不同长度的棒状有序介孔炭的双电层电容性能
刘 娜,余吕强,陈晓红,廖丽芳,周继升,马兆昆,宋怀河
(北京化工大学 化工资源有效利用国家重点实验室,材料电化学过程与技术北京市重点实验室,北京100029)
不同长度的棒状有序介孔炭的双电层电容性能
刘娜,余吕强,陈晓红,廖丽芳,周继升,马兆昆,宋怀河
(北京化工大学 化工资源有效利用国家重点实验室,材料电化学过程与技术北京市重点实验室,北京100029)
将三嵌段共聚物P123既充当结构导向剂又作为碳源,通过硫酸处理,并采用直接炭化硅/ P123复合材料的方法制备出棒状有序介孔炭,避免了传统硬模板法中需要除去昂贵的表面活性剂与反复浸渍的过程。通过改变合成参数,制备出不同长度的、从一微米到几十微米变化的棒状有序介孔炭材料。采用SEM,HR-TEM,XRD与N2吸脱附等对有序介孔炭材料的形态、结构以及孔特点进行表征,并将其作为双电层电容器的电极材料进行电化学测试,以期关联形貌、结构(尤其是棒长度)与其电化学性能的关系。结果表明在这些炭材料中,最长的介孔炭具有最高的比容量170 F/g。在2 000 mA/g电流密度下,具有双孔径的介孔炭表现出最高的容量保持率(92%)。
有序介孔炭;棒状;模板;三嵌段共聚物;超级电容器
1 Introduction
Electric double-layer capacitor (EDLC) is a next-generation energy storage device,which can be applied to an auxiliary power supply and space flight technology.The double layer is formed at electrode/electrolyte interface,where electric charges are accumulated on the electrode surfaces[1,2].Compared with conventional capacitors,EDLCs can store much more energy because there is a very small charge separation distance at the interface between electrode and electrolyte and a large amount of charges on electrode of the large surface area[3].On the basis of the double-layer energy-storage mechanism,the key to enhance the specific capacitance is to enlarge the specific surface area[4]and to control the pore size and its distribution of the electrode materials[5].
Since the first report in 1999[6],ordered mesoporous carbons (OMCs) have been widely studied as the electrode materials for EDLC owing to their well-ordered pore channels,high specific surface areas and narrow pore size distributions[7-10].Recently,Tang et al[11]synthesized an OMC through a facile way without any templates and the carbon showed a high specific capacitance (259 F·g-1) and high rate capability (189 F·g-1at 100 A·g-1).Jurewicz et al[12]investigated the electrochemical performance of carbon materials with a highly ordered mesoporous structure.The highest capacitance values are obtained for the carbons with the highest total surface area,the highest total pore volume and the most marked microporous character.It is obvious that the presence of interconnected mesopores and micropores makes the active surface more available for charge accumulation on EDLC than in a strictly microporous material.Xing et al[13]presented the EDLC performance of the OMCs with 3-D cubic and 2-D hexagonal mesopore structures.It was found that the 2-D hexagonal OMC exhibited better high-rate capability than the 3-D cubic OMC.This is attributed to the favorable ion transport in mesopores of the 2-D hexagonal OMC.Gao[14]synthesized a 3-D cubic OMC with a high energy density of 6.53 Wh·kg-1at a power density of 5 000 W·kg-1,indicating a promising application for the high performance supercapacitors.Wang et al[15]studied the ion transport behavior in hexagonal OMC rods with diverse mesopore diameters and lengths by evaluating the dynamic process of inner-pore electric double layer formation.They considered that the ion transport behavior was affected by the ratio between mesopore length and diameter,and the behavior can be enhanced by minimizing the aspect ratio of the mesopores.Xiao[16]studied the fiber-like and rod-like OMC performance in EDLC and the latter showed a better property because the short rod-like morphology and the well-defined pore size distribution favor the ions penetration into their pores.Liang et al[17]compared the EDLC performance of the OMC with an interconnected channel structure to the OMC with an unconnected channel structure,and found that the former has better performance than the latter owing to rapid mass transport in the former.
Our group have compared the EDLC behavior of three types of OMCs with different pore characteristics and found that the OMC with a high surface area and appropriate pore size distribution (centered at 3.6 nm) exhibits the lowest resistance and highest specific capacitance[5].In this work,we fabricated four types of OMC rods from the carbonization of silica/triblock copolymer composites.By changing the synthesis parameters,the rod length of the carbons can be controlled from one to tens of micrometers.The electrochemical performance of the capacitor electrodes prepared from the OMC rods have been investigated and are tentatively correlated with their structures and pore characteristics
2 Experimental
2.1Preparation of OMCs
Rod-type OMCs were synthesized using a triblock copolymer P123 as the carbon source and tetraethoxysilicon (TEOS) as the silica source.The P123 (EO20PO70EO20,Mav= 5 800) was purchased from Sigma.All commercial chemicals were used without further purification.The typical experiment[18-20]procedure is shown in the Fig.1.
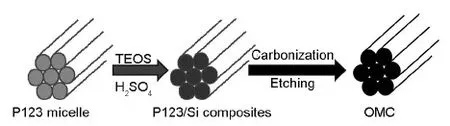
Fig.1 Synthesis of OMCs.
The micelle was formed after the P123 was dissolved in water.The P123/SiO2composite was formed in the presence of the inorganic precursor and sulfuric acid.OMCs were obtained after carbonization of the composites and etching of the SiO2with a HF solution.In a typical run,5.0 g P123 was dissolved in 130 mL distilled water at 38 ℃,then 6.4 mL sulfuric acid (98 wt%) and 9.2 mL TEOS were added to the solution under vigorous stirring.After the stirring for 5 min,the mixture was kept statically at 38 ℃ for 24 h,followed by aging at 100 ℃ for 24 h.The solid product was filtered and dried at 100 ℃ for 6 h and 160 ℃ for 6 h,respectively to get the P123/silica composite.The dark powder was carbonized under N2flow at 850 ℃ for 2 h.The obtained silica/carbon composite was treated by a diluted HF solution to remove the silica to get the OMC.By changing the adding order of sulfuric acid and P123,we obtained two samples,C1 (sulfuric acid added at the same time with P123) and C2 (sulfuric acid added after P123 dissolved).By varying the amount of TEOS with the same adding order as C1 but stop stirring 5 min after the addition of TEOS,two samples were synthesized,C3 (9.2 mL TEOS) and C4 (13.8 mL TEOS) and.
2.2Characterization
The OMCs were characterized by X-ray diffraction (XRD),scanning electron microscopy (SEM),high resolution transmission electron microscopy (HR-TEM) and nitrogen adsorption.XRD patterns were recorded on a Rigaku D/max-2500B2+/PCX system operating at 40 kV and 20 mA using CuKαradiation (λ= 0.154 06 nm).The interplanar spacings of the OMCs are calculated from the Bragg’s equation:λ= 2dhklsinθ.SEM images were obtained using a Zeiss Supra 55 electron microscope operating at 20 kV.Nitrogen adsorption were performed with an ASAP 2020 Micromeritics Instrument at 77 K.The pore size distributions were calculated from the desorption branch of the isotherms using the BJH (Barrett-Joyner-Halenda) method.The specific surface areas were calculated from the adsorption data in the relative pressure interval from 0.04 to 0.2 using the Brunauer-Emmett-Teller (BET) method.The total pore volumes were estimated at a relative pressure of 0.98.HR-TEM images were obtained using a JEOL JEM-2100 electron microscope operating at 200 kV.The samples were prepared by dispersing the products in ethanol with an ultrasonic bath for 20 min and then a few drops of the resulting suspension were spread on a copper grid.
The EDLC electrodes were obtained by pressing a mixture of the OMC (80 wt%),graphite (10 wt%),and polytetrafluoroethylene (10 wt%) to the nickel foam as a current collector.The electrodes had a surface of 100 mm2and thickness of 0.4 mm.A platinum wire and the Hg/HgO electrode were used as the counter and reference electrodes,respectively.The electrolyte was a 30 wt% KOH aqueous solution.The galvanostatic charge/discharge capacitance (C) of the electrode was measured using a Program Testing System (produced by Wuhan LAND Co.Ltd.,China).Charge and discharge were carried out between 0.9 and 0.01 V.The C in Farad was calculated on the basis of the equation:C = (IΔt)/(mΔV)[21],where C is the capacitance,I the constant discharge current,△t the discharge time,m is the mass of active material within the electrode andΔVis the potential range.
The cyclic voltammetry and AC impedance were carried out with a CHI 660B electrochemical working station.For the cyclic voltammetric measurements,the sweep rate ranged from 1 to 10 mV·s-1within a potential range of -0.3 to 0.2 V.For the AC impedance measurements,the potential amplitude of AC was kept as 30 mV and the frequency range was from 10 kHz to 1 Hz.The impedance spectra were fitted to an equivalent circuit model[22]by ZView software.
3 Results and discussion
3.1Pore characteristics of the OMCs
Small-angle XRD patterns of C1,C2,C3 and C4 are shown in Fig.2.As can be seen,the powder XRD patterns of the samples exhibit one intense peak indexed as (100) reflection and two weak peaks indexed as (110) and (200) reflections,associated with a 2-D hexagonal symmetry (p6mm)[23],implying the long range order of the OMCs.The (100) diffraction peaks of the samples are all around 2θ= 1.00°,which indicates that the samples have the similar interplanar spacings (d100) and unit cell parameters (a).The interplanar spacing (d100) and unit cell parameter (a) of C4 are calculated to be 8.83 nm and 10.2 nm,respectively.

Fig.2 XRD patterns of samples (a) C1,(b) C2,(c) C3, (d) C4 and (e) an enlarged XRD pattern of C4 from 2θ= 1.3-2.3°.
Fig.3 shows the SEM images of the samples.From the images we can see that the rod lengths of the OMCs are very sensitive to the synthesis parameters.As can be seen,C1 (Fig.3a) consists of bundles of rods with the length up to tens of micrometers.By changing the adding order of sulfuric acid and TEOS,dramatic change happens with its morphology.Short and bended rods with the length of 1-4 μm can be observed in C2 (Fig.3b).In the case of C3 (Fig.3c),the rods become shorter and more straight than C2,and the rod length is about 2-3 μm.C4 (Fig.3d) shows the shortest rod in the four samples with a length of about 1 μm.The HRTEM images of the samples are shown in Fig.4.The images show well-ordered mesopores with a 2-D hexagonal mesoporous structure in all the four samples.C1,C2 and C3 exhibit a rod-like morphology with the channels paralleling along the long axis.For 2-D hexagonal OMCs,their mesopore lengths correspond with their rod lengths[24,25].Pore lengths of samples are summarized in Table 1.The pore sizes of the samples are estimated to be about 3-4 nm,which is in good agreement with the following nitrogen sorption analysis.

Fig.3 SEM images of samples (a) C1,(b) C2,(c) C3 and (d) C4.

Fig.4 HRTEM images of samples (a) C1,(b) C2,(c) C3 and (d) C4.
The pore structures of the samples were further analyzed using nitrogen sorption.Fig.5 displays the nitrogen adsorption-desorption isotherms and BJH pore size distributions.Whatever the synthesis route was,the isotherms of the OMCs exhibit a typical IV shape,indicating their mesoporous characteristics[26].C3 presents two pore systems with sizes centered at 3.7 and 14.2 nm.The small mesopores may be derived from the removal of template skeleton and the large mesopores originate from the coalescence of pores once the template was removed[27].C1,C2 and C4 contain narrow pore size distributions with pore sizes mainly centered at 3.7,3.6 and 3.6 nm,respectively.The BET surface areas,average pore sizes and pore volumes of samples are summarized in Table 1.
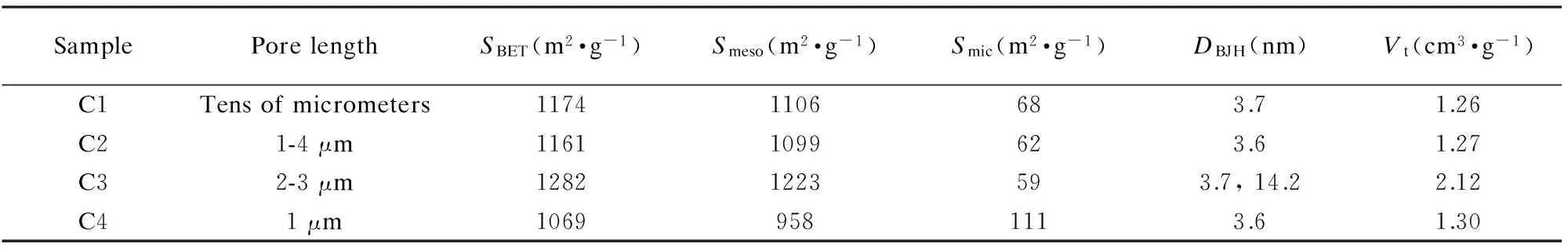
Table 1 Pore parameters of samples calculated from the nitrogen sorption isotherms.
Note:SBET,BET surface area; Smeso,mesopore surface area; Smic,micropore surface area; DBJH,average pore diameter; Vt,total pore volume.
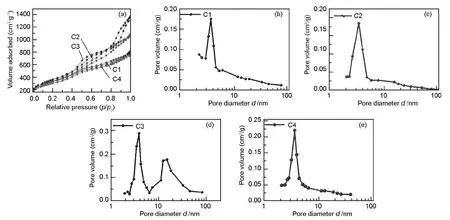
Fig.5 (a) Nitrogen adsorption-desorption isotherms and BJH pore size distributions of samples (b) C1,(c) C2,(d) C3 and (e) C4.
3.2Electrochemical characterization
To investigate the electrochemical performance of the OMCs as electrodes for supercapacitors,galvanostatic charge/discharge cycling measurements were performed.The result for carbon C1 at a current load of 100 mA/g is shown in Fig.6a.A direct apparent feature is that the electrode exhibits an ideal capacitor behavior from the typical triangular-shaped curve.Another important characteristic is the well-retained shape during cycling,reflecting a good reversibility and demonstrating that double layer behavior results from electrostatic attraction without Faradaic reactions.Fig.6b displays the capacitance versus cycle number of the OMCs under a current density of 100 mA/g,and all the electrodes exhibit stable capacitances with little fading after 500 cycles.The good cycle performance of the OMCs implies their stable energy-storage performance during the long cycle charging/discharging.
The specific capacitances of the OMCs at various current densities are shown in Fig.6c.Theoretically,in order to achieve a high capacitance,the electrode material should have a high surface area,since the charge storage ability (expressed as capacitance) is proportional to surface area[28].However,actually,materials may have different porosities and pore structures.Not all the surface area is electrochemically accessible,and there are many factors affecting the double-layer capacitor behavior,such as untramicropore volume[29]and pore size distribution[30].
For the four OMCs,the specific surface areas are in the range of 1 069-1 282 m2/g,the specific capacitances are 140 to 176 F/g at the current density of 100 mA·g-1,correspondingly to the specific surface capacitances in the range of 12-15 μF/cm2,which are higher than the value 7-10 μF/cm2for the general microporous activated carbons[31],implying the mesoporous structure is more accessible for ions.C1 exhibits the highest specific capacitance of 176 F·g-1among the four samples at the current density of 100 mA·g-1,and decreases from 176 to 150 F·g-1with the increase of current density from 100 to 2 000 mA·g-1.Although the surface areas of C1 and C2 are very close,the capacitance of C2 (159 F·g-1at the current density of 100 mA·g-1) is lower than that of C1,implying that the ion-accessible surface area of C2 is lower than C1.C3 with the highest surface area of 1 282 m2/g shows a capacitance of 152 F·g-1at the current density of 100 mA·g-1,which is lower than that of C1,suggesting that the accessible ratio of the surface area of C3 with two pore systems is much lower than that of C1.C4 with the lowest surface area of 1 069 m2/g has the lowest capacitance among the carbon materials,which decreases from 140 to 127 F·g-1with the increase of current density from 100 to 2 000 mA·g-1.
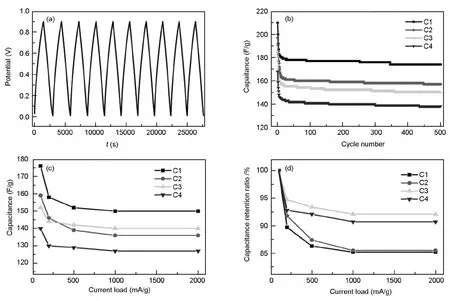
Fig.6 Electrochemical performance of the OMCs.(a) galvanostatic charge/discharge cycling for C1 at a current density of 100 mA/g; (b) capacitances of the OMCs at a current density of 100 mA/g; (c) the specific capacitance and (d) capacitance retention ratio of OMCs at various current densities.
The capacitance retention ratio is used to evaluate the ion transport behavior of the carbon materials,and the larger the retention ratio,the better the ion transport behavior[13].The ion transport behavior of the material is affected by many factors,such as pore size[15],pore length[15],pore order[32],and pore continuity[32].The capacitance retention ratios (relative to capacitance at a current density of 100 mA·g-1) of the OMCs at various current densities are shown in Fig.6d.As the current density rises to 2 000 mA/g,the capacitance retention ratios of the four samples are all above 85%,while the retention ratio of the activated carbons is only about 60%[33,34],which suggests that the OMCs have better ion transport behavior than activated carbons.The capacitance retention ratio of C3 is the highest among the four samples at the current density of 2 000 mA/g,retaining 92% of its initial capacitance at 100 mA/g,which is a little higher than that of C4 (91%).Besides the existence of similar pore size with C4 (3.6 nm),C3 contains larger pores (14.2 nm),which would make the ion transport more easily in C3 and thus a better ion transport behavior.Although C1,C2 and C4 have similar pore sizes (3.6 nm),the rank of capacitance retention ratio at the current density of 2 000 mA/g is C4 > C2 (86%) > C1 (85%),which could be partly ascribed to their different pore lengths.Shorter pore length needs shorter transport time when the ion transport coefficient is prescribed.The rank of pore length is C4 < C2 < C1,so the ion transport behavior in the rapid charging/discharging operation at high current densities is C4 > C2 > C1.
Cyclic voltammetry measurements were carried out within the potential range of -0.3-0.2 V to analyze the electrochemical behavior of the supercapacitors.Fig.7a exhibits the cyclic voltammograms of C1 recorded at different sweep rates.It is known that an ideal mesostructure should be capable of providing very fast ion transport pathways,and thus the electrical double layer can be re-organized quickly at the switching potentials,resulting in a rectangular-shaped CV curve[35].
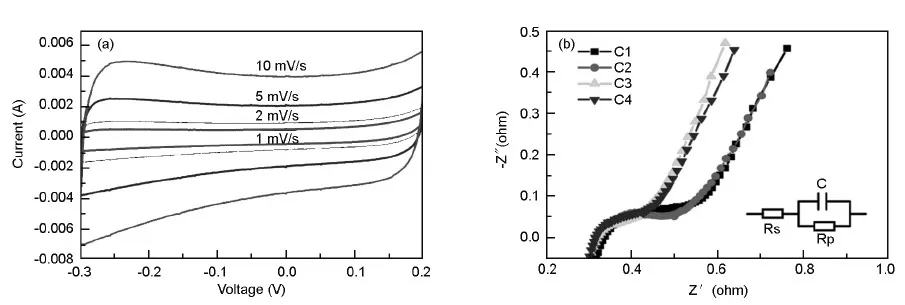
Fig.7 (a) Cycle voltammograms of C1 at different scan rates and (b) Nyquist impedance plots for different electrodes (insert:equivalent circuit model.)
The rectangle degree of CV curve can reflect the ion diffusion rate within a carbon mesostructure.The higher the rectangle degree has,the faster the ion diffusion rate is.It can be found that from 1 to 10 mV/s,the sample gives a good rectangular-shaped CV curve,indicating that the mesopores are able to provide fast ion transport pathways at such sweep rates.The current clearly increases with sweep rate,indicating a good rate capability.
Electrochemical impedance spectroscopy has also been used to check the ability of carbon materials to store electrical energy[36,37].The impedance plots (Fig.7b) exhibit two distinct parts,a semicircle in the high frequency range and a sloped line in the low frequency range.The magnitude of the resistance can be estimated from the curvature of the high-frequency loop,whereas the diffusion is characterized by the linear area at low frequencies.The impedances on electrode/electrolyte interface (Rs) of C1,C2,C3 and C4 are 0.36,0.34,0.32 and 0.33 Ω,respectively.Lower impedance leads to better ion transport behavior,thus the rank of ion transport behavior is C3> C4 > C2 > C1,which is in accordance with the results from capacitance retention ratios.
4 Conclusions
OMCs with different rod lengths were synthesized by changing the operational parameters during the synthesis,using TEOS as the silica source and P123 as the carbon precursor.Their electrochemical performance was investigated in alkaline electrolytic solution using galvanostatic charge-discharge test and AC impedance spectroscopy.It was found that the OMCs exhibit ideal capacitor behaviors.The ion transport behavior of the carbons is affected by pore structures,such as pore length and pore size.Materials with two pore systems and shorter pore channels show better ion transport behavior.
[1]Kötz R,Carlen M.Principles and applications of electrochemical capacitors[J].Electrochimica Acta,2000,45(15):2483-2498.
[2]Frackowiak E,Beguin F.Carbon materials for the electrochemical storage of energy in capacitors[J].Carbon,2001,39(6):937-950.
[3]Pandolfo A G,Hollenkamp A F.Carbon properties and their role in supercapacitors[J].Journal of Power Sources,2006,157(1):11-27.
[4]Lewandowski A,Galinski M.Practical and theoretical limits for electrochemical double-layer capacitors[J].Journal of Power Sources,2007,173(2):822-828.
[5]Li L,Song H,Chen X.Pore characteristics and electrochemical performance of ordered mesoporous carbons for electric double-layer capacitors[J].Electrochimica Acta,2006,51(26):5715-5720.
[6]Ryoo R,Joo S H,Jun S.Synthesis of highly ordered carbon molecular sieves via template-mediated structural transformation[J].The Journal of Physical Chemistry B,1999,103(37):7743-7746.
[7]Leyva-García S,Lozano-Castelló D,Morallón E,et al.Silica-templated ordered mesoporous carbon thin films as electrodes for micro-capacitors[J].Journal of Materials Chemistry A,2016,4(12):4570-4579.
[8]Alvarez S,Blanco-Lopez M C,Miranda-Ordieres A J,et al.Electrochemical capacitor performance of mesoporous carbons obtained by templating technique[J].Carbon,2005,43(4):866-870.
[9]WANG Da-wei,LI Feng,LIU Min,et al.Improved capacitance of SBA-15 templated mesoporous carbons after modification with nitric acid oxidation[J].New Carbon Materials,2007,22(4):307-314.
(王大伟,李峰,刘敏,等.硝酸氧化改性SBA-15 模板合成的中孔炭电容性能研究[J].新型炭材料,2007,22(4):307-314.)
[10]宋怀河,李丽霞,陈晓红.有序介孔炭的模板合成进展[J].新型炭材料,2006,21(4):374-383.
(SONG Huai-he,LI Li-xia,CHEN Xiao-hong.The synthesis of ordered mesoporous carbons via a template method[J].New Carbon Materials,2006,21(4):374-383.)
[11]Tang D,Hu S,Dai F,et al.Self-templated synthesis of mesoporous carbon from carbon tetrachloride precursor for supercapacitor electrodes[J].ACS Applied Materials & Interfaces,2016,8(11):6779-6783.
[12]Jurewicz K,Vix-Guterl C,Frackowiak E,et al.Capacitance properties of ordered porous carbon materials prepared by a templating procedure[J].Journal of Physics and Chemistry of Solids,2004,65(2):287-293.
[13]Xing W,Qiao S Z,Ding R G,et al.Superior electric double layer capacitors using ordered mesoporous carbons[J].Carbon,2006,44(2):216-224.
[14]Gao J,Wang X,Zhao Q,et al.Synthesis and supercapacitive performance of three-dimensional cubic-ordered mesoporous carbons[J].Electrochimica Acta,2015,163:223-231.
[15]Wang D W,Li F,Liu M,et al.Mesopore-aspect-ratio dependence of ion transport in rod type ordered mesoporous carbon[J].The Journal of Physical Chemistry C,2008,112(26):9950-9955.
[16]Xiao Y,Dong H,Lei B,et al.Ordered mesoporous carbons with fiber-and rod-like morphologies for supercapacitor electrode materials[J].Materials Letters,2015,138:37-40.
[17]Liang Y,Wu D,Fu R.Preparation and electrochemical performance of novel ordered mesoporous carbon with an interconnected channel structure[J].Langmuir,2009,25(14):7783-7785.
[18]Yan X,Song H,Chen X.Synthesis of spherical ordered mesoporous carbons from direct carbonization of silica/triblock-copolymer composites[J].Journal of Materials Chemistry,2009,19(26):4491-4494.
[19]Liu C,Li L,Song H,et al.Facile synthesis of ordered mesoporous carbons from F108/resorcinol-formaldehyde composites obtained in basic media[J].Chemical Communications,2007(7):757-759.
[20]张煜,王同华,米盼盼.双模板结构导向剂制备有序介孔炭[J].新型炭材料,2012,27(4):301-306.
(ZHANG Yu,WANG Tong-hua,MI Pan-pan.Synthesis of ordered mesoporous carbon with dual templates as structure directing agents[J].New Carbon Materials,2012,27(4):301-306.)
[21]Lin G,Wang F,Wang Y,et al.Enhanced electrochemical performance of ordered mesoporous carbons by a one-step carbonization/activation treatment[J].Journal of Electroanalytical Chemistry,2015,758:39-45.
[22]Pröbstle H,Schmitt C,Fricke J.Button cell supercapacitors with monolithic carbon aerogels[J].Journal of Power Sources,2002,105(2):189-194.
[23]Zhu J,Yang J,Miao R,et al.Nitrogen-enriched,ordered mesoporous carbons for potential electrochemical energy storage[J].Journal of Materials Chemistry A,2016,4:2286-2292.
[24]Li H Q,Luo J Y,Zhou X F,et al.An ordered mesoporous carbon with short pore length and its electrochemical performances in supercapacitor applications[J].Journal of the Electrochemical Society,2007,154(8):A731-A736.
[25]Wang D W,Li F,Fang H T,et al.Effect of pore packing defects in 2-D ordered mesoporous carbons on ionic transport[J].The Journal of Physical Chemistry B,2006,110(17):8570-8575.
[26]Tang Y,Yuan S,Guo Y,et al.Highly ordered mesoporous Si/C nanocomposite as high performance anode material for Li-ion batteries[J].Electrochimica Acta,2016,200:182-188.
[27]Fuertes A B,Pico F,Rojo J M.Influence of pore structure on electric double-layer capacitance of template mesoporous carbons[J].Journal of Power Sources,2004,133(2):329-336.
[28]Osaka T,Liu X,Nojima M,et al.An electrochemical double layer capacitor using an activated carbon electrode with gel electrolyte binder[J].Journal of the Electrochemical Society,1999,146(5):1724-1729.
[29]Vix-Guterl C,Frackowiak E,Jurewicz K,et al.Electrochemical energy storage in ordered porous carbon materials[J].Carbon,2005,43(6):1293-1302.
[30]Gryglewicz G,Machnikowski J,Lorenc-Grabowska E,et al.Effect of pore size distribution of coal-based activated carbons on double layer capacitance[J].Electrochimica Acta,2005,50(5):1197-1206.
[31]Du X,Guo P,Song H,et al.Graphene nanosheets as electrode material for electric double-layer capacitors[J].Electrochimica Acta,2010,55(16):4812-4819.
[32]Wu D,Chen X,Lu S,et al.Study on synergistic effect of ordered mesoporous carbon and carbon aerogel during electrochemical charge-discharge process[J].Microporous and Mesoporous Materials,2010,131(1):261-264.
[33]Tamai H,Kouzu M,Morita M,et al.Highly mesoporous carbon electrodes for electric double-layer capacitors[J].Electrochemical and Solid-state Letters,2003,6(10):A214-A217.
[34]Alvarez S,Blanco-Lopez M C,Miranda-Ordieres A J,et al.Electrochemical capacitor performance of mesoporous carbons obtained by templating technique[J].Carbon,2005,43(4):866-870.
[35]Fang B,Binder L.A novel carbon electrode material for highly improved EDLC performance[J].The Journal of Physical Chemistry B,2006,110(15):7877-7882.
[36]王六平,周颖,邱介山.硝酸氧化对沥青烯基有序介孔炭电化学性能的影响[J].新型炭材料,2011,26(3):204-210.
(WANG Liu-ping,ZHOU Ying,QIU Jie-shan.The influence of nitric acid oxidation on the electrochemical performance of asphaltene-based ordered mesoporous carbon[J].New Carbon Materials,2011,26(3):204-210.)
[37]Frackowiak E,Beguin F.Carbon materials for the electrochemical storage of energy in capacitors[J].Carbon,2001,39(6):937-950.
Electrochemical performance of rod-type ordered mesoporous carbons with different rod lengths for electric double-layer capacitors
LIU Na,YU Lv-qiang,CHEN Xiao-hong,LIAO Li-fang,ZHOU Ji-sheng,MA Zao-kun,SONG Huai-he
(State Key Laboratory of Chemical Resource Engineering,Beijing Key Laboratory of Electrochemical Process and Technology for Materials,Beijing University of Chemical Technology,Beijing100029)
Rod-type ordered mesoporous carbons were synthesized by the direct carbonization of sulfuric-acid-treated silica/triblock copolymer composites,followed by etching the silica with a HF solution.The morphologies,microstructures and pore structures of the mesoporous carbons were investigated by scanning electron microscopy,high resolution transmission electron microscopy,X-ray diffraction and nitrogen sorption.Their electrochemical performance as electrodes for supercapacitors was investigated by impedance spectroscopy and charge/discharge tests.It was found that the rod length of the mesoporous carbons can be changed from one to tens of micrometers by changing the synthesis parameters.The sample with the longest rod length has the highest specific capacitance.The sample with two pore sizes has the highest capacitance retention ratio of 92% at a high current density of 2 A/g.
Ordered mesoporous carbon; Rod-type; Template; Triblock copolymer; Supercapacitor
date:2016-05-08;Revised date:2016-06-10
National Natural Science Foundation of China (50872006,51272016).
SONG Huai-he.Professor.E-mail:songhh@mail.buct.edu.cn
introduction:LIU Na.Ph.D.E-mail:1552881023@qq.com
1007-8827(2016)03-0328-08
TQ127.1+1
A
国家自然科学基金(50872006,51272016).
宋怀河,教授.E-mail:songhh@mail.buct.edu.cn
刘娜,博士.E-mail:1552881023@qq.com
English edition available online ScienceDirect (http:www.sciencedirect.comsciencejournal18725805).
10.1016/S1872-5805(16)60016-1

