聚苯胺复合石墨烯纳米卷的制备及其在超级电容器中的应用
郑冰娜,高 超
(浙江大学 高分子科学与工程学系,高分子合成与功能构造教育部重点实验室,浙江 杭州310027)
聚苯胺复合石墨烯纳米卷的制备及其在超级电容器中的应用
郑冰娜,高超
(浙江大学 高分子科学与工程学系,高分子合成与功能构造教育部重点实验室,浙江 杭州310027)
石墨烯纳米卷是一种具有开放式螺旋状纳米卷结构的管状石墨烯。以石墨烯纳米卷为模板,利用原位聚合的方法,将聚苯胺生长在石墨烯纳米卷表面。通过对材料形貌进行表征,发现聚苯胺均匀地分布在石墨烯纳米卷表面。分别对3种不同单体浓度的聚苯胺复合石墨烯纳米卷进行电化学性能考察,结果发现石墨烯纳米卷和聚苯胺产生的协同效应使得复合卷在继承石墨烯纳米卷良好的倍率特性同时显著地提升了比电容,在1 A/g时比电容可达320 F/g,100 A/g时仍可以保持92.1%的初始电容,为制备高比容、快速充放电的石墨烯纳米卷基超级电容器奠定了基础。
石墨烯纳米卷;聚苯胺;超级电容器
1 Introduction
Graphene nanoscrolls (GNSs) are new topological structures of graphene materials with the unique one-dimensional tubular morphology and open inner cavity[1-5].The specialty of the structure provides GNSs with a good ion-transfer capability and high electrochemical performances[5-9].Electric double-layer capacitances (EDLC) of GNS-based supercapacitors are usually limited in the range of 100-200 F/g due to the intrinsic nature of carbon.Incorporating materials that have pseudo-capacitances like metal oxide and conducting polymer is an efficient way to improve specific capacitance of carbon-based supercapacitors.Attempts that combining metal oxide such as MnO2and Co3O4have already been made[8,10].Mai’s group reported the nanowire templated semi-hollow bicontinuous GNSs and the MnO2templated GNSs exhibited 317 F/g at 1 A/g[10].Unfortunately,the electrochemical performance of other aspects was not satisfying and conducting polymer hybrid GNSs have never been reported.Moreover,the rate capability of GNS-based composite materials has scarcely been reported[9].
Polyaniline (PANI) is a famous conducting polymer that has been extensively studied and used in electrochemical devices.The facile synthesis,good environmental stability,electroactivity,simple doping/dedoping chemistry and light weight make PANI an ideal material for energy conversion/storage applications[11-14].In this paper,we report a novel method for preparing PANI coated GNS (PANI@GNS) by in-situ polymerization.Mat-like PANI@GNSs was produced via filtration of the mixture.By adjusting the feed radios,three kinds of PANI@GNSs were obtained.The electrochemical performance of GNS and PANI@GNSs samples were systematically tested and compared.The synergistic effect of PANI and GNS endows PANI@GNS2 much higher specific capacitance (320 F/g at 1 A/g) and excellent rate capability (92.1% retention rate at 100 A/g).This method can be applied to other conducting polymers to improve the performance of GNS-modified conducting polymers for use in high performance supercapacitors.
2 Experimental
2.1Materials
Giant graphene oxide (GGO,Average sheet size:~71 μm; Thickness:0.8-1.0 nm; Dispersion ratio:0.45) was purchased from C6G6 (www.c6g6.com).85% N2H4·H2O solution,ethanol,ammonium persulfate,sulfuric acid and perchloric acid were purchased from Sinopharm Chemical Reagent Co.,Ltd and used as received.Aniline (>98%) was purchased from Shanghai Wulian Chemicals and used as received.
2.2Preparation of GNSs
N2H4·H2O solution was added dropwise into GGO dispersions (0.2 mg/mL) and the weight ratio of 85% N2H4·H2O solution to GGO was about 15∶1.After 5 min magnetic stirring,the above GGO dispersions were transformed into chemical reduced graphene (CRG) at 60 ℃ for 30 min with stirring in an oil bath.CRG dispersions were sprayed into liquid nitrogen bath and freeze dried in a lyophilizer with an optimized temperature profile.The as received samples were kept in a sealed container with N2H4at 90 ℃ for 12 h to obtain GNSs.
2.3Preparation of PANI@GNS
Different amounts of aniline were dissolved in 10 mL ethanol/H2O mixed solutions and added to GNS/ethanol suspension (10 mg GNS was immersed in 3 mL ethanol),resulting in GNS/aniline mixture solutions.After magnetic stirring for 1 h at room temperature,the solutions were transferred to ice-water bath.Ammonium persulfate was dissolved in 1 M perchloric acid and added dropwise to the GNS/aniline mixture solutions (mass ratio of aniline to ammonium persulfate is 1∶1.5).The polymerization lasts for 18 h at -10 ℃.The PANI@GNS samples were separated from the suspensions by vacuum filtration and repeatedly washed by 1 M perchloric acid to get rid of excess monomers.1 M H2SO4was used to displace the perchloric acid for the following electrochemical tests.According to the mass ratios of GNS/aniline (1∶1.8,1∶3.6 and 1∶7.7),PANI@GNSs samples were named as PANI@GNS1,PANI@GNS2 and PANI@GNS3,respectively.
2.4Assembly of supercapacitor
Two pieces of testing samples were sealed into a detachable stainless steel cell (custom-made from Institute of Physics,CAS) as two electrodes with a mixed cellulose acetate ester membrane as a separator (Yaxing Purification Materials Factory,Φ=0.45 μm) and 1 M H2SO4as an electrolyte.
2.5Characterization
SEM images were taken on a Hitachi S4800 field-emission SEM system.TGA was conducted on a PerkinElmer Pris 1 system in nitrogen flow at a heating rate of 10 ℃/min.Electrochemical impedance spectroscopy (EIS),cyclic voltammetry (CV) and galvanostatic charge-discharge (GCD) measurements were performed using an electrochemical workstation (CHI 660e,CH Instruments,Inc.).All electrochemical tests of supercapacitors are based on two-electrode system.
3 Results and discussion
The composition and structure of GNS and PANI@GNSs were studied by thermogravimetric analysis (TGA) as shown in Fig.1.The peaks of GNS are attributed to the removal of absorbed water (~200 ℃),removal of residual oxygenate groups (~350 ℃) and further carbon sketch loss (~600 ℃)[15,16].The total weight loss of GNS at 800 ℃ is 28%.For PANI@GNS samples,the peaks at around 300-350 ℃ attributed to the removal of residual oxygenate groups.The stages at around 500 ℃ correspond to the carbonization of PANI.The final peaks at 600 ℃ indicate the subsequent loss of carbon sketches.With the increase of monomer amount,the final weight losses of PANI@GNS1 to PANI@GNS3 are 32%,36% and 44%,respectively.
Fig.2a is the characteristic morphology of GNS without compression.The length of one GNS is tens of micron and the diameter is about 100-500 nm.There are many entanglements and junctions between GNSs,which result in the aerogel at macro scale.Due to the weak interactions,compression and solvent infiltration will lead to the transformation from 3D aerogel to 2D film.The 1D GNS can be clearly observed on the surface of the film (Fig.2b),which is like a GNS mat.At the break cross-section,GNSs were pulled out from the film (Fig.2c).
After polymerization,the surface of PANI@GNS1 film (Fig.2d) shows the similar mat-like morphology as GNS film.As a matter of long time stirring,reaction and filtration,GNSs are swollen in solvents and a small part of scrolls is unfold to sheet morphology,which is inevitable.The nano-cones of PANI are evenly distributed on the surface of GNSs as observed in a high magnification.PANI nanoparticles are successfully grown onto GNSs (Fig.2e,f).
With the increase of monomer amounts,PANI clusters are gradually formed.As shown in Fig.3,although PANI distributes uniformly on GNSs,PANI custers exist in both PANI@GNS2 and PANI@GNS3.
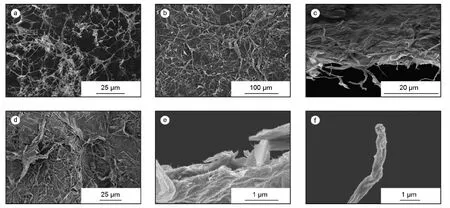
Fig.2 Morphologies of (a) GNS foam,(b) surface of GNS film and (c) cross-section of GNS film; (d) surface morphology of PANI@GNS1 film,(e) cross-section of PANI@GNS1 and (f) single fiber of PANI@GNS1 pulled from PANI@GNS1 film.
The capacitive performance of symmetric supercapacitors composed of GNSs and PANI@GNSs were evaluated by using cycle voltammetry (CV) and galvanostatic charge/discharge tests.Fig.4a shows the CV curves of GNSs and PANI@GNS samples at a scan rate of 10 mV/s.The curve of GNSs is rectangular shape,showing the EDLC property.The integral areas of PANI@GNSs are all larger than that of GNSs due to the contribution of pseudo-capacitance.The two pairs of redox peaks are attributed to the redox of PANI,corresponding to the leucoemeraldine/emeraldine and emeraldine/pernigraniline structural conversions.The specific capacitances of GNSs,PANI@GNS1,PANI@GNS2 and PANI@GNS3 are 91,301,347,352 F/g,respectively.
Fig.4b demonstrates the galvanostatic charge/discharge curves of the supercapacitors tested at a current density of 1 A/g.GNSs exhibit a triangular-shape curve,implying EDLC is the main contribution to capacitance.The discharging curve of PANI@GNSs show two voltage ranges.The range of 0.8-0.45 V relates to the EDLC and the range of 0.45-0 V is ascribed to a combination of EDLC and faradic capacitance of PANI.The specific capacitances of GNSs,PANI@GNS1,PANI@GNS2 and PANI@GNS3 are 87,253,320,326 F/g,respectively.
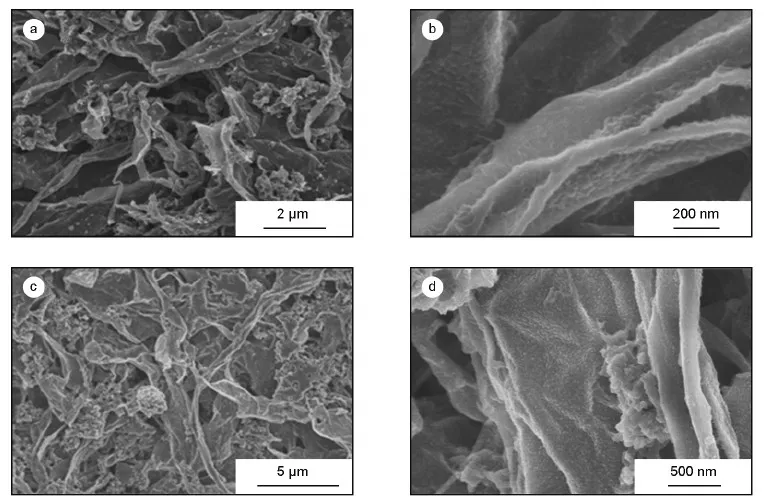
Fig.3 Morphologies of (a,b) PANI@GNS2 and (c,d) PANI@GNS3.

Fig.4 (a) CV curves of GNS and PANI@GNS samples at 10 mV/s and (b) galvanostatic charge/discharge curves of GNS and PANI@GNS samples at 1 A/g.
According to the tests,electrochemical performance of PANI@GNSs is all superior to that of pure GNSs.Moreover,the specific capacitance increase with the monomer amounts.Nevertheless,the rate capabilities of GNSs and PANI@GNSs show different tendencies.As mentioned by our former report,GNSs have a good rate capability as a matter of the special 1D spiral open structure.GNSs have reversible swelling behavior in solvents and electrolytes,which is beneficial to the fast transformation of ions.From 10 mV/s to 2 000 mV/s,GNSs show a 56% capacitance retention rate (Fig.5a).And a 84% retention rate is observed by galvanostatic charge/discharge from 1 to 100 A/g (Fig.5b) for PANI@GNSs.After the decoration of PANI,the flexibility and interaction of graphene sheets are changed.PANI@GNS1 and PANI@GNS2 inherit the good rate property of GNSs,showing similar capacitance retention rates with GNSs in both CV and galvanostatic charge/discharge tests (Fig.5a,b).However,a further increase of PANI in PANI@GNS3 hinders the ion transporting routes and deteriorates the rate capability.
Normalized performances in CV tests of PANI@GNS2 are shown in Fig.5c.From 10 mV/s to 1 000 mV/s,the redox peaks become weak due to the hysteresis of redox reaction of PANI.However,the deterioration of integral area is little.Same tendencies are also observed in galvanostatic charge/discharge test (Fig.5d).The excellent rate capability of PANI@GNS2 is also superior to that of other literature (Fig.5e)[17-21].
Electrochemical impedance spectroscopy was conducted from 0.01 Hz to 100 kHz and the Nyquist plots of all samples are shown in Fig.5f.The Nyquist plot of GNSs is more vertical to the x axis than others,implying the ideal EDLC behavior.Compared with PANI@GNS1 and PANI@GNS2,the excess PANI in PANI@GNS3 hinders the effective entanglements of GNS,which lowers its electrical conductivity and leads to an increased equivalent series resistance.At the high frequency range,the semi-circle of PANI@GNS2 is smaller than others,proving the small charge-transport resistance.

Fig.5 Electrovchemical performance comparisons of GNS and PANI@GNSs tested by (a) CV method and (b) galvanostatic charge/discharge method; (c) CV curves of PANI@GNS2 at different scan rates; (d) galvanostatic charge/discharge curves of PANI@GNS2 at different current densities; (e) Comparions of PANI@GNS2 with data obtained from other literatures; (f) electrochemical impedance spectroscopy comparisons of GNS and PANI@GNSs.
4 Conclusions
Polyaniline has been successfully grown onto GNSs by in-situ polymerization.The nano-cone like PANI is evenly distributed on the surface of GNSs and the as-prepared PANI@GNS films seem like mats constructed by interconnected scrolls.PANI@GNS2 inherits the excellent rate capability of GNSs (92.1% retention rate from 1 A/g to 100 A/g) and has an obvious elevated specific capacitance (320 F/g at 1 A/g) compared with GNSs.This method can be applied to other conducting polymers and paves the way of high performance GNS-modified conducting polymer supercapacitors.
[1]Viculis L M,Mack J J,Kaner R B.A chemical route to carbon nanoscrolls[J].Science,2003,299:1361.
[2]Quintana M,Gizelcazk M,Spyrou K,et al.A Simple road for the transformation of few-layer graphene into MWNTs[J].J Am Chem Soc,2012,134:13310-13315.
[3]Xie X,Ju L,Feng X,et al.Controlled fabrication of high-quality carbon nanoscrolls from monolayer graphene[J].Nano Lett,2009,9:2565-2570.
[4]Schaper A K,Wang M S,Xu Z,et al.Comparative studies on the electrical and mechanical behavior of catalytically grown multiwalled carbon nanotubes and scrolled graphene[J].Nano Lett,2011,11:3295-3300.
[5]Zeng F,Kuang Y,Wang Y,et al.Facile preparation of high-quality graphene scrolls from graphite oxide by a microexplosion method[J].Adv Mater,2011,23:4929-4932.
[6]Zeng F,Kuang Y,Liu G,et al.Supercapacitors based on high-quality graphene scrolls[J].Nanoscale,2012,4:3997-4001.
[7]Fan T,Zeng W,Niu Q,et al.Fabrication of high-quality graphene oxide nanoscrolls and application in supercapacitor[J].Nanoscale Res Lett,2015,10:192.
[8]Zhou W,Liu J,Chen T,et al.Fabrication of Co3O4-reduced graphene oxide scrolls for high-performance supercapacitor electrodes[J].Phys Chem Chem Phys,2011,13:14462-14465.
[9]Zheng B,Xu Z,Gao C.Massive production of graphene nanoscrolls and their assist for high rate performance supercapacitors[J].Nanoscale,2016,8:1413.
[10]Yan M,Wang F,Han C,et al.Nanowire templated semihollow bicontinuous graphene scrolls:designed construction,mechanism,and enhanced energy storage performance[J].J Am Chem Soc,2013,135:8176-18182.
[11]Li D,Huang J X,Kaner R B.Polyaniline nanofibers:A unique polymer nanostructure for versatile applications[J].Acc Chem Res,2009,42:135-145.
[12]Bai H,Shi G Q.Gas sensors based on conducting polymers[J].Sensors,2007,7:267-307
[13]Kang E T,Neoh K G,Tan K L.Polyaniline:A polymer with many Interesting intrinsic redox states[J].Prog Polym Sci,1998,23:277-324.
[14]Ryu K S,Kim K M,Park N G,et al.Symmetric redox supercapacitor with conducting polyaniline electrodes[J].J Power Sources,2002,103:305-309.
[15]McAllister M J,Li J L,Adamson D H,et al.Single sheet functionalized graphene by oxidation and thermal expansion of graphite[J].Chem Mater,2007,19:4396- 4404.
[16]Zhang J,Jiang J,Li H,et al.A high-performance asymmetric supercapacitor fabricated with graphene-based electrodes[J].Energy Environ Sci,2011,4:4009-4015.
[17]Xie K,Qin X,Wang X,et al.Carbon nanocages as supercapacitor electrode materials[J].Adv Mater,2012,24:347-352.
[18]Liu F,Song S,Xue D,et al.Folded structured graphene paper for high performance electrode materials[J].Adv Mater,2012,24:1089-1094.
[19]Bo Z,Zhu W,Ma W,et al.Vertically oriented graphene bridging active-layer/current-collector interface for ultrahigh rate supercapacitors[J].Adv Mater,2013,25:5799-5806.
[20]Huang T,Zheng B,Liu Z,et al.High rate capability supercapacitors assembled from wet-spun graphene films with a CaCO3template[J].J Mater Chem A,2015,3:1890-1895.
[21]Yoon Y,Lee K,Baik C,et al.Anti-solvent derived non-stacked reduced graphene oxide for high performance supercapacitors[J].Adv Mater,2013,25:4437-4444.
Preparation of graphene nanoscroll/polyaniline composites and their use in high performance supercapacitors
ZHENG Bing-na,GAO Chao
(MOE Key Laboratory of Macromolecular Synthesis and Functionalization,Department of Polymer Science and Engineering,Zhejiang University,Hangzhou310027,China)
The graphene nanoscroll is a kind of tubular graphene with an open end and a helical nanostructure.Different amounts of polyaniline were formed on the surface of the nanoscroll by the in-situ polymerization of aniline.SEM observation shows thatthe polyaniline nanoparticles are evenly distributed on the nanoscrolls and the number of free polyaniline clusters increases with the amount of monomer used.The electrochemical performance of three composites with different polyaniline/graphene nanoscroll ratios was evaluated.The best specific capacitance of the composites reaches 320 F/g at 1 A/g and a 92.1% retention capacitance rate is obtained at 100 A/g,indicating that the composite has a rate performance as good as graphene nanoscrolls and a higher specific capacitance.
Graphene nanoscrolls; Polyaniline; Supercapacitors
date:2016-05-12;Revise date:2016-06-10
National Natural Science Foundation of China (21325417,51533008).
GAO Chao,Professor.E-mail:cgao18@163.com
introduction:ZHENG Bing-na,Ph.D.E-mail:zhengbn87@163.com
1007-8827(2016)03-0315-06
TQ127.1+1
A
国家自然科学基金(21325417,51533008).
高超,教授,E-mail:cgao18@163.com
郑冰娜,博士,E-mail:zhengbn87@163.com
English edition available online ScienceDirect ( http:www.sciencedirect.comsciencejournal18725805 ).
10.1016/S1872-5805(16)60015-X

