志愿者(男、女各3人),年龄24~26岁,无传染性疾病,无相关血液疾病,静脉血血小板计数大于105/μL,采血前3个月未服用影响血小板功能的药物,如阿司匹林等。本研究已通过北京大学口腔医院生物医学伦理委员会的审核(PKUSSIRB-201519008),所有志愿者知情并同意采血用于本研究。每名志愿者采集静脉血9 mL/支,共2支,立即放入Medifuge离心机(SilfradentSrl,意大利)内以CGF程序离心(加速启动,30 s;2 700 r/min,2 min;2 400 r/min,4 min;2 700 r/min,4 min;3 000 r/min,3 min;减速停止,36 s),分别留取上清层、CGF凝胶层和RBC层样品。
1.2 CGF凝胶层与RBC层样本的免疫组织化学分析
将制备即刻留取的CGF凝胶层与RBC层样品置于10%(体积分数)甲醛溶液固定48 h后石蜡包埋,每个样本切片机下分切20片(约7 μm厚)备用。
1.2.1 免疫组织化学 切片脱蜡:石蜡包埋的切片经二甲苯处理,浓度梯度乙醇,去离子水再水化。置于0.05 mol/L柠檬酸钠盐酸缓冲液(pH 6.0)中,在微波炉650 W条件下处理5 min两次,400 W条件下处理3 min一次,进行抗原修复。在3%(体积分数)过氧化氢溶液内处理30 min后,阻断内源性过氧化氢酶的活性。切片在3%(质量分数)牛血清白蛋白(bovine serum albumin,BSA,北京中杉金桥生物技术有限公司)室温封闭30 min以上,滴加兔抗人TGF-β1单克隆抗体(稀释比1 ∶50,北京中杉金桥生物技术有限公司)、兔抗人VEGF多克隆抗体(稀释比1 ∶50,北京中杉金桥生物技术有限公司),并设置不加一抗的空白对照,置于4 ℃过夜。分别滴加兔超敏二步法免疫组织化学检测试剂(Polink-2 plus®,Golden Bridge International, Inc.)一液和二液,各室温孵育30 min,滴加DAB显色液(北京中杉金桥生物技术有限公司)显色。所有切片用苏木素复染,脱水,封片。
1.2.2 TGF-β1、VEGF的半定量分析 40倍光学显微镜(BX51,DP72,Olympus)下每张切片随机选取5个视野拍摄数码照片,固定显微镜光强,设定手动曝光时间为556 μs,关闭自动白平衡。所有照片导入Image-ProPlus 6.0(Media Cybernetics,Inc.),分别测量CGF凝胶层累积光密度值(integrated optical density,IOD)、CGF凝胶层与RBC层平均光密度值(average optical density,AOD),其中IOD值可反映待测生长因子在总面积内相对含量的变化,AOD值可反映待测生长因子单位面积浓度的变化[14-15]。
1.3 上清层与CGF凝胶层析出液的酶联免疫吸附测定分析
制备CGF后,立即分装各样本上清层于EP管中,-80 ℃保存备用。无菌条件下将CGF凝胶样品分装EP管内,置于37 ℃培养箱内贮存1 d,随后留取CGF凝胶层析出液于EP管中,-80 ℃保存备用。
使用酶联免疫吸附测定(enzyme-linked immunosorbent assays,ELISA)试剂盒(上海蓝基生物科技有限公司)对上清层与CGF凝胶层析出液中TGF-β1、VEGF的浓度进行检测,按照试剂盒说明书进行操作。取出试剂盒,于室温(20~25 ℃)放置15~30 min。实验过程应在室温(20~25 ℃)内进行,取出酶标板,按照标准品的次序分别加入100 μL的标准品溶液于空白微孔中。空白微孔中加入100 μL的样品(稀释比:TGF-β1,1∶1 000;VEGF,1∶10),空白对照加入100 μL的蒸馏水;在各孔中加入50 μL的酶标记溶液(不含空白对照孔),将酶标板用封口胶密封后,37 ℃孵育反应1 h(在孵育箱中保持稳定的温度与湿度),充分清洗酶标板5次,保持各孔有充足的水压(浓缩洗涤液以1∶100的比例用蒸馏水稀释),酶标板洗涤后用吸水纸彻底拍干;各孔加入显色剂A、B液各50 μL,37 ℃下避光反应15 min,各孔加入50 μL终止液,终止反应。于波长450 nm的酶标仪(Elx808,BioTek,USA)上读取各孔的光密度值,用标准物的浓度与光密度值拟合出标准曲线的回归方程式,将样品的光密度值代入方程式,计算出样品浓度,再乘以稀释倍数,即为样品的实际浓度。
1.4 数据统计分析
应用SPSS 16.0软件(Statistical Product and Service Solutions,Inc.)对CGF层中TGF-β1、VEGF表达的IOD值,CGF层与RBC层中TGF-β1、VEGF表达的AOD值,上清层与CGF凝胶层析出液中TGF-β1、VEGF的浓度测量结果进行独立样本t检验,检验水准α=0.05,以P<0.05为差异有统计学意义。
2 结果
2.1 免疫组织化学
2.1.1 大体观察 CGF凝胶层可见大量纤维蛋白束,其中TGF-β1的表达(图1A)明显高于VEGF(图1B),TGF-β1表达的区域与纤维蛋白束所在区域几乎一致,而VEGF仅表现为散在分布,空白对照中均未出现TGF-β1和VEGF的表达(图1C、D)。RBC层中TGF-β1(图2A)和VEGF(图2B)的表达仅局限于多核细胞胞浆和散在的血小板内,且靠近CGF凝胶层与RBC层的交界区域的多核细胞和血小板分布较多(图3A、B),多核细胞胞浆内VEGF的表达稍高于TGF-β1(图3C、D),在红细胞内无TGF-β1和VEGF的表达,空白对照亦未出现TGF-β1和VEGF的表达(图2C、D)。在CGF凝胶层与RBC层的交界区域(图3E、F)可见大量聚集的血小板与白细胞,其中血小板层靠近CGF凝胶层,且TGF-β1含量较高,而VEGF则鲜见表达;白细胞层近RBC层,未见TGF-β1和VEGF的表达。
Significantly higher expression level of TGF-β1 (A) was observed in CGF gel than that of VEGF (B) did, while no expressions of TGF-β1 (C) and VEGF (D) in blank control (samples without the primary antibodies).
图1 CGF凝胶层中TGF-β1和VEGF的表达(×40)
Figure 1 The expressions of TGF-β1 and VEGF in CGF gel layer (×40)
The expression level of TGF-β1 (A) and VEGF (B) only present in polykaryocytes and sporadic platelets of RBC layer, but not in erythrocytes. No expressions of TGF-β1 (C) and VEGF (D) in blank controls.
图2 RBC层中TGF-β1和VEGF的表达(×40)
Figure 2 The expressions of TGF-β1 and VEGF in RBC layer (×40)
Relatively higher distributions of platelets and polykaryocytes were observed in RBC layer close to the junction of CGF gel and RBC layer. TGF-β1 (A,×40) and VEGF (B,×40) only present in polykaryocytes and sporadic platelets of RBC layer, the expressions of TGF-β1 (C,×100) were lower than VEGF (D,×100) in the endochylema of polykaryocytes. Platelets and leukocytes were concentrated in between CGF gel and RBC layer with high expression of TGF-β1 (E,×20), but little VEGF (F,×20) can be observed in this zone. No expressions of TGF-β1 (E,×20) and VEGF (F,×20) in the leukocyte layer.
图3 不同层中TGF-β1和VEGF的表达情况
Figure 3 The expressions of TGF-β1 and VEGF in different layers
2.1.2 IOD值与AOD值测量 CGF凝胶层TGF-β1的IOD值约为VEGF的149.3倍(93 963.14±28 644.99vs. 629.15±348.06,t=35.690,P<0.001),TGF-β1的AOD值约为VEGF的6.7倍(P<0.001,表1),但RBC层中TGF-β1和VEGF的AOD值大致相等。
2.2 ELISA测定结果
在上清层和CGF凝胶层析出液中,TGF-β1的浓度分别约为VEGF的61.8倍(P<0.001)和90.4倍(P<0.001),CGF凝胶层析出液TGF-β1和VEGF的浓度分别约为上清层的2.67倍(P<0.001)和1.8倍(P<0.001,表2)。
CGF, concentrated growth factors; TGF-β1, transforming growth factor-β1; VEGF, vascular endothelial growth factor; RBC, red blood cells.
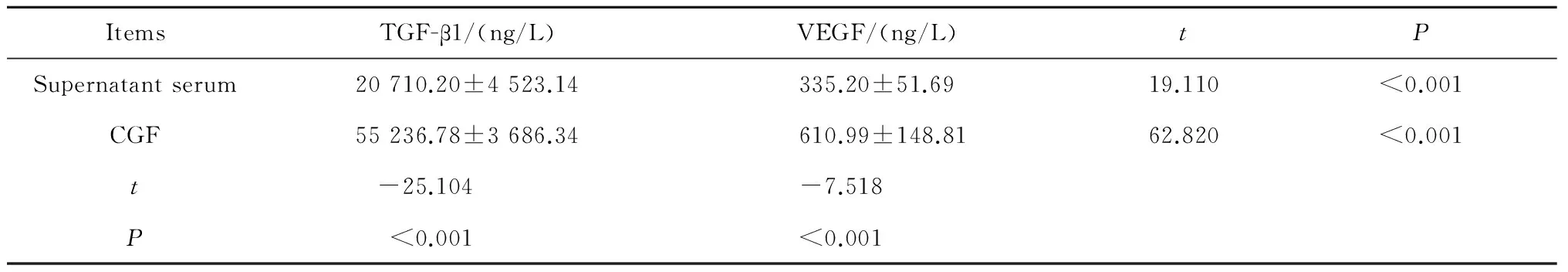
表2 上清层及CGF凝胶层析出液中TGF-β1、VEGF的浓度Table 2 Concentrations of TGF-β1 and VEGF in supernatant serumand CGF gel
Abbreviations as in Table 1.
3 讨论
3.1 CGF凝胶层与RBC层中TGF-β1和VEGF免疫表达的分布
Rodella等[1]对6名志愿者CGF凝胶块中TGF-β1和VEGF进行了免疫组织化学半定量分析,结果显示,在CGF层与RBC层中都有广泛的免疫染色,通过测定其IOD,发现在以上各层中两种生长因子成分含量水平相似。本研究结果发现,TGF-β1在CGF凝胶层中有广泛的免疫染色,但VEGF仅表现为散在分布,且在RBC层中TGF-β1和VEGF的表达仅局限于多核细胞胞浆和散在的血小板内,并非广泛染色,提示离心作用有效地使TGF-β1等生长因子浓缩于CGF层内,应当作为临床应用的主要部分。另外,本研究观察到CGF凝胶层与RBC层的交界区域有大量血小板与白细胞聚集,并有TGF-β1的较高表达,与PRF纤维蛋白网内可观察到大量蓝染的白细胞核不同[16],提示在CGF的临床应用中,可将此交界区域一并使用,从而发挥促进成骨与抗感染的作用。
3.2 CGF凝胶层与RBC层中TGF-β1和VEGF的含量与浓度
本研究结果显示,CGF制备即刻,其凝胶层中TGF-β1的IOD值约为VEGF的149.3倍,AOD值约为VEGF的6.7倍;TGF-β1在CGF凝胶层析出液中的浓度约为VEGF的90.4倍,在上清层中约为VEGF的61.8倍,表明TGF-β1的相对含量与浓度均明显高于VEGF。静脉血中的TGF-β1主要存在于血小板的α-颗粒内,且含量很高,在离心制备CGF的过程中,血小板经脱颗粒释放大量的TGF-β1于血浆内[17-18],而VEGF则主要存在于血管内皮细胞、巨噬细胞及肿瘤细胞内,且总体含量较少[6, 19],因此血浆内存在的VEGF含量明显少于TGF-β1。此前对PRF的研究报道揭示,其纤维蛋白网眼内和聚合物可捕获并缓慢释放生长因子[20-22],通过电子显微镜观察,在CGF中同样具备此纤维蛋白网眼和聚合物[1],因此血浆内的TGF-β1和VEGF等生长因子能被纤维蛋白网眼和聚合物大量捕获,最终形成富含生长因子和纤维蛋白的CGF凝胶层,并被缓慢释放出来。本研究中TGF-β1和VEGF在上清层中的浓度分别为(20 710.20±4 523.14) ng/L和(335.20±51.69) ng/L,与Rodella等[1]报道的实验结果相似。制备1 d后TGF-β1和VEGF在CGF凝胶层析出液中的浓度分别约为其在上清层浓度的2.67倍和1.8倍,提示经离心制备后,大量生长因子经血小板脱颗粒后聚集于CGF凝胶层内,并且随时间的推移持续析出,从而发挥促进成骨的作用[23-24]。对比本研究与已报道文献中PRP和PRF的浓度数据可见,CGF中TGF-β1与VEGF的浓缩程度均较高[21, 24],在临床应用中能够为成骨和组织愈合提供更强且更持久的生长因子调控,从而达到促进新骨形成和加速组织愈合的目的。
综上所述,CGF凝胶层析出液中TGF-β1和VEGF的浓度均显著高于上清层,可以为植入术区提供大量的生长因子,发挥促进成骨的作用,分离的上清层也可与骨替代材料混合使用,使其所含的生长因子也能发挥作用。离心作用有效地使TGF-β1等生长因子浓缩在凝胶层,而在RBC层中TGF-β1与VEGF仅位于多核细胞胞浆和散在的血小板内,因此临床应用中应主要取用凝胶层。CGF凝胶层与RBC层的交界区域有大量血小板与白细胞聚集,并有较高的TGF-β1表达,因此,在临床应用中,建议将整个CGF凝胶层与下方RBC层交界区域一并使用,发挥促进成骨与抗感染的作用。
[1]Rodella LF, Favero G, Boninsegna R, et al. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction [J]. Microsc Res Tech, 2011, 74(8): 772-777.
[2]Honda H, Tamai N, Naka N, et al. Bone tissue engineering with bone marrow-derived stromal cells integrated with concentrated growth factor in Rattus norvegicus calvaria defect model [J]. J Artif Organs, 2013, 16(3): 305-315.
[3]Yu B, Wang Z. Effect of concentrated growth factors on beagle periodontal ligament stem cellsinvitro[J]. Mol Med Rep, 2014, 9(1): 235-242.
[4]Nikolidakis D, Meijer GJ, Oortgiesen DA, et al. The effect of a low dose of transforming growth factor beta1 (TGF-beta1) on the early bone-healing around oral implants inserted in trabecular bone [J]. Biomaterials, 2009, 30(1): 94-99.
[5]Ozkan K, Eralp L, Kocaoglu M, et al. The effect of transforming growth factor beta1 (TGF-beta1) on the regenerate bone in distraction osteogenesis [J]. Growth Factors, 2007, 25(2): 101-107.
[6]Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases [J]. J Biochem, 2013, 153(1): 13-19.
[7]Ferrara N. Vascular endothelial growth factor: basic science and clinical progress [J]. Endocr Rev, 2004, 25(4): 581-611.
[8]Zhang W, Zhu C, Wu Y, et al. VEGF and BMP-2 promote bone regeneration by facilitating bone marrow stem cell homing and differentiation [J]. Eur Cell Mater, 2014(27): 1-11.
[9]Kim TH, Kim SH, Sandor GK, et al. Comparison of platelet-rich plasma (PRP), platelet-rich fibrin (PRF), and concentrated growth factor (CGF) in rabbit-skull defect healing [J]. Arch Oral Biol, 2014, 59(5): 550-558.
[10]柳宏志, 李超, 王天祥, 等. CGF联合骨代用品在即刻种植中修复种植体周围骨缺损的实验研究 [J]. 口腔颌面外科杂志, 2012, 22(4): 277-282.
[11]柳宏志, 邹高峰, 王天祥, 等. 浓缩生长因子促进犬软组织损伤修复的实验研究 [J]. 口腔颌面外科杂志, 2013, 23(1): 28-31.
[12]Sohn DS, Heo JU, Kwak DH, et al. Bone regeneration in the maxillary sinus using an autologous fibrin-rich block with concentrated growth factors alone [J]. Implant Dent, 2011, 20(5): 389-395.
[13]Kim JM, Sohn DS, Bae MS, et al. Flapless transcrestal sinus augmentation using hydrodynamic piezoelectric internal sinus elevation with autologous concentrated growth factors alone [J]. Implant Dent, 2014, 23(2): 168-174.
[14]Andrade WC, da Silva LF, Coelho MC, et al. Effects of the administration of pentoxifylline and prednisolone on the evolution of portal fibrogenesis secondary to biliary obstruction in growing animals: immunohistochemical analysis of the expression of TGF-β and VEGF [J]. Clinics, 2012, 67(12): 1455-1461.
[15]Zhang JX, Yang JR, Chen GX, et al. Sesamin ameliorates arterial dysfunction in spontaneously hypertensive ratsviadownregulation of NADPH oxidase subunits and upregulation of eNOS expression [J]. Acta Pharmacol Sin, 2013, 34(7): 912-920.
[16]孙洁, 张剑明, 李彦秋. 富血小板纤维蛋白超微结构的观察与探讨 [J]. 口腔医学研究, 2010, 26(1): 98-101.
[17]Meyer A, Wang W, Qu J, et al. Platelet TGF-beta1 contributions to plasma TGF-beta1, cardiac fibrosis, and systolic dysfunction in a mouse model of pressure overload [J]. Blood, 2012, 119(4): 1064-1074.
[18]Toma I, McCaffrey TA. Transforming growth factor-beta and athe-rosclerosis: interwoven atherogenic and atheroprotective aspects [J]. Cell Tissue Res, 2012, 347(1): 155-175.
[19]Kim HA, Seo KH, Kang YR, et al. Mechanisms of platelet-activating factor-induced enhancement of VEGF expression [J]. Cell Physiol Biochem, 2011, 27(1): 55-62.
[20]Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part Ⅱ: platelet-related biologic features [J]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2006, 101(3): e45-50.
[21]杨世茂, 王明国, 李静, 等. 富血小板纤维蛋白与富血小板血浆体外释放生长因子的比较及其对脂肪干细胞增殖分化的影响 [J]. 华西口腔医学杂志, 2012, 30(6): 641-649.
[22]Dohan Ehrenfest DM, Bielecki T, Jimbo R, et al. Do the fibrin architecture and leukocyte content influence the growth factor release of platelet concentrates? An evidence-based answer comparing a pure platelet-rich plasma (P-PRP) gel and a leukocyte- and platelet-rich fibrin (L-PRF) [J]. Curr Pharm Biotechnol, 2012, 13(7): 1145-1152.
[23]Su CY, Kuo YP, Tseng YH, et al.Invitrorelease of growth factors from platelet-rich fibrin (PRF): a proposal to optimize the clinical applications of PRF [J]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2009, 108(1): 56-61.
[24]Roussy Y, Bertrand Duchesne MP, Gagnon G. Activation of human platelet-rich plasmas: effect on growth factors release, cell division andinvivobone formation [J]. Clin Oral Implants Res, 2007, 18(5): 639-648.
(2015-02-11收稿)
(本文编辑:赵 波)
Distribution and content of transforming growth factor-β1 and vascular endothelial growth factor in each layer of concentrated growth factors
CHEN Fei1,2, PAN Shao-xia1△, FENG Hai-lan1
(1. Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China; 2. Department of Stomatology, Chinese PLA General Hospital, Beijing 100853, China)
Objective:To investigate the distribution and content of transforming growth factor-β1 (TGF-β1) and vascular endothelial growth factor (VEGF) in concentrated growth factors (CGF) gel, and to clarify the difference among different layers of CGF. Methods: Venous blood samples were collected from 6 healthy volunteers to prepare CGF. The distribution, integrated optical density (IOD) and average optical density (AOD) of TGF-β1 and VEGF in CGF gel and red blood cell (RBC) layer were measured using immunohistochemistry. The concentrations of TGF-β1 and VEGF in the supernatant se-rum at baseline and the CGF releasate after 1 day were evaluated with enzyme-linked immunosorbent assays.Results:Abundant TGF-β1 and VEGF were concentrated in CGF gel. However, only a little could be found in polykaryocytes and sporadic platelets in RBC layer. Platelets and leukocytes were concentra-ted in between the two layers with high expression of TGF-β1. The concentrations of TGF-β1 and VEGF in the CGF releasate(55 236.78±3 686.34), (610.99±148.81) ng/L were significantly higher than those in the supernatant serum(20 710.20±4 523.14), (335.20±51.69)ng/L (P<0.001).Conclusion: CGF contains high quantities of TGF-β1 that can promote new bone formation and tissue healing. We suggest that CGF gel should be used right after being prepared. Supernatant serum and the area between CGF gel and RBC layer could also be mixed with bone substitute materials.
Concentrated growth factors; Transforming growth factor beta1; Vascular endothelial growth factors; Osteogenesis
国家临床重点专科建设项目(2011)和教育部留学回国人员科研启动基金(2012-940)资助Supported by the National Key Clinical Specialist Construction Programs of China (2011) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, Ministry of Education of China(2012-940)
时间:2016-1-5 14:47:21
http://www.cnki.net/kcms/detail/11.4691.R.20160105.1447.018.html
R329
A
1671-167X(2016)05-0860-06
10.3969/j.issn.1671-167X.2016.05.021
△ Corresponding author’s e-mail, sx_pan@hotmail.com

