多孔CuCo2S4/石墨烯复合材料的合成及其在超级电容器上的应用
刘立乐,K.P.Annamalai,陶有胜
(1.福州大学 化学学院,福建 福州350108;2.中国科学院海西研究院 中国科学院功能纳米结构设计与组装重点实验室,福建 福州350002)
多孔CuCo2S4/石墨烯复合材料的合成及其在超级电容器上的应用
刘立乐1,2,K.P.Annamalai2,陶有胜2
(1.福州大学 化学学院,福建 福州350108;2.中国科学院海西研究院 中国科学院功能纳米结构设计与组装重点实验室,福建 福州350002)
采用水热法合成了纳米带状多孔CuCo2S4/石墨烯复合材料,并通过扫描电镜、透射电镜、X-射线衍射、77 K氮气吸附等方法对其进行了表征分析。该复合材料具有0.7~1.2 nm 微孔和2~10 nm 介孔,其总孔容为0.1 cm3·g-1。电化学性能研究表明,该材料应用于超级电容器具有良好的电化学储能性能。
石墨烯;金属硫化物;复合材料;微孔;介孔;超级电容器
1 Introduction
Supercapacitors (SCs),a class of energy storage devices,can store a large amount of energy and then release when it is needed by a faradaic and non faradaic electrochemical reactions.They have attracted great attention owing to their fast charging-discharging rates,high power density and long cycle lifespan,high reliability and low cost[1-3].They are promising in the areas of uninterruptible power supplies,hybrid electric vehicles,aerospace,emergency lighting and renewable energies[4-8].Generally,SCs are classified into two types based on the charge storage mechanisms,non-Faradaic double layer capacitors (EDLCs) or reversible Faradaic redox electrochemical capacitors (pseudocapacitors)[9-10].The pseudocapacitors with metal oxides,metal sulfides and conductive polymers as active materials can provide much higher specific capacitance and higher energy density than EDLCs,attracting widespread attentions[11-13].
Transition-metal oxides,hydroxides,and conducting polymers are applied in supercapacitors with their pseudocapacitive properties[14-18].More recently,metal sulfides have attracted extensive attention owing to their high specific capacitance,low cost,safety and environment benignity and high electrochemical activity.They have the very potential applications such as catalysis,sensors,solar energy and batteries[19-28].However,most of metal sulfides posses low electronic conductivity and undergo a large volume change during repetitive cycling.To overcome these problems,one of most effective way is to combine metal sulfides with carbon matrix[29].
In carbon-based composite electrodes,two dimensional (2-D) graphene (GR) is flexible to integrate with metal compounds.GR nanomaterials serve as the conductive support to prevent electroactive nanomaterials from agglomerating.Moreover the notable synergistic effect between the graphene and electroactive nanomaterials can improve the diffusion rate of ions,leading to a high specific capacitance and rate capability[30].Thus,efforts have been devoted to GR-based electrode materials of SCs.
Because of the richer redox reactions and high conductivity of GR based metal sulfide than the mono-metal sulfide,there are numerous research studies concerning the preparation of ternary compounds for supercapacitors[31-33].A combination of the metal sulfides and graphene synergizes the electrochemical properties of the ternary metal sulfide/graphene composite.Therefore,it is of significant importance and challenging to exploit multi-component materials for SCs[34].Several metal sulfides were synthesized by a hydrothermal anion-exchange reaction method[35].However,this anion exchange method is difficult to control the nanostructure because the uncontrollable exchange reaction dislocates the crystal growing significantly.Here we proposed a method to control the nanostructure and synthesized a uniform nano-belt- like nanocomposites.
Among metal sulfides,a few researches reported on the synthesis of copper and cobalt-based sulfide nanomaterials recently.Moosavifard et al.prepared Ni-foam templated nanoneedle array[36].Shen et al.fabricated three dimensional nanocomposite with graphene and CNTs[37].It is necessary to explore their properties for capacitive performance.In this work,we prepared nanoporous CuCo2S4/rGO nanocomposite with a nano-belt structure by a facile hydrothermal method and used the material as supercapacitor electrode.Electrochemical tests showed that the CuCo2S4/rGO electrode had a maximum specific capacitance of 525 F·g-1at 1 A·g-1and a capacitance retention of 58% at 20 A·g-1.A capacitance retention of 83% after 1 000 charge-discharge cycles at 4 A·g-1indicated their excellent cycling stability.The hierarchically nanoporous structure of the CuCo2S4/rGO nanocomposites would be advantageous for such an excellent electrochemical performance.
2 Experimental
2.1Materials and reagents
Co(acetate)·4H2O,Cu(acetate)·H2O,Sodium carbonate (Na2CO3),Na2S·9H2O,potassium hydroxide(KOH),N-methyl-2-pyrrolidone (NMP) and polyvinylidene fluoride (PVDF) were purchased from Aladdin,China.Trimethylamine (TMA) was purchased from Sinopharm Co.,Ltd in analytical grade.All chemcals were used without further purification.Distilled water was used in this work.
2.2Preparation of CuCo2S4/rGO nanocomposite
CuCo2S4/rGO nanocomposites were prepared by a hydrothermal synthesis method as follows.Graphene oxide (GO) was synthesized from graphite powder by the improved Hummers’ method[38,39].3 mg/mL GO was dispersed in 15 mL distilled water by sonication for 3 h to obtain a homogeneous dispersion.5 mM of Cu(acetate)·H2O and 10 mM of Co(acetate)·4H2O were dissolved in 15 mL ethanol.The above metal ion solution was slowly dropped into the GO dispersion under vigorous stirring.Then 68 mM Na2CO3and 1.5 mL TMA were added drop wise into the above solution and stirred for 1 h at room temperature.The reaction mixture was transferred into a Teflon-lined autoclave and heated to 453 K for 12 h.After cooled down to room temperature,the composite precursors were obtained by washing with distilled water and ethanol separately,dried at 313 K and annealed at 573 k for 3 h in air.The annealed composites were dispersed in water at a concentration of 1 mg/mL and Na2S·9H2O water solution was added under stirring.The mixture was then treated at 453 K for 6 h in the autoclave.The product was washed with water,ethanol and dried at 313K to obtain the CuCo2S4/rGO nanocomposite.
2.3Characterization
The field emission scanning electron micrographs (FE-SEM) of the sample was obtained by a JSM-6700 (JEOL) scanning electron microscope,operating at 5 kV.Transmission electron micrographs (TEM) were obtained on a TecnaiF20 (Philips) transmission electron microscope,operating at 100 kV.Before the test,sample was dispersed in ethanol and placed in TEM grid.The powder X-ray diffraction (XRD) patterns were obtained on a M18XHF X-ray automatic diffractometer (MacScience) using a monochromatized X-ray 25 beam from nickel-filtered CuKα(λ=0.154 050 nm) radiation and operated at 50 kV and 250 mA from 10-70 degree at 0.2(°)/min.The nitrogen adsorption isotherms were measured at 77 K using a ASAP2020 (Micromeritics) gas adsorption analyzer.The samples were evacuated at 10-4Pa and 393 K for 2 h before the measurement.
To test the electrochemical performance of the CuCo2S4/rGO nanocomposite,cyclic voltammetry (CV),galvanostatic charge-discharge (GCD) were conducted in 3 M KOH between the potential range of 0.01 to 0.6 V with a conventional three-electrode cell using a CHI 660E electrochemical workstation (Shanghai CH Instruments,China).Active material and polyvinylidene difluoride (PVDF) binder were uniformly mixed in the weight ratio of 80∶10 and made as a slurry using N-methyl 2-pyrrollidone.The slurry was coated on 1 cm2×1 cm2Ni-foam,dried in vacuum oven at 343 K overnight and then pressed under 5 MPa.As-prepared active material electrode,Hg/HgO electrode,and a platinum electrode were used as the working electrode,reference electrode and counter electrode,respectively.
The capacitance of the electrode can be evaluated according to the following equations:
Cs=[(∫IdV)/2(S*ΔV*m)]
Cp= I Δt/m Δv
Where both Csand Cp(F·g-1) are the specific capacitance,∫IdV is the area under the CV curve,I (A) is the current during the discharge process,Δt (s) is the discharge time,ΔV (V) is the potential window and m (g) is the mass of the active materials.S (mV·s-1) is the scan rate.
3 Results and discussion
Morphology architectures of the prepared nanocomposite were studied with FE-SEM and are shown in Fig.1.CuCo2S4was of a nano-belt structure with the relatively uniform widths.This nano-belt-like structure was mainly initiated by the formation of metal ions-TMA complex.The growth of nano-belt was controlled by the carbonate anions,while the final hydrothermal treatment alleviated the crystalline nature of the nanostructure.The magnified SEM (inset in Fig.1a) showed a highly crystalline nano-belt structure.TEM image of CuCo2S4/rGO as shown in Fig.1b further confirmed the crystalline nano-belt structure of the sample.Compared with the vertically-grown nanostructure,our sample of the robust and horizontal nanostructure can provide easy access to a wide range of electrolytes[34].Rough surface and defective edges additionally allowed the excess electrolyte interaction and boosted up the redox reaction at high current densities.

Fig.1 (a) FE-SEM image and (b) TEM image of the CuCo2S4/rGO composite. Insets in (a) and (b) are the high magnification images of dotted line in the corresponding images.
XRD analysis gave the composition and purity of the sample.Fig.2a shows a XRD pattern of the CuCo2S4/rGO.The diffraction peaks located at 16.1°,26.6°,31.3°,37.9°,46.9°,49.9°,54.8°,57.4°,61.9°,64.4°,and 68.6°,can be indexed to the (111),(022),(113),(224),(115),(044),(135),(026),(335),(444) and (511) planes of CuCo2S4,respectively (JCPDS no.42-1450).Weak peak around 25° indicated the presence of graphene in the composites.This result revealed that as-obtained sample was constructed with graphene and CuCo2S4.Absence of other peaks indicated that prepared nanocomposites were highly pure without any impurities.Composition and purities of the nanocomposite were studied by energy dispersive X-ray (EDX) spectroscopy.Fig.2b indicates the presence of copper,cobalt and sulfur.Their weight percentage ratio of 1∶2∶4 further confirmed that present nano-belt structure was CuCo2S4.Presence of carbon and oxygen in the composites proved the existence of graphene in the composite.
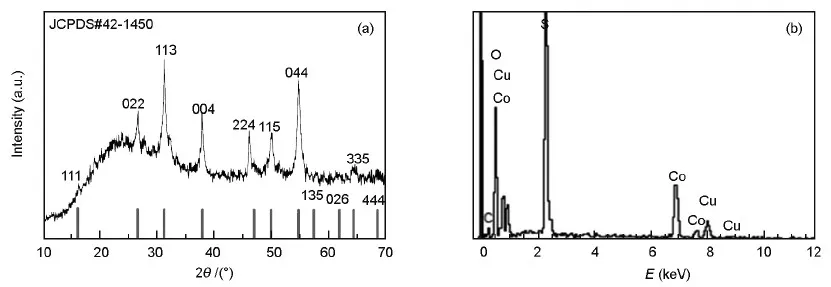
Fig.2 (A) XRD pattern and (B) EDX spectrum of the CuCo2S4/rGO nanocomposite.
Fig.3 shows the N2adsorption isotherms for the CuCo2S4/rGO and GO.Both isotherms had a gradual N2uptake below p/p0= 0.2 and a small hysteresis loop extending from p/p0= 0.45 to p/p0= ~1,suggesting the co-existence of random packed micropores and mesopores in the samples.The micropore and mesopore size distributions (insets in Fig.3) of the CuCo2S4/rGO indicated that it had micropores of 0.7-1.2 nm and mesopores of 2-10 nm.The total pore volume of the CuCo2S4/rGO was 0.1 cm3·g-1,much higher than that of GO.
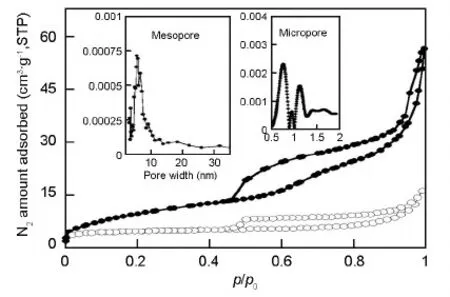
Fig.3 Adsorption/desorption isotherms of nitrogen at 77 K on CuCo2S4/rGO nanocomposite (●) with that of GO (○) included for the comparison.Insets show HK micropore and DH mesopore size distributions of the CuCo2S4/rGO.
The electrochemical properties of CuCo2S4/rGO nanocomposites were investigated by CV and GCD in a three electrode system.Fig.4a shows the CV curves of the CuCo2S4/rGO sample.It was found that all the CV curves had a similar shape and the current increased with the scan rates from 7.5 to 100 mV·s-1in the potential window of 0.01 to 0.6 V.According to these curves,the distinct pairs of redox peaks presented in the CV curves,suggesting the pseudocapacitive characteristics of the CuCo2S4active material.With the increase of sweep rates,the current density increased and the position of anodic and cathodic peaks shifted to high and low potentials,respectively.The composite exhibited a maximum capacitance of 665 F·g-1at 7.5 mV·s-1,as shown in Fig.3b.However,the capacitance gradually decreased with the current density and reached 284 F·g-1at a scan rate of 100 mV·s-1.This capacitance drop is mainly attributed to the incomplete reversible redox reaction within the potential range at fast scan rates.GCD measurements were conducted in 3 M KOH electrolyte in a potential window of 0.01 to 0.5 V.Fig.3c shows the charge-discharge curves with the current densities ranging from 1 to 20 A·g-1.Charge and discharge rates were almost identical in all the current densities,indicating the high columbic efficiency under various current densities.The triangular charge-discharge curves at low current densities showed two obvious platforms,which corresponded to the redox reactions in alkaline medium.At a current density of 1 A·g-1,the electrode delivered a specific capacitance of 525 F·g-1(Fig.3d).The superior performance of the electrode must be benefited from the short diffusion paths of ions in the hierarchically nanoporous nano-belts of CuCo2S4/rGO and the increased electrical conductivity of the CuCo2S4/rGO nano-architecture.It is worth to note that the CuCo2S4/rGO electrode demonstrated a remarkable specific capacitance of 425 and 370 F·g-1even at the current density as high as 5 and 10 A·g-1,respectively.Because with the increasing of current density the mobility of the OH-ions increased and the utilization ratio of the active material was significantly decreased[40],the capacitance gradually decreased at high current densities.
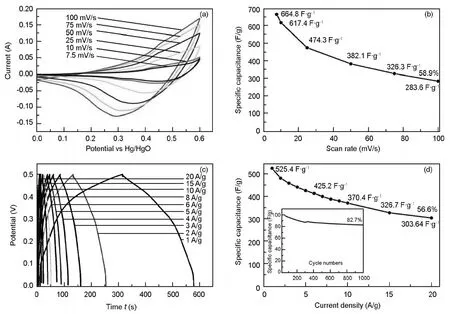
Fig.4 (a) Cyclic voltammetry (CV) measurement at different scan rates; (b) Summary of capacitance vs.scan rate; (c) Galvanostatic charge-discharge (GCD) curves from 1 to 20 A·g-1current densities and (d) Specific capacitance as a function of current density (Inset:cycle stability over 1 000 cycles).
Long cycling life is an important requirement for supercapacitors.The cycling performance of the CuCo2S4/rGO electrode at a current density of 4 A·g-1is shown in Fig.3d.After 1 000 successive cycles,83% capacitance was retained.The sudden capacitance drop around 200 cycle was due to poor wetting nature of the electrode.After that there was gradual decrement in the capacitance mainly because of the dissolution and conversion of metal sulfide to hydroxide under alkaline medium as well as detachment of active materials from the electrode surface.This high performance and excellent cycle stability were ascribed to the presence of conductive graphene support and highly active CuCo2S4of hierarchically nanoporous structure.
4 Conclusions
Hierarchically nanoporous CuCo2S4/rGO composite of a nano-belt structure was prepared with a facile hydrothermal method.The material showed an enhanced capacitance and desirable rate performance (525 F·g-1at 1 A·g-1and 304 F·g-1at 20 A·g-1) and a high specific capacitance retention ( 83%) after 1 000 cycles at 4 A·g-1.Such excellent electrochemical properties makes the CuCo2S4/rGO nanocomposite a promising electrode material for supercapacitors.
[1]Chen Y J,Qu B h,Hu L L,et al.High-performance supercapacitor and lithium-ion battery based on 3D hierarchical NH4F-induced nickel cobaltate nanosheet-nanowire cluster arrays as self-supported electrodes[J].Nanoscale,2013,5(20):9812-9820.
[2]Xia X H,Tu J P,Zhang Y Q,et al.High-quality metal oxide core/shell nanowire arrays on conductive substrates for electrochemical energy storage[J].ACS Nano,2012,6(6):5531-5538.
[3]Shim B S,Chen W,Doty C,et al.Smart electronic yarns and wearable fabrics for human biomonitoring made by carbon nanotube coating with polyelectrolytes[J].Nano Letters,2008,8(12):4151-4157.
[4]Chen L F,Huang Z H,Liang H W,et al.Bacterial-cellulose-derived carbon nanofiber@MnO2and nitrogen-doped carbon nanofiber electrode materials:An asymmetric supercapacitor with high energy and power density[J].Advanced Materials,2013,25(34):4746-4752.
[5]Boukhalfa S,Evanoff K,Y S Gleb.Atomic layer deposition of vanadium oxide on carbon nanotubes for high-power supercapacitor electrodes[J].Energy & Environmental Science,2012,5(5):6872-6879.
[6]Liu D Q,Wang Q,Qiao L,et al.Preparation of nano-networks of MnO2shell/Ni current collector core for high-performance supercapacitor electrodes[J].Journal of Materials Chemistry,2012,22(2):483-487.
[7]Zhang G Q,Lou X W.General solution growth of mesoporous NiCo2O4nanosheets on various conductive substrates as high-performance electrodes for supercapacitors[J].Advanced Materials,2013,25(7):976-979.
[8]Wang S B,Pu J,Tong Y,et al.ZnCo2O4nanowire arrays grown on nickel foam for high-performance pseudocapacitors[J].Journal of Materials Chemistry A,2014,2(48):5434-5440.
[9]Simon P,Gogotsi Y.Materials for electrochemical capacitors[J].Nature Materials,2008,7(11):845-854.
[10]Pendashteh A,Rahmanifar M S,Richard B,et al.Facile synthesis of nanostructured CuCo2O4as a novel electrode material for high-rate supercapacitors[J].Chemistry Communications,2014,50(16):1972-1975.
[11]Zhang Y F,Ma M Z,Yang J,et al.Shape-controlled synthesis of NiCo2S4and their charge storage characteristics in supercapacitors[J].Nanoscale,2014,6(16):9824-9830.
[12]Lei Z B,Christov N,Zhao X S,et al.Intercalation of mesoporous carbon spheres between reduced graphene oxide sheets for preparing high-rate supercapacitor electrodes[J].Energy & Environmental Science,2011,4(5):1866-1873.
[13]Chen Z,Qin Y C,Weng D,et al.Design and synthesis of hierarchical nanowire composites for electrochemical energy storage[J].Advanced Functional Materials,2009,19(21):3420-3426.
[14]Chen H,Zhou S X,Wu L M.Porous nickel hydroxide-manganese dioxide-reduced graphene oxide ternary hybrid spheres as excellent supercapacitor electrode materials[J].ACS Applied Materials & Interfaces,2014,6(11):8621-8630.
[15]Chen H,Hu L F,Yan Y,et al.One-step fabrication of ultrathin porous nickel hydroxide-manganese dioxide hybrid nanosheets for supercapacitor electrodes with excellent capacitive performance[J].Advanced Energy Materials,2013,3(12):1636-1646.
[16]Yuan C Z,Wu H B,Xie Y,et al.Mixed transition-metal oxides:Design,synthesis,and energy-related applications[J].Angewandte Chemie International Edition,2014,53(6):1488-1504.
[17]Chen H,Hu L F,Chen M,et al.Nickel-cobalt layered double hydroxide nanosheets for high-performance supercapacitor electrode materials[J].Advanced Functional Materials,2014,24(7):934-942.
[18]Snook G A,Kao P,Best A S.Conducting-polymer-based supercapacitor devices and electrodes[J].Journal of Power Sources,2011,196(1):1-12.
[19]Thounthong P,Chunkag V,Sethakul P,et al.Energy management of fuel cell/solar cell/supercapacitor hybrid power source[J].Journal of Power Sources,2011,196(1):313-324.
[20]Danks T N,Slade R C T,Varcoe J R.Comparison of PVDF-and FEP-based radiation-grafted alkaline anion-exchange membranes for use in low temperature portable DMFCs[J].Journal of Materials Chemistry,2002,12(12):3371-3373.
[21]Liu H S,Song C J,Zhang L.A review of anode catalysis in the direct methanol fuel cell[J].Journal of Power Sources,2006,155(2):95-110.
[22]Boggs B K,Botte G G.Optimization of Pt-Ir on carbon fiber paper for the electro-oxidation of ammonia in alkaline media[J].Electrochimica Acta,2010,55(19):5287-5293.
[23]Jiang H,Zhao T,Ma J,et al.Ultrafine manganese dioxide nanowire network for high-performance supercapacitors[J].Chemical Communications,2011,47(4):1264-1266.
[24]Reddy A L M,Shaijumon M M,Gowda S R,et al.Coaxial MnO2/Carbon nanotube array electrodes for high-performance lithium batteries[J].Nano Letters,2009,9(3):1002-1006.
[25]Pendashteh A,Rahmanifar M S,Kanerc R B,et al.Facile synthesis of nanostructured CuCo2O4as a novel electrode material for high-rate supercapacitors[J].Chemical Communications,2014,50(16):1972-1975.
[26]Lai C H,Lu M Y,Chen L J.Metal sulfide nanostructures:Synthesis,properties and applications in energy conversion and storage[J].Journal of Materials Chemistry,2012,22(1):19-30.
[27]Xia X H,Zhu C R,Luo J S,et al.Synthesis of free-standing metal sulfide nanoarrays via anion exchange reaction and their electrochemical energy storage application[J].Small,2014,10(4):766-773.
[28]Xiao J W,Wan L,Yang S L,et al.Design hierarchical electrodes with highly conductive NiCo2S4nanotube arrays grown on carbon fiber Paper for High-Performance Pseudocapacitors[J].Nano Letters,2014,14(2):831-838.
[29]Wang H L,Dai H J.Strongly coupled inorganic-nano-carbon hybrid materials for energy storage[J].Chemical Society Reviews,2013,42(24):3088-3113.
[30]Du W M,Wang Z Y,Zhu Z Q,et al.Facile synthesis and superior electrochemical performances of CoNi2S4/graphene nanocomposite suitable for supercapacitor electrodes[J].Journal of Materials Chemistry A,2014,2(25):9613-9619.
[31]Rui X H,Tan H T,Yan Q Y,Nanostructured metal sulfides for energy storage[J].Nanoscale,2014,6(17):9889-9924.
[32]Gao Y,Mi L W,Wei W T,et al.Double metal ions synergistic effect in hierarchical multiple sulfide microflowers for enhanced supercapacitor performance[J].ACS Applied Materials & Interfaces,2015,7 (7):4311-4319.
[33]Zheng X S,Liu J D,Chen L,et al.Metal sulfides/graphene nanocomposites:An overview of preparation and applications[J].Recent Patents on Chemical Engineering,2013,6(3):152-160.
[34]Peng S J,Li L L,Li C C,et al.In situ growth of NiCo2S4nanosheets on graphene for high-performance supercapacitors[J].Chemcial Communications,2013,49(86):10178-10180.
[35]Yu X Y,Yu L,Lou X W.Metal sulfide hollow nanostructures for electrochemical energy storage[J].Advance Energy Material,2015:1501333.
[36]Moosavifard S E,Fanib S,Rahmanianc M.Hierarchical CuCo2S4hollow nanoneedle arrays as novel binder-free electrodes for high-performance asymmetric supercapacitors[J].Chemical Communications,2016,52(24):4517-4520.
[37]Shen J F,Tang J H,Dong P,et al.Construction of three-Dimensional CuCo2S4/CNT/graphene nanocomposite for high performance supercapacitors[J].RSC Advances,2016,6(16):13456-13460.
[38]Marcano D C,Kosynkin D V,Berlin J M,et al.Improved synthesis of graphene oxide[J].ACS Nano,2010,40(8):4806-4814.
[39]Annamalai K P,Gao J P,Liu L L,et al.Nanoporous graphene/single wall carbon nanohorn heterostructures with enhanced capacitance[J].Journal of Materials Chemistry A,2015,3(22):11740-11744.
[40]M.Toupin,Brousse T,Belanger D.Influence of microstucture on the charge storage properties of chemically synthesized manganese dioxide[J].Chemistry of Materials,2002,14:3946-3952.
A hierarchically porous CuCo2S4/graphene composite as an electrode material for supercapacitors
LIU Li-le1,2,K.P.Annamalai2,TAO You-sheng2
(1.College of Chemistry,Fuzhou University,Fuzhou350108,China;2.CAS Key Laboratory of Design and Assembly of Functional Nanostructures,Haixi Institutes,Chinese Academy of Sciences (CAS),Fuzhou350002,China)
A CuCo2S4/graphene composite was synthesized using a simple hydrothermal method.The sample was characterized by field emission scanning electron microscopy,transmission electron microscopy,X-ray diffraction,nitrogen adsorption and electrochemical tests.The composite had a hierarchical porous structure with micropores of 0.7-1.2 nm,mesopores of 2-10 nm and a total pore volume of 0.1 cm3·g-1,and the CuCo2S4had a nano-belt structure.As the electrode of a supercapacitor the composite showed a high specific capacitance of 665 F/g at 7.5 mV/s,and excellent rate capability and cycling stability.
Graphene; Metal sulfide; Composite; Micropore; Mesopore; Supercapacitor
date:2016-05-08;Revised date:2016-06-12
National Natural Science Foundation of China (21273236); Science and Technology Planning Projects of Fujian Province of China (2014H2008,2015I0008); “100 Talents” Program of Chinese Academy of Sciences; “100 Talents” Program of Fujian Province,China.
TAO You-sheng,Ph.D,Professor.E-mail:taoys@tom.com,taoyoushengjp@yahoo.co.jp
s introduction:LIU Li-le,Master Student,E-mail:liull342921@163.com;
1007-8827(2016)03-0336-07
TB333
A
国家自然科学基金(21273236);福建省科技计划(2014H2008,2015I0008);中国科学院“百人计划”;福建省“百人计划”.
陶有胜,教授.E-mail:taoys@tom.com,taoyoushengjp@yahoo.co.jp
刘立乐,硕士研究生.E-mail:liull342921@163.com; K.P.Annamalai,博士后.E-mail:kpannamalai@outlook.com
K.P.Annamalai,Post-doctorial Researcher,E-mail:kpannamalai@outlook.com
English edition available online ScienceDirect (http:www.sciencedirect.comsciencejournal18725805 ).
10.1016/S1872-5805(16)60017-3
——李振声

