基于聚芴及阳离子铱配合物的白光发光电化学池
吴家祺, 李福山, 聂 晨, 曾群英, 郭太良
(福州大学物理与信息工程学院 光电显示技术研究所, 福建 福州 350002)
基于聚芴及阳离子铱配合物的白光发光电化学池
吴家祺, 李福山*, 聂晨, 曾群英, 郭太良
(福州大学物理与信息工程学院 光电显示技术研究所, 福建 福州350002)
发光电化学池在柔性显示和大面积发光面板的低成本制造方面具有显著优势,但是白光发光电化学池器件的制备一直是一个难题。本文报道了基于阳离子铱离子配合物的柔性黄光发光电化学池,其在6 V时的电流效率高达11.6 cd/A。并将此铱离子配合物与蓝光聚芴材料以一定比例混合,基于此材料制备了白光发光电化学池,其色坐标为(0.31,0.33),接近标准白光。
发光电化学池; 白光; 阳离子铱配合物; 聚芴
1 引 言
1995年,Pei等首次将离子型导电聚合物和固态电解液混合,而后发现了共轭聚合物的电化学氧化-还原机制,第一个发光电化学池(LECs)器件被制作出来[1-2]。与有机电致发光二极管(Organic light-emitting diode,OLED)相比,LECs由于更优越的性能,如溶液可处理、对阴极功函数没要求、单层器件结构等,使其更能实现像壁纸似的发光上的应用[3-6]。一般地,根据发光材料的不同可以将LECs分为两类:在这里我们提到的聚合物发光电化学池(Polymer LECs, PLECs)指的是发光材料为聚合物的LECs,而离子型过渡金属配合物发光电化学池(Ionic transition-metal complex LECs, iTMC-LECs)指的是发光材料为离子型过渡金属配合物的LECs[3-5]。1997年,Yang等[7]报道了第一个基于聚芴衍生物与聚(环氧乙烷)(PEO)的相分离混合物的白光LECs。由于荧光材料自身的特性,其最终的电致发光效率会受到限制。所以近年来,白光LECs大多以离子型过渡金属配合物为主。由于铱离子配合物具有高的发光效率、发光颜色可调、短的激发态寿命等优点,现今iTMC-LECs主要采用的发光材料为双环金属的铱配合物[8-10]。2008年,Su等[11]首次报道了以蓝绿光配合物为主体、红光配合物为客体主客体掺杂的白色发光电化学池。2011年,Su等[12]将蓝绿光发射的配合物和红光发射的配合物还有黄光发射的配合物双掺杂(红光和黄光作为客体),得到白光发射的LEC。2014年,Takeo等[13]使用串联结构制备了白光发光器件。对于大多数颜色的iTMC-LECs,高亮度和效率已经得到实现,然而高纯蓝光的LECs器件却一直很难实现,而这对于白光LECs器件的发展是极其重要的[10,14-16]。本文对黄光LECs进行了研究分析,并以OLED中常用的聚芴材料作为LECs中的蓝光材料,将其与黄光阳离子铱配合物混合制备了白光LECs器件,提供了一种新的制备白光LECs的方法。
2 实 验
本实验采用的黄光铱离子配合物和蓝光聚芴材料分别为Ir(ppz)2Mptz(Y1),聚9,9-二辛基芴(poly(9,9-dioctylfluorene),PFO)。材料的结构式如图1(a)所示,器件的结构为Al(100 nm)/发光层/PEDOT∶PSS/ITO阳极/柔性基底,如图1(b)所示。
器件的阳极为柔性氧化铟锡(ITO),分别用去离子水、丙酮、酒精、去离子水超声20 min,将清洗后的器件放入干燥箱烘干,用等离子处理基片2 min以提高柔性ITO的表面浸润性。将poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) (PEDOT∶PSS)溶液旋涂在ITO上,转速为3 000 r/min,再放到120 ℃的加热台上热处理20 min。将黄光材料Y1溶于乙腈溶液(20 mg/mL),并将1-丁基-3-甲基咪唑六氟磷酸盐(BMIMPF6)以1∶0.2的量比混合。添加BMIMPF6是为了提供额外的阴离子以降低开启电压和减少器件的响应时间[17]。以3 000 r/min的转速将溶液旋涂于PEDOT∶PSS上,随后将其放置于70 ℃的加热台上加热40 min。白光材料则由PFO三氯甲烷溶液(10 mg/mL)与Y1三氯甲烷溶液(20 mg/mL)按 80∶1的量比混合。以4 000 r/min的转速将该混合溶液旋涂在PEDOT∶PSS上,随后将其放到60 ℃的加热台上加热1 h。以上步骤都在大气中完成。最后,将基片放入真空腔体中,蒸镀100 nm的Al电极。蒸发室的工作真空度为3×10-4Pa,沉积速度和厚度采用石英振荡器监控。
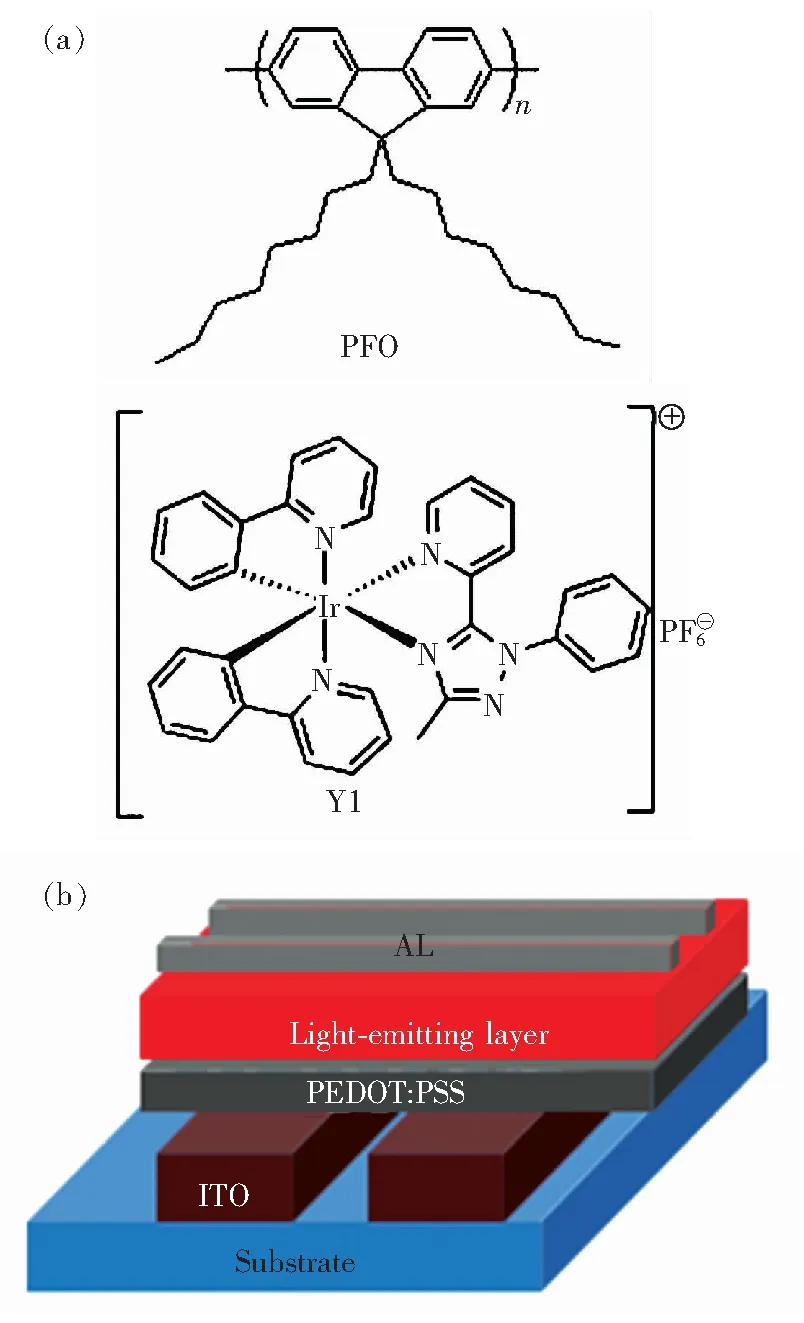
图1 (a)发光材料结构式;(b)器件结构式。
Fig.1(a) Chemical structure of the light-emitting material.(b) As-fabricated device configuration.
材料的光致发光光谱(Photoluminescence,PL)和器件的电致发光光谱(Electroluminescent,EL)由日本日立公司的F-4600测试,亮度和色坐标由日本拓普康SR-3A分光辐射光度计测得。器件的I-V曲线由Kithley4200SCS半导体测试仪测得。所有测试均在室温中进行。
3 结果与讨论
3.1发光光谱分析
图2(a)、(b)分别为黄光器件的电致发光光谱及其薄膜的光致发光光谱和白光器件的电致发光光谱。从图中可以看出黄光的电致发光峰在572 nm,相对于光致发光光谱基本重合,稍微蓝移了9 nm,并且在长波区域出现拖尾现象。这可能是由于铱离子配合物分子存在强相互作用和分子所处环境的变化所导致的[18-19]。白光器件的电致发光峰分别在430 nm和560 nm左右。它的色坐标(Commission International de L’Eclairage,CIE)为x=0.31和y=0.33。

图2 (a)黄光器件的电致发光光谱及其薄膜的光致发光谱;(b)白光器件的电致发光光谱,插图为所制备的白光器件。
Fig.2(a) Normalized EL spectrum of the yellow LECs and PL spectrum of the light-emitting layer. (b) Normalized EL spectrum of the white LECs. Inset shows the luminous LECs of the yellow and white LECs.
3.2发光性能分析
黄光器件的结构为PET/ITO/PEDOT∶PSS/Y1∶BMIMPF6(1∶x)/Al,表1为器件的详细的电学性能数值。如图3(a)所示,在常压(6 V)下,黄光LECs的电流密度和亮度一开始随时间的增加而增大,然后在达到最大亮度或最大电流时开始衰减。随着时间的增加,施加的电压促使更多的阳离子在阳极附近累积而后在阴极耗尽,从而促进载流子的注入和复合,最后导致器件的电流和亮度增大。但是随着电流密度的持续增大,载流子注入逐渐失衡,器件的亮度开始衰减。对于这个现象,Costa等[20]认为器件的稳定性与发光阶段直接相关,而与载流子注入和传输阶段无关。从表1可以看出,对于引入离子液体BMIMPF6的黄光LECs,只需0.43 h就达到最大亮度53 cd/m2,但同时器件的寿命衰减到0.91 h。离子液体虽然会促进载流子的复合但是与此同时会导致更多激子的猝灭,从而降低器件的稳定性[21-23]。在实际应用中,稳定的电学性能也是柔性器件所必备的。因此本文对黄光器件进行了弯曲性测试,曲率半径为10 mm,弯曲次数为200次,得出的曲线如图3(b)所示。黄光器件的最大电流效率随弯曲次数的变化基本保持不变,说明黄光柔性器件具有很好的机械柔韧性。
图3(c)为白光LECs的电流密度曲线和最大亮度曲线。器件结构为ITO/PEDOT∶PSS/PFO∶Y1(80∶1)/Al。器件的起亮电压为6 V,随着电压的增大,亮度逐渐增大,达到亮度最大值。图3(d)为白光器件的电流效率曲线,当电压为10 V时,器件达到效率最大值0.92 cd/A。我们注意到器件的整体效率比较低,这可能是由于所采用的聚芴材料与铱离子配合物的能级匹配度不高,材料性质差异性较大,导致微相分离,器件内部载流子输出不平衡,还产生过多的热量;并且PFO作为主体材料,其器件的发光效率不高,使得器件整体的效率较低[7,24-25]。
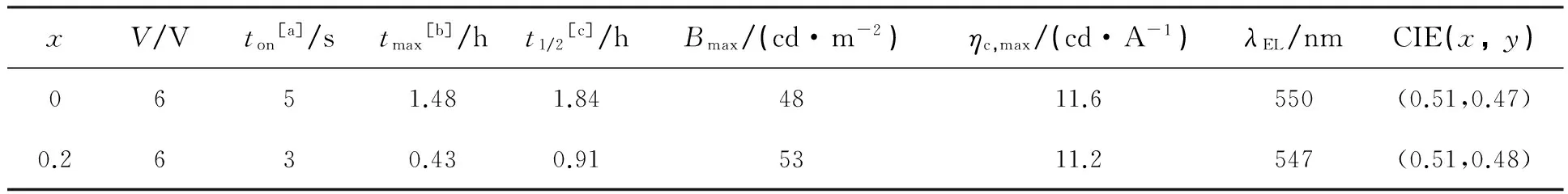
表1 基于配合物Y1的发光电化学池的电学性能
[a]亮度达到1 cd/m2所需时间;
[b]达到最大亮度所需时间(响应时间);
[c]在常压下从最大亮度衰减到一半亮度所需时间。
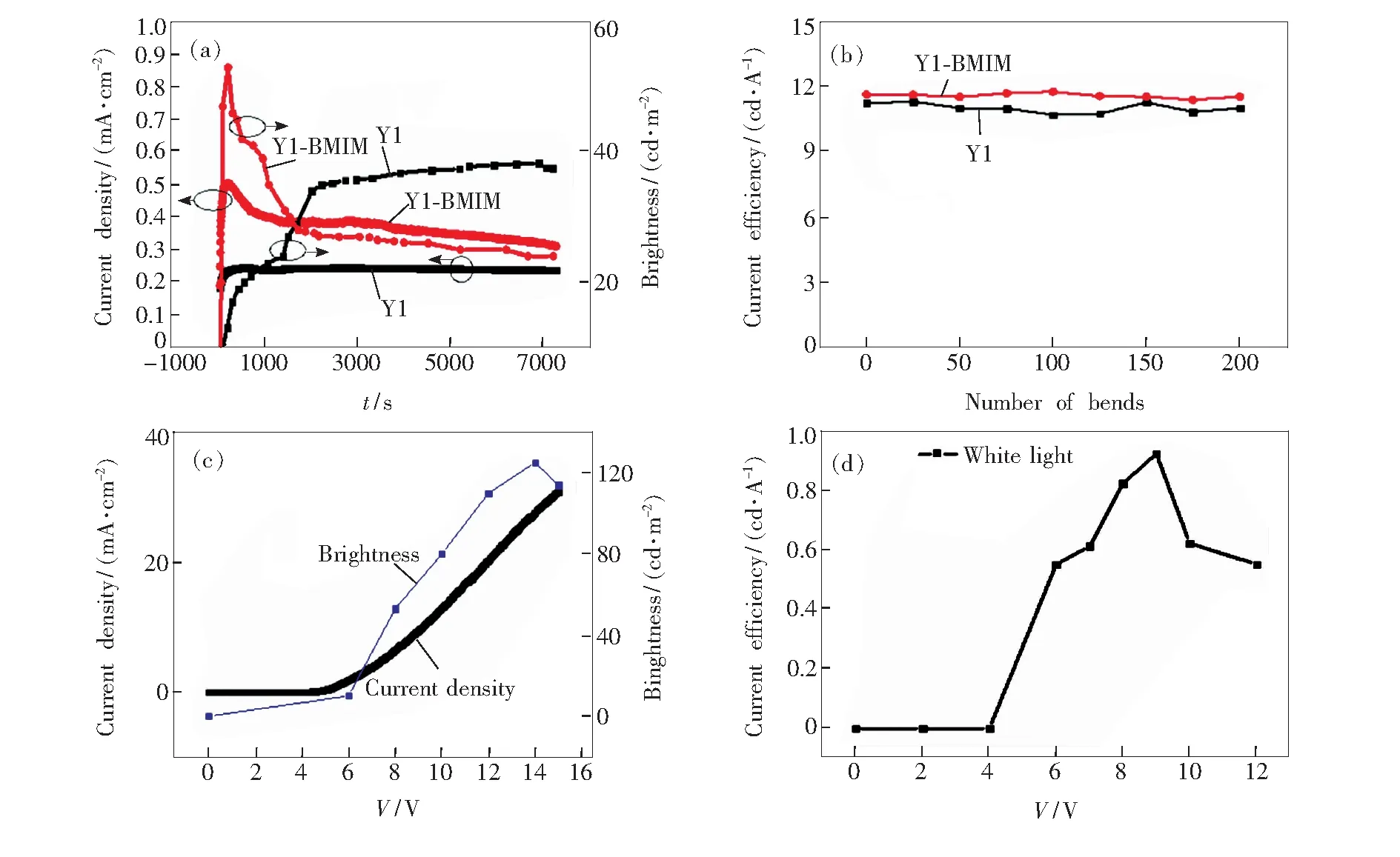
图3(a)6 V电压下,有无添加离子液体的黄光LECs器件的电流密度和亮度随时间的变化曲线;(b)有无添加离子液体的黄光LECs在10 mm曲率半径下弯曲测试时的电流效率变化曲线;(c)白光LECs器件的电流密度曲线和最大亮度曲线;(d)白光LECs器件的电流效率曲线。
Fig.3(a) Time-dependent current-density and brightness curves of the yellow LECs and its device with ion liquid biased at 6 V. (b) Current efficiency behavior of the yellow LECs and its device with ion liquid biased at 6 V, as a function of the bending repetitions at 10 mm curvature radius. (c) Current density and peak brightness as a function of bias voltage for white LECs. (d) Current efficiency as a function of bias voltage for white LECs.
4 结 论
研究了基于铱离子配合物的柔性黄光发光电化学池。器件在6 V时的最高效率为11.6 cd/A,CIE (Commission International de L’Eclairage) 坐标为(0.51,0.47)。器件在曲率半径为10 mm的弯曲性测试中显示出了良好的发光稳定性。将该铱离子配合物与聚芴材料混合,制备了白色发光电化学池,它在10 V时的最高效率为0.91 cd/A,CIE坐标为(0.31,0.33)。这些结果都表明,发光电化学池在下一代照明应用上具有良好的前景。
[1] PEI Q, YU G, ZHANG C,etal.. Polymer light-emitting electrochemical cells [J].Science, 1995, 269(5227):1086-1088.
[2] YANG Y, PEI Q. Voltage controlled two color light-emitting electrochemical cells [J].Appl.Phys.Lett., 1996, 68(19):2708-2710.
[3] SLINKER J, BERNARDS D, HOUSTON P L,etal.. Solid-state electroluminescent devices based on transition metal complexes [J].Chem.Commun., 2003(19):2392-2399.
[4] SLINKER J D, RIVNAY J, MOSKOWITZ J S,etal.. Electroluminescent devices from ionic transition metal complexes [J].J.Mater.Chem., 2007, 17(29):2976-2988.
[5] HU T, HE L, DUAN L,etal.. Solid-state light-emitting electrochemical cells based on ionic iridium (Ⅲ) complexes [J].J.Mater.Chem., 2012, 22(10):4206-4215.
[6] MATYBA P, YAMAGUCHI H, CHHOWALLA M,etal.. Flexible and metal-free light-emitting electrochemical cells based on graphene and PEDOT-PSS as the electrode materials [J].AcsNano, 2010, 5(1):574-580.
[7] YANG Y, PEI Q. Efficient blue-green and white light-emitting electrochemical cells based on poly [9, 9-bis (3, 6-dioxaheptyl)-fluorene-2, 7-diyl] [J].J.Appl.Phys., 1997, 81(7):3294-3298.
[8] SLINKER J D, GORODETSKY A A, LOWRY M S,etal.. Efficient yellow electroluminescence from a single layer of a cyclometalated iridium complex [J].J.Am.Chem.Soc., 2004, 126(9):2763-2767.
[9] LOWRY M S, BERNHARD S. Synthetically tailored excited states: phosphorescent, cyclometalated iridium (Ⅲ) complexes and their applications [J].Chem.—AEur.J. , 2006, 12(31):7970-7977.
[10] HE L, DUAN L, QIAO J,etal.. Highly efficient blue-green and white light-emitting electrochemical cells based on a cationic Iridium complex with a bulky side group [J].Chem.Mater., 2010, 22(11):3535-3542.
[11] SU H C, CHEN H F, FANG F C,etal.. Solid-state white light-emitting electrochemical cells using iridium-based cationic transition metal complexes [J].J.Am.Chem.Soc., 2008, 130(11):3413-3419.
[12] SU H C, CHEN H F, SHEN Y C,etal.. Highly efficient double-doped solid-state white light-emitting electrochemical cells [J].J.Mater.Chem., 2011, 21(26):9653-9660.
[13] AKATSUKA T, ROLDN-CARMONA C, ORTE,etal.. Dynamically doped white light emitting tandem devices [J].Adv.Mater., 2014, 26(5):770-774.
[14] SU H C, CHENG C Y. Recent advances in solid-state white light-emitting electrochemical cells [J].IsraelJ.Chem., 2014, 54(7):855-866.
[15] TAMAYO A B, GARON S, SAJOTO T,etal.. Cationic bis-cyclometalated iridium (Ⅲ) diimine complexes and their use in efficient blue, green, and red electroluminescent devices [J].Inorg.Chem., 2005, 44(24):8723-8732.
[16] HE L, QIAO J, DUAN L,etal.. Toward highly efficient solid-state white light-emitting electrochemical cells: blue-green to red emitting cationic iridium complexes with imidazole-type ancillary ligands [J].Adv.Funct.Mater., 2009, 19(18):2950-2960.
[17] PARKER S T, SLINKER J D, LOWRY M S,etal.. Improved turn-on times of iridium electroluminescent devices by use of ionic liquids [J].Chem.Mater., 2005, 17(12):3187-3190.
[18] WANG Y M, TENG F, HOU Y B,etal.. Copper (Ⅰ) complex employed in organic light-emitting electrochemical cells: device and spectra shift [J].Appl.Phys.Lett., 2005, 87(23):233512.
[19] BOLINK H J, CAPPELLI L, CHEYLAN S,etal.. Origin of the large spectral shift in electroluminescence in a blue light emitting cationic iridium (Ⅲ) complex [J].J.Mater.Chem., 2007, 17(48):5032-5041.
[20] COSTA R D, ORTIE, BOLINK H J,etal.. Intramolecular π-stacking in a phenylpyrazole-based iridium complex and its use in light-emitting electrochemical cells [J].J.Am.Chem.Soc., 2010, 132(17):5978-5980.
[21] HABRARD F, OUISSE T, STEPHAN O,etal.. Conjugated polymer/molten salt blends: the relationship between morphology and electrical aging [J].J.Appl.Phys., 2004, 96(12):7219-7224.
[22] KOSILKIN I V, MARTENS M S, MURPHY M P,etal.. Polymerizable ionic liquids for fixed-junction polymer light-emitting electrochemical cells [J].Chem.Mater., 2010, 22(17):4838-4840.
[23] NORELL BADER A J, ILKEVICH A A, KOSILKIN I V,etal.. Precise color tuning via hybrid light-emitting electrochemical cells [J].NanoLett., 2010, 11(2):461-465.
[24] LEE C, KIM J J. Enhanced light out-coupling of OLEDs with low haze by inserting randomly dispersed nanopillar arrays formed by lateral phase separation of polymer blends [J].Small, 2013, 9(22):3858-3863.
[25] SHAO Y, BAZAN G C, HEEGER A J. Long-lifetime polymer light-emitting electrochemical cells [J].Adv.Mater., 2007, 19(3):365-370.

吴家祺(1990-),男,福建漳州人,硕士研究生,2013年于集美大学获得学士学位,主要从事有机电致发光器件的研究。

E-mail: wjq2009536020@126.com李福山(1978-),男,福建莆田人,博士,研究员,2005年于北京大学获得博士学位,主要从事纳米电子材料与器件的研究。
E-mail: fushanli@hotmail.com
基金项目: “863”国家高技术研究发展计划(2014AA032606); 国家自然科学基金(61376090)资助项目
文章编号: 1000-7032(2016)05-0578-05
Abstract: This essay has reported the fabrication of a metal-oxide-semiconductor AlGaN/GaN high electron mobility transistor (MOS-HEMT) with an Al2O3insulator layer which was deposited by atomic layer deposition (ALD) as the gate dielectric. The MOS-HEMT with a gate-drain distance of 10 μm exhibits a drive current density of 680 mA/mm at a gate-source bias (Vgs) of +3 V and a specific on-resistance of 1.47 mΩ·cm2. Under a negative gate bias of -20 V, the gate leakage current of the MOS HEMT is over four orders of magnitude, which is lower than that of the Schottky-gate HEMT. The off-state breakdown voltage is 640 V at drain leakage current of 27 μA/mm withVgs=-14 V. The Schottky-gate HEMT leakage current is 191 μA at the gate bias of +2 V and the MOS HEMT leakage current is as low as 23.6 nA at the gate bias of +20 V, which is approximately seven orders of magnitude lower than that of the Schottky-gate HEMT with similar gate dimensions. The on/off drain-current ratio (Ion/Ioff) is over 109for the MOS-HEMT.
Key words: AlGaN/GaN; Al2O3; high breakdown voltage; MOS-HEMT
White Light-emitting Electrochemical Cells Based on Polyfluorene and Cationic Iridium Complexes
WU Jia-qi, LI Fu-shan*, NIE Chen, ZENG Qun-ying, GUO Tai-liang
((InstituteofOptoelectronicDisplay,CollegeofPhysicsandInformationEngineering,FuzhouUniversity,Fuzhou350002,China)
*CorrespondingAuthor,E-mail:fushanli@hotmail.com
Light-emitting electrochemical cells (LECs) have a crucial benefit in low cost fabrication processes in flexible and large area illumination panels, but the white light emission remains to be a problem. The fabrication of flexible yellow LECs based on a cationic iridium complex was reported in this paper, which showed yellow electroluminescence with high current efficiency of 11.6 cd/A at 6 V. White light-emitting electrochemical cells were fabricated by blending material mixing polyfluorene with cationic iridium complexes. The cells show white electroluminescence with CIE coordinates of (0.31, 0.33), which is close to standard white emission.
light-emitting electrochemical cells; white light; cationic iridium complexes; polyfluorene
Al2O3/AlGaN/GaN MOS-HEMT with High On/Off Drain Current Ratio
ZHAO Yong-bing1,2,3,4, ZHANG Yun1,2,3*, CHENG Zhe1,2,3,HUANG Yu-liang1,2,3,4, ZHANG Lian1,2,3, LIU Zhi-qiang1,2,3,YI Xiao-yan1,2,3, WANG Guo-hong1,2,3, LI Jin-min1,2,3
(1.ResearchandDevelopmentCenterforSolidStateLighting,InstituteofSemiconductors,ChineseAcademyofSciences,Beijing100083,China;2.StateKeyLaboratoryofSolidStateLighting,Beijing100083,China;3.BeijingEngineeringResearchCenterforThe3rdGenerationSemiconductorMaterialsandApplication,Beijing100083,China;4.UniversityofChineseAcademyofSciences,Beijing100049,China)
*CorrespondingAuthor,E-mail:yzhang34@semi.ac.cn
TN3; TN4; TN5 Document code: A
10.3788/fgxb20163705.0578
1000-7032(2016)05-0573-05
2015-11-03;
2016-03-07
国家自然科学基金(61377027); 福建省自然科学基金(2013J01233)资助项目
TN383+.1
ADOI: 10.3788/fgxb20163705.0573
16-01-18; 修订日期: 2016-03-03

