罗格列酮预处理对凝血酶激活的小胶质细胞PPARγ、Nrf2及HO-1表达的影响*
杭 航, 王丽琨, 伍国锋, 陈星宇
(贵州医科大学附属医院急诊医学科/急诊医学教研室, 贵州 贵阳 550004)
罗格列酮预处理对凝血酶激活的小胶质细胞PPARγ、Nrf2及HO-1表达的影响*
杭航,王丽琨,伍国锋△,陈星宇
(贵州医科大学附属医院急诊医学科/急诊医学教研室, 贵州 贵阳 550004)
目的: 采用凝血酶激活新生大鼠神经胶质细胞,观察罗格列酮预处理对小胶质细胞过氧化物酶体增殖物活化受体γ(PPARγ)、核因子E2相关因子2(Nrf-2)及血红素加氧酶-1(HO-1)表达的影响。方法: 用新生SD大鼠的脑组织,体外培养原代小胶质细胞14 d左右分离收集细胞,分为:正常对照组、凝血酶刺激组、罗格列酮干预组(罗格列酮+凝血酶组)和维甲酸干预组(维甲酸+凝血酶组)进行实验。分别采用免疫组化染色、real-time PCR和Western blot检测PPARγ、Nrf2和HO-1的表达并进行统计分析。结果: 免疫组化染色显示,与对照组比较,刺激组、罗格列酮+凝血酶组及维甲酸+凝血酶组的PPARγ、Nrf2和HO-1染色细胞数均增多。Real-time PCR结果显示罗格列酮+凝血酶组PPARγ、Nrf2及HO-1的mRNA表达均显著高于刺激组、对照组及维甲酸+凝血酶组(P<0.01),维甲酸+凝血酶组Nrf2及HO-1的mRNA表达均较刺激组和罗格列酮+凝血酶组降低(P<0.01)。Western blot 结果显示,罗格列酮+凝血酶组PPARγ、Nrf2及HO-1的 蛋白表达也明显高于刺激组、对照组及维甲酸+凝血酶组(P<0.01),维甲酸+凝血酶组Nrf2及HO-1的蛋白表达均较刺激组和罗格列酮+凝血酶组降低(P<0.01)。结论: 罗格列酮预处理后可增加凝血酶激活的小胶质细胞PPARγ、Nrf2及HO-1的表达,通过维甲酸预处理抑制Nrf2的表达后,其下游基因HO-1表达也受影响,说明PPARγ抗氧化作用可能是通过Nrf2调控下游基因实现的。
小胶质细胞; 脑出血; 罗格列酮; 过氧化物酶体增殖物活化受体γ; 核因子E2相关因子2; 血红素加氧酶-1
脑出血占脑卒中发病率的8%~15%,其病理生理过程包括脑实质中血肿的形成及继发性脑损害,其发病率、死亡率及致残率均高居不下,目前尚无特效的治疗方案[1]。脑出血后凝血系统被激活,所释放的凝血酶(thrombin,TH)主要通过破坏血脑屏障、造成神经损伤以及参与炎症反应这几方面起作用,而产生一些细胞毒性物质,通过激活小胶质细胞和巨噬细胞产生氧自由基,从而诱发氧化应激反应,造成继发性脑损伤[2-4]。过氧化物酶体增殖物活化受体γ(peroxisome proliferator-activated receptor γ,PPARγ)在激活小胶质细胞/巨噬细胞和降低氧化应激方面都起着非常重要的作用[5],增加PPARγ的作用将有助于提高小胶质细胞/巨噬细胞的吞噬功能及降低氧化应激反应从而减轻继发性脑损害。不少研究已经发现PPARγ激动剂可减轻继发性脑损伤,同时有一定的神经细胞保护作用[6-7]。罗格列酮(rosiglitazone,RGZ)是PPARγ的有效激动剂[8],脑出血后使用RGZ激活PPARγ可在转录水平上调抗氧化剂和铜锌超氧化物歧化酶[9-10],如血红素加氧酶-1(heme oxygenase 1,HO-1),从而增加细胞的氧化应激能力。核因子E2相关因子2(nuclear factor E2-related factor 2,Nrf2)是氧化应激的感受器,参与体内氧化应激反应,是氧化应激重要的转录激活因子。脑出血后PPARγ表达增加[8], Nrf2表达也增高,参与脑内氧化应激反应[11]。本研究组先前的工作发现,罗格列酮预处理可使凝血酶刺激后的小胶质细胞PPARγ及HO-1表达增加[12],然而这些变化是否与Nrf2有关联尚不清楚。
本实验的目的在于采用罗格列酮预处理小胶质细胞,观察凝血酶激活的小胶质细胞PPARγ、Nrf2和HO-1表达的影响,并初步探讨其相互关系。
材 料 和 方 法
1实验材料
1.1动物来源健康SD大鼠乳鼠,雌雄均可,1~3日龄,由贵州医科大学动物实验中心提供。
1.2主要试剂与材料北美胎牛血清(fetal bovine serum,FBS)、DMEM/F12培养基、胰酶(含EDTA,0.25%)、青霉素-链霉素溶液及GlutaMax(Gibco);多聚L-赖氨酸、DNase I和罗格列酮(Sigma);TH(湖南一格公司);OX42抗体(Abcam);4%多聚甲醛、山羊抗兔Ⅱ抗FITC、封闭山羊血清和抗荧光衰减封片剂(索莱宝公司);DAPI染色液(泛博公司);PPARγ、Nrf2和HO-1抗体(Proteintech);哺乳动物蛋白抽提试剂盒、SDS-PAGE上样缓冲液、Tris-Glycine转膜缓冲液和Tris-Glycine SDS电泳缓冲液(康为世纪公司);cDNA合成试剂盒和real-time PCR试剂盒(Thermo)。
2实验方法
2.1原代细胞培养新生1~3 d的SD大鼠(110只),由贵州医科大学动物实验中心提供。将出生1~3 d的SD大鼠无菌条件下处死,取出脑组织,剥离脑膜和血管,用0.25%的胰酶消化液消化后过滤离心,用完全培养基进行细胞沉淀悬浮后计数细胞,以2×105个接种至培养瓶内。24 h后全量换液并根据细胞代谢情况,约3 d换液1次,继续培养至14 d左右,采用恒温摇床振摇法分离细胞,将上层培养液收集后以4×104/cm2的密度接种至预先用0.01%的多聚赖氨酸包被好的培养板中培养。24 h后细胞贴壁完全,用小胶质细胞特异性抗体OX42进行免疫细胞化学染色鉴定纯度。阳性率>95%即可用于后续实验。
将培养成功的细胞,随机分为4个组,分别为正常对照组、凝血酶刺激组、罗格列酮干预组(RGZ+TH组)和维甲酸干预组(RA+TH组)。给药方案:对照组加入与刺激组所加凝血酶等体积的培养基,刺激组加入凝血酶(2×104U/L)处理24 h,RGZ+TH组予25 μmol/L的罗格列酮对小胶质细胞预处理1 h,之后加入凝血酶(2×104U/L),共同作用小胶质细胞24 h,RA+TH组用维甲酸(1 μmol/L)预处理1 h后再用凝血酶(2×104U/L)刺激小胶质细胞24 h。
2.2原代小胶质细胞的鉴定将分离后的细胞接种于细胞爬片上24 h,经PBS液洗涤,用4%的多聚甲醛固定15 min后,用0.25% Triton通透20 min,PBS洗3遍,每次3 min,10%正常山羊血清封闭;加入I抗为小鼠抗大鼠CD11b/c 单克隆抗体(1∶50),37 ℃孵育2 h,PBS洗3遍,每次3 min;加入II抗为山羊抗小鼠FITC (1∶100)37 ℃避光孵育1 h,PBS洗3遍,每次3 min;DAPI染核5 min,最后加入抗荧光淬灭封片剂封片。用激光共聚焦显微镜观察、摄片。以上染色均用PBS及正常牛血清代替I抗作为对照染色。2.3免疫细胞化学染色观察PPARγ、Nrf2和HO-1的表达 分别将对照组、刺激组、RGZ+TH组和RA+TH组4组已爬片细胞予PBS液洗涤,4%多聚甲醛固定15 min,再用0.25% Triton通透20 min,PBS洗涤,10%正常山羊血清封闭;加入I 抗为PPARγ抗体(1∶50),4 ℃孵育过夜,PBS洗3遍,每次3 min;加入 II抗为山羊抗小鼠FITC (1∶100)37 ℃避光孵育1 h,PBS洗3遍,每次3 min;DAPI染核5 min,最后加入抗荧光淬灭封片剂封片。用激光共聚焦显微镜观察、摄片。以上染色均用PBS及正常牛血清代替I抗作为对照染色。同样方法,将I抗换成Nrf2和HO-1抗体(1∶50),观察各组Nrf2和HO-1变化情况。2.4Real-time PCR检测PPARγ、Nrf2和HO-1的mRNA表达将分离后的原代培养的小胶质细胞以每孔1×106个接种于6孔板,培养24 h后,分为4组处理。处理后的细胞用预冷PBS洗2次后,加入TRIzol 1 mL裂解细胞,用枪头充分吹打混匀。室温放置后,加入氯仿,剧烈振荡,再次室温静置后,4 ℃、12 000 r/min离心10 min;吸取上清加入异丙醇沉淀RNA,轻轻混匀,室温静置;以12 000 r/min、4 ℃离心10 min;弃上清, 75%乙醇洗涤RNA 2次, 4 ℃、7 500 r/min 离心5 min,弃上清,室温放置干燥5 min;加入20 μL DEPC水,用枪头吹打充分; 取2 μL RNA样品于ND2000下测核酸浓度以及A260/A280比值以了解RNA纯度,比值在1.8~2.2者可用于下一步实验;根据A260值计算RNA含量。将提出的RNA按逆转录试剂盒说明书进行逆转录成cDNA,PPARγ、Nrf2及HO-1引物(上海生工设计并合成)序列见表1,以β-actin为内参照,按照PCR反应说明书进行PCR反应。
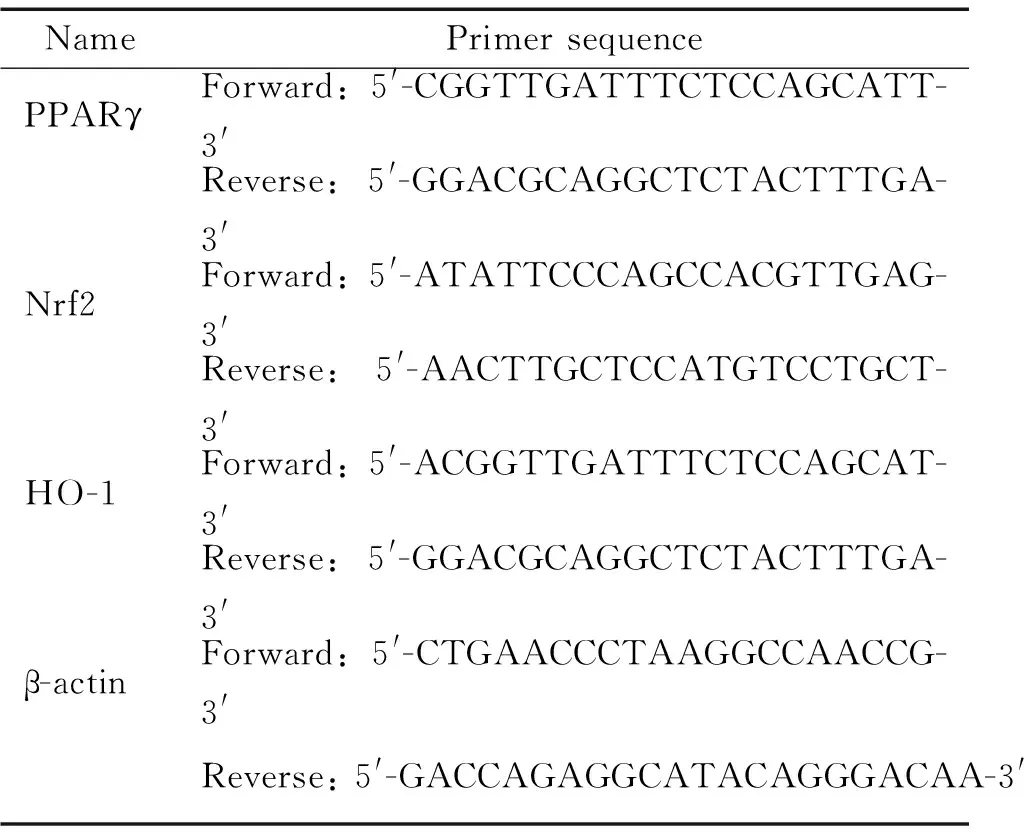
表1 引物序列
2.5蛋白印迹法检测PPARγ、Nrf2和HO-1的蛋白表达 根据细胞覆盖率按照一定比例加入细胞抽提试剂,吹打均匀,吸出蛋白液至1.5 mL EP管中,冰上孵育20 min,10 000 r/min离心20 min后收集上清,-80 ℃冰箱保存。蛋白定量后取4倍体积样品缓冲液,95 ℃变性10 min。蛋白上样量为每孔30 μg,10% SDS-PAGE 电泳,将蛋白电转移至0.45 μm PVDF膜上,依分子量大小切取条带,加入封闭液室温下震荡2 h 后,取相应条带分别加入 I 抗兔抗鼠PPARγ多克隆抗体(1∶500)、兔抗鼠Nrf2多克隆抗体(1∶200)和兔抗鼠HO-1多克隆抗体(1∶200)4 ℃过夜。室温孵育1 h后, 分别加入 II 抗(生物素标记羊抗兔IgG,1∶200)室温孵育1 h,将孵育好 II 抗的膜在漂洗液中洗涤5次,每次5 min。ECL反应5 min,曝光,显影定影。经X胶片曝光显影。图片扫描保存电脑文件。
3统计学处理
使用SPSS 13.0 统计软件进行统计学分析。计量资料数据均采用均数±标准差(mean±SD)表示。多样本均数比较采用单因素方差分析,其中满足方差齐性的采用Fisher方差分析,方差不齐的采用近似F检验Welch法;多样本均数间两两比较满足方差齐性的采用SNK法检验,方差不齐的采用Dunnett’s T3检验。以P<0.05 为差异有统计学意义。
结 果
1细胞培养结果
胶质细胞混合培养至9 d左右细胞分层明显,相差显微镜下观察底层细胞充分铺展,紧密接触,为星形胶质细胞,其上层主要为散在分布的阿米巴样细胞,胞体圆形,透光性较底层细胞好,经振摇法分离出来的小胶质细胞产量为1.2×106个,存活率≥95%, OX42染色阳性,阳性细胞(纯度)≥95%,见图1。
2PPARγ的表达
2.1免疫组化结果对照组、刺激组、RGZ+TH组及RA+TH组PPARγ均着色,对照组PPARγ可见胞质均匀着色(绿色),DAPI染核可见蓝色;刺激组PPARγ染色细胞数较对照组增多,胞质着色加深;RGZ+TH组PPARγ染色细胞数较对照组和刺激组明显增多,且以细胞核染色为显著,说明罗格列酮干预后可促进PPARγ表达增多并且使得其表达向核内转移, RA+TH组PPARγ染色细胞数与刺激组无明显差别,部分细胞着色在胞核加深,见图2。

Figure 1.The expression of OX42 detected by immunofluorescence for identification of microglia (×200).
图1免疫组化法检测OX42的表达鉴定小胶质细胞

Figure 2.PPARγ expression in the microglias observed by immunofluorescence (×400).
图2免疫组化检测PPARγ的表达
2.2PPARγ的mRNA表达4组间PPARγ的mRNA表达量相对值差异具有统计学意义(P<0.01);刺激组与RA+TH组之间差异无统计学显著性,其余组两两间比较差异有统计学显著性(P<0.05),见图3。
2.3PPARγ蛋白的表达4组间PPARγ蛋白表达量相对值差异有统计学显著性(P<0.01);刺激组与RA+TH组之间差异无统计学显著性,其余组两两间比较差异有统计学显著性(P<0.05),见图4。
3Nrf2的表达
3.1免疫组化结果 对照组Nrf2染色为阴性,FITC可见几乎没有细胞着色,DAPI染色后可见细胞核,融合后见染色细胞数低;刺激组Nrf2染色细胞数较对照组明显增多,胞质呈绿色,着色均匀,融合后可见细胞染色率较高;RGZ+TH组Nrf2染色细胞数较正常组、刺激组及RA+TH组增多,且胞质深染,提示罗格列酮干预后可促进Nrf2的表达;RA+TH组Nrf2染色细胞数及染色程度较RGZ+TH组及刺激组明显减少,见图5。
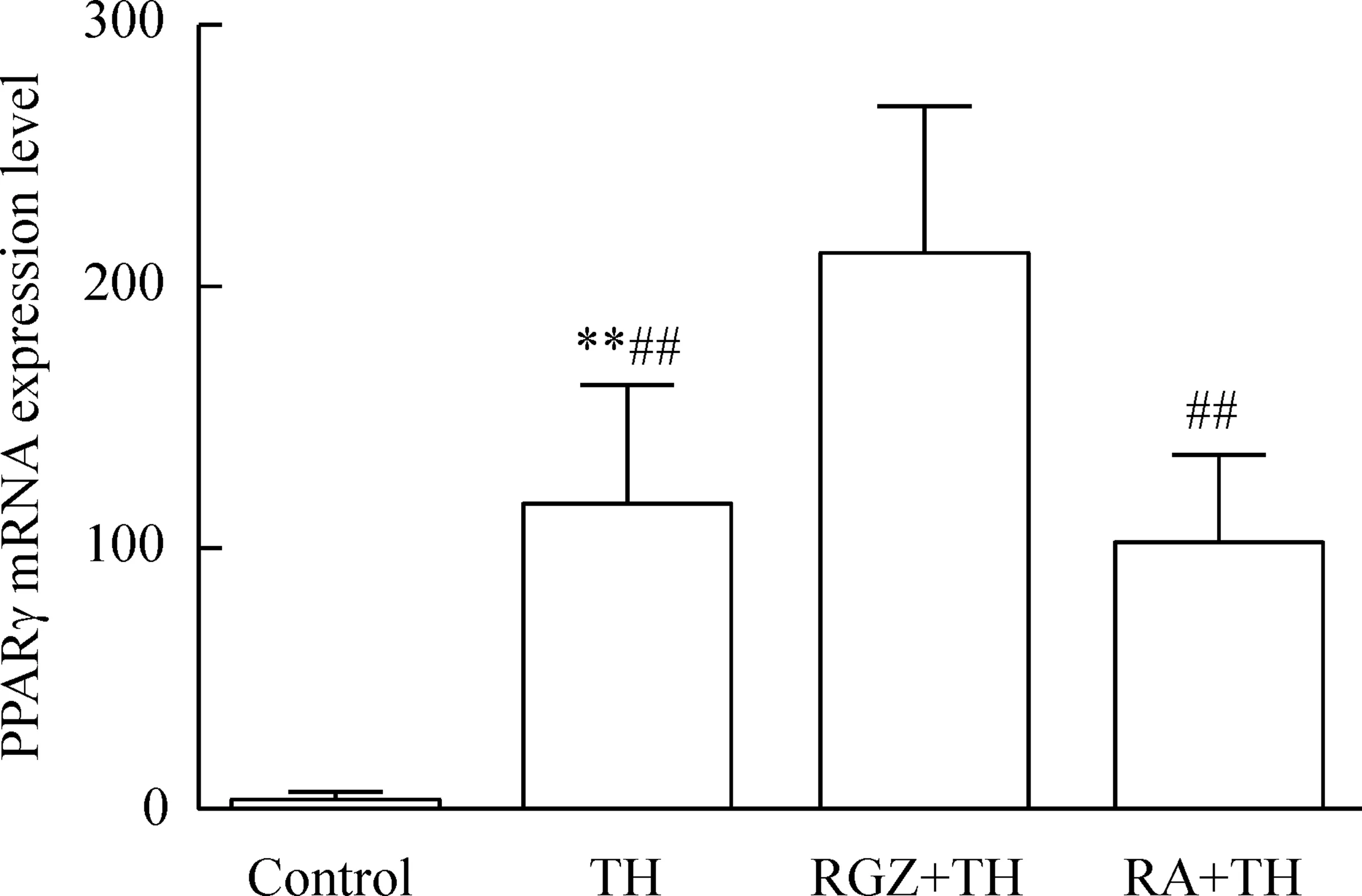
Figure 3.The mRNA expression of PPARγ in the microglia with different treatments. Mean±SD.n=20.**P<0.01vscontrol;##P<0.01vsRGZ+TH.
图3Real-time PCR检测PPARγ的mRNA表达

Figure 4.The protein expression of PPARγ in the microglia with different treatments. Mean±SD.n=10.**P<0.01vscontrol;##P<0.01vsRGZ+TH.
图4Western blot检测PPARγ蛋白的表达

Figure 5.Nrf2 expression in the microglia observed by immunofluorescence (×400).
图5免疫组化检测Nrf2的表达
3.2Nrf2的mRNA表达4组间Nrf2 mRNA表达量相对值差异具有统计学显著性(P<0.01);各组两两间比较差异有统计学显著性(P<0.05),见图6。3.3Nrf2蛋白的表达4组间Nrf2蛋白表达量相对值差异具有统计学显著性(P<0.01);各组两两间比较差异有统计学显著性(P<0.05),见图7。
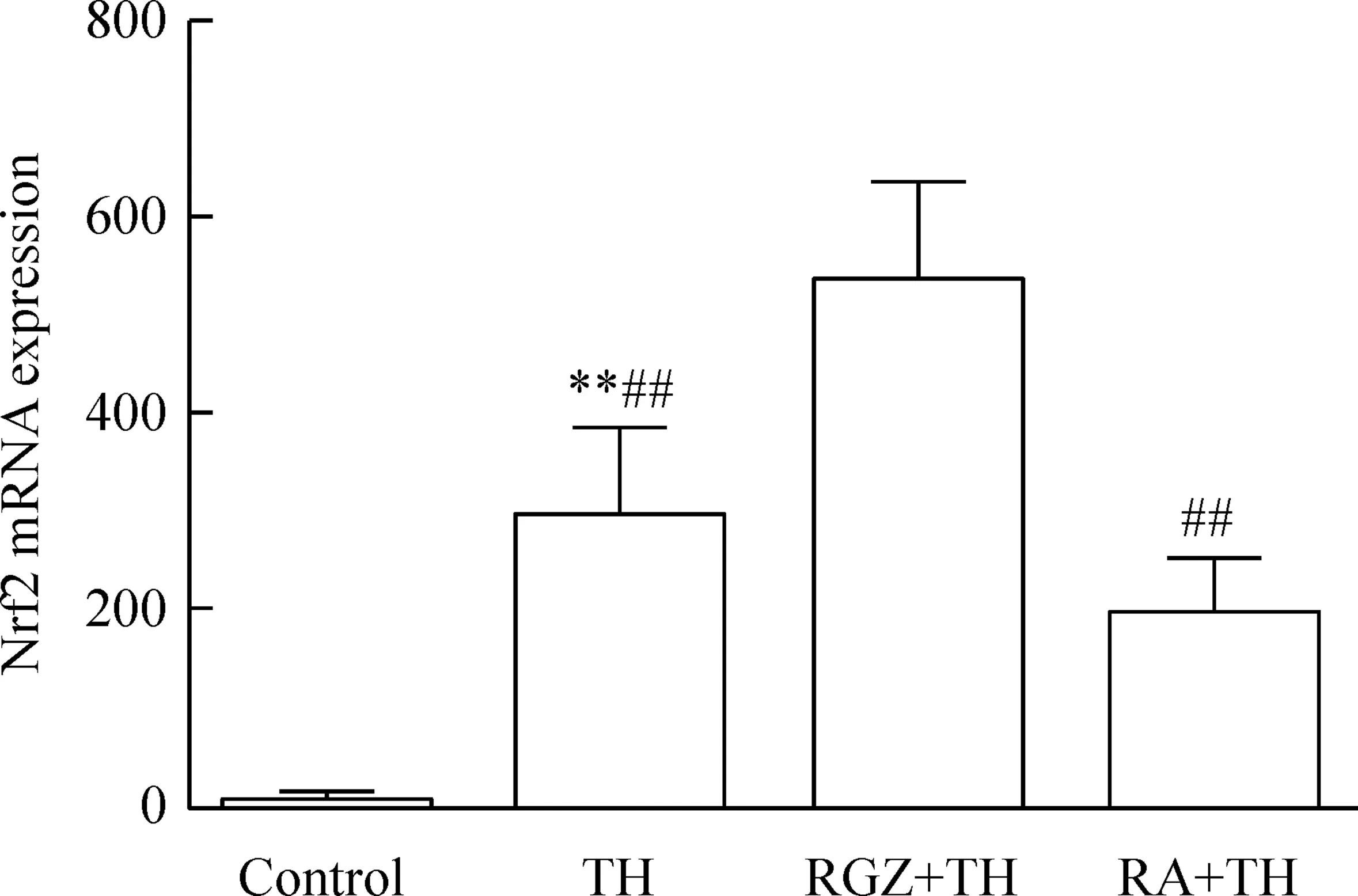
Figure 6.The mRNA expression of Nrf2 in the microglia with different treatments. Mean±SD.n=10.**P<0.01vscontrol;##P<0.01vsRGZ+TH.
图6Real-time PCR 检测Nrf2 mRNA的表达

Figure 7.The protein expression of Nrf2 in the microglia with different treatments.Mean±SD.n=10.**P<0.01vscontrol;##P<0.01vsRGZ+TH.
图7Western blot检测Nrf2蛋白的表达
4HO-1的表达
4.1免疫组化结果对照组HO-1染色为阴性,FITC可见几乎没有细胞着色,DAPI染色后可见细胞核,融合后见染色细胞数低;刺激组HO-1染色细胞数较对照组明显增多,胞质呈绿色,融合后可见细胞染色率较高;RGZ+TH组HO-1染色细胞数较正常组、刺激组增多,胞质、胞核均着色,部分细胞胞核深染,提示罗格列酮干预后可促进HO-1的表达,RA+TH组细胞染色率较RGZ+TH组减少,染色程度也较浅,见图8。
4.2HO-1的mRNA表达4组间HO-1 mRNA表达量相对值差异具有统计学显著性(P<0.01);各组两两间比较差异有统计学显著性(P<0.05),见图9。
4.3HO-1蛋白的表达4组间HO-1蛋白表达量相对值差异具有统计学显著性(P<0.01);各组两两间比较差异有统计学显著性(P<0.05),见图10。
讨 论
综合上述实验结果,提示凝血酶刺激小胶质细胞可诱导PPARγ、Nrf2和HO-1的表达,而罗格列酮预处理后PPARγ、Nrf2和HO-1的表达增加更为显著,维甲酸预处理后Nrf2和HO-1的表达下降。Nrf2和PPARγ均参加脑内氧化应激反应,Nrf2有助于神经保护和脑缺血后脑损伤的改善[13-14],而脑出血后PPARγ则主要通过减少氧化应激、炎症和解毒的分子的生成,从而中和许多有毒物质介导的继发性脑伤害。PPARγ在小胶质细胞的激活和降低氧化应激方面都起着至关重要的作用[9]。PPARγ被激活后,一方面可通过转录水平上调细胞表面清道夫受体CD36,来帮助小胶质细胞和巨噬细胞通过吞噬或内吞作用来清除坏死或凋亡的细胞残骸[15-16];另一方面,激活的PPARγ可通过转录水平上调抗氧化剂和铜锌超氧化物歧化酶[5],如HO-1、SOD等,从而增加细胞的抗氧化功能。噻唑烷二酮类药物(如罗格列酮)是PPARγ有效的激动剂[17]。本课题组前期研究表明,脑出血后血肿周围脑组织发生一系列的病理生理变化,包括谷氨酸含量增加、基质金属蛋白酶表达增多、血脑屏障通透性升高等,而且使用罗格列酮激活PPARγ从而减轻继发性脑损害方面的研究取得了一定的实验成果[18-23]。然而,对于使用罗格列酮激活PPARγ减轻氧化应激反应从而降低脑损伤程度的机制尚不明确。本实验中,使用罗格列酮后PPARγ表达增加,其下游HO-1 mRNA及蛋白表达均增加,这样便增加了脑细胞的抗氧化应激能力。
在脑出血后的抗氧化应激反应过程中,Nrf2可以诱导抗氧化应激有关组件的产生、减少过氧化物的形成、增加CD36的吞噬功能以及增加对红细胞碎片的吞噬作用。近期研究表明,Nrf2在抗氧化应激能力的提升、增加吞噬功能以及促进血肿吸收方面发挥重要作用[7]。Nrf2-ARE通路是体内内源性抗氧化系统中非常重要的一条通路,其中抗氧化反应元件(antioxidant response element,ARE)与Nrf2结合并起着关键作用[24],而PPARγ和Nrf2的相互作用可能涉及多种机制。首先,PPAR反应元件(PPAR response element, PPRE)和ARE共存在相同的基因,如CD36[25-27]和过氧化氢酶;其次,Nrf2和PPARγ之间存在着相互的转录调控,如Nrf2基因包含着PPRE[28],而PPARγ基因也包含着ARE[29-30]。前期有研究发现维甲酸可以抑制Nrf2的表达[31-32]。 本实验中,凝血酶激活后的小胶质细胞PPARγ和Nrf2增加,其下游基因HO-1也增加,使用罗格列酮后见PPARγ、Nrf2和HO-1增加更为显著,然而使用维甲酸后,PPARγ表达与凝血酶激活表达差异无统计学显著性,Nrf2和HO-1的表达下降明显,PPARγ的下游基因是HO-1,因此推断PPARγ有可能通过Nrf2调控后者的产生,从而发挥抗氧化应激作用。
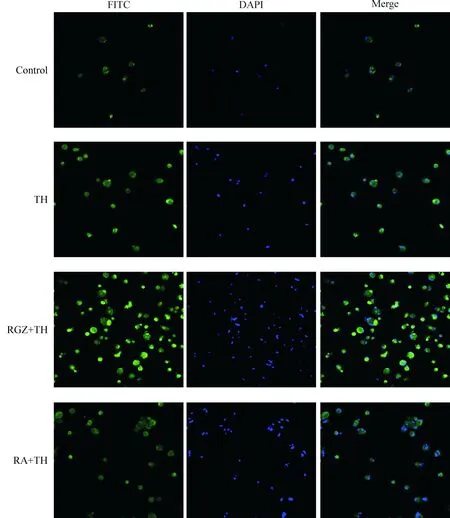
Figure 8.HO-1 expression in the microglia observed by immunofluorescence (×400).
图8免疫组化检测HO-1的表达

Figure 9.The mRNA expression of HO-1 in the microglia with different treatments. Mean±SD.n=10.**P<0.01vscontrol;##P<0.01vsRGZ+TH.
图9Real-time PCR检测HO-1的mRNA表达
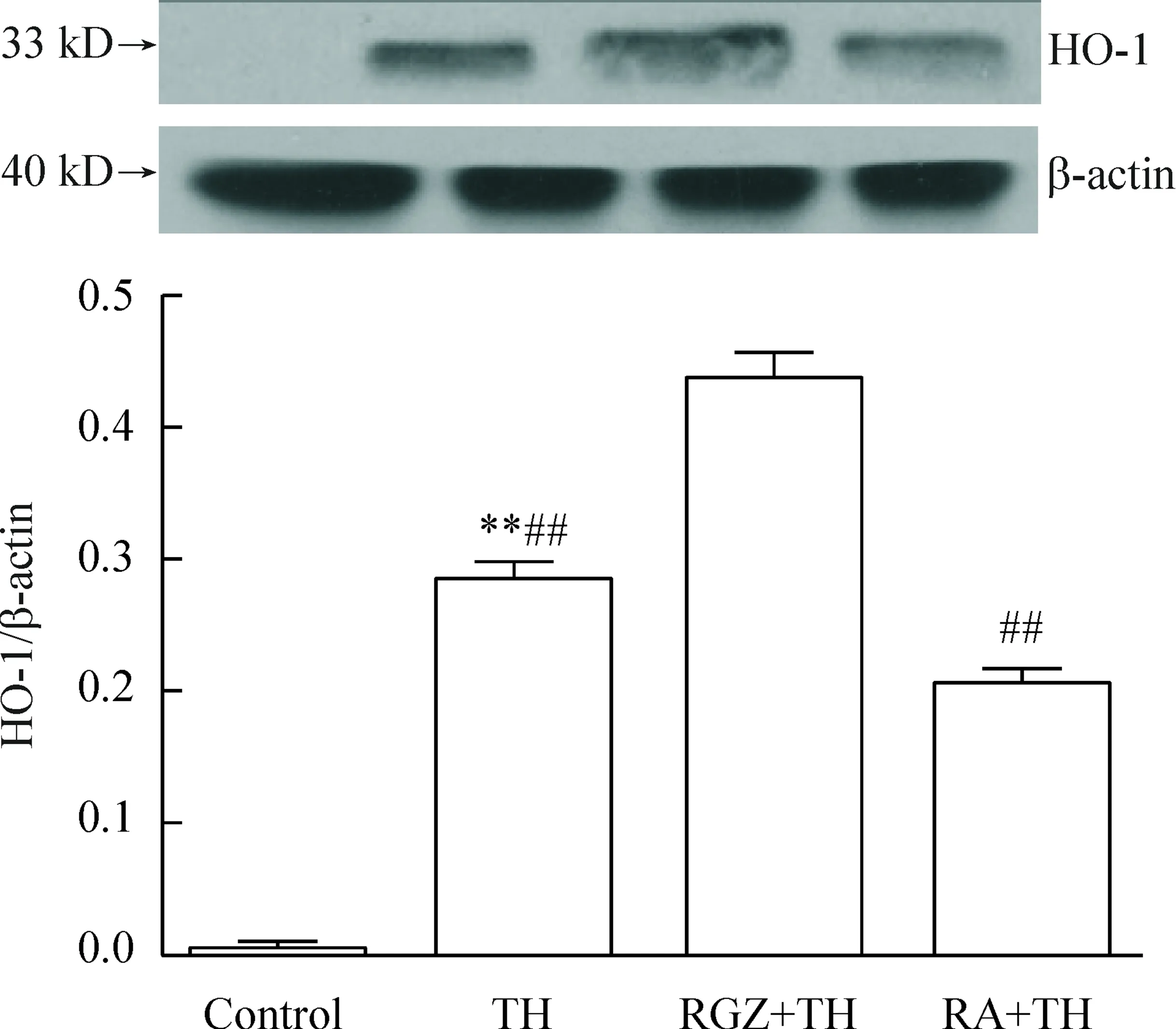
Figure 10.The protein expression of HO-1 in the microglia with different treatments. Mean±SD.n=10.**P<0.01vscontrol;##P<0.01vsRGZ+TH.
图10Western blot检测HO-1蛋白的表达
综上所述,凝血酶激活小胶质细胞可以诱导PPARγ、Nrf2和HO-1表达增加;罗格列酮预处理后,凝血酶激活的小胶质细胞PPARγ、Nrf2和HO-1的表达明显增加;使用维甲酸预处理后Nrf2下降,其下游基因HO-1也下降;推测PPARγ可能部分通过Nrf2作用其下游基因,从而发挥其抗氧化作用,这一课题有待进一步研究。
[1]Zhao XR, Gonzales N, Aronowski J. Pleiotropic role of PPARγ in intracerebral hemorrhage: an intricate system involving Nrf2, RXR, and NF-κB[J]. CNS Neurosci Ther, 2015, 21(4):357-366.
[2]Mracsko E, Veltkamp R. Neuroinflammation after intra-cerebral hemorrhage[J]. Front Cell Neurosci, 2014, 8:388.
[3]Jesberger JA, Richardson JS. Oxygen free radicals and brain dysfunction[J]. Int J Neurosci, 1991, 57(1-2):1-17.
[4]Han N, Ding SJ, Wu T, et al. Correlation of free radical level and apoptosis after intracerebral hemorrhage in rats[J]. Neurosci Bull, 2008, 24(6):351-358.
[5]Zhao X, Aronowski J. The role of PPARγ in stroke[M]//Chen J, Hu X, Stenzel-Poore M, et al. Immunological mechanisms and therapies in brain injuries and stroke. New York: Springer, 2014:301-320.
[6]张婧媛,张艳桥,张一娜,等. PPAR-γ激动剂减轻缺氧性大鼠神经细胞损伤的作用机制[J]. 中国病理生理杂志, 2009, 25(1):89-92.
[7]乔保华,高建新,王芬,等. PPARγ激动剂吡格列酮减少大鼠创伤性脑损伤后的神经损伤和胶质增殖[J]. 中国病理生理杂志, 2010, 26(5):912-916.
[8]Zhao X, Sun G, Zhang J, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages[J]. Annal Neurol, 2007, 61(4):352-362.
[9]Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury[J]. Stroke, 2011, 42(6):1781-1786.
[10]Jin J, Albertz J, Guo Z, et al. Neuroprotective effects of PPAR-γ agonist rosiglitazone in N171-82Q mouse model of Huntington′s disease[J]. J Neurochem, 2013, 125(3):410-419.
[11]Zhao X, Sun G, Ting SM, et al. Cleaning up after ICH: the role of Nrf2 in modulating microglia function and hematoma clearance[J]. J Neurochem, 2014, 133(1):144-152.
[12]陈星宇,伍国锋. 罗格列酮对凝血酶激活的小胶质细胞保护作用的研究[C]//中华医学会,中华医学会神经病学分会.中华医学会第十七次全国神经病学学术会议论文汇编(下). 2014:672.
[13]Itoh K, Wakabayashi N, Katoh Y, et al. Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles[J]. Genes Cells, 2003, 8(4):379-391.
[14]Giudice A, Montella M. Activation of the Nrf2-ARE signaling pathway: a promising strategy in cancer prevention[J]. Bioessays, 2006, 28(2):169-181.
[15]Villegas I, Martín AR, Toma W, et al. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor gamma, protects against gastric ischemia-reperfusion damage in rats: role of oxygen free radicals generation[J]. Eur J Pharmacol, 2004, 505(1-3):195-203.
[16]Asada K, Sasaki S, Suda T, et al. Antiinflammatory roles of peroxisome proliferator-activated receptor gamma in human alveolar macrophages[J]. Am J Respir Crit Care Med, 2004, 169(2):195-200.
[17]Krentz AJ, Friedmann PS. Type 2 diabetes, psoriasis and thiazolidinediones[J]. Int J Clin Pract, 2006, 60(3):362-363.
[18]Wu G, Li C, Wang L, et al. Minimally invasive procedures for evacuation of intracerebral hemorrhage reduces perihematomal glutamate content, blood-brain barrier permeability and brain edema in rabbits[J]. Neurocrit Care, 2011, 14(1):118-126.
[19]Wu G, Sheng F, Wang L, et al. The pathophysiological time window study of performing minimally invasive procedures for the intracerebral hematoma evacuation in rabbit[J]. Brain Res, 2012, 1465:57-65.
[20]Wu G, Wang L, Hong Z, et al. Effects of minimally invasive procedures for removal of intracranial hematoma on matrix metalloproteinase expression and blood-brain barrier permeability in perihematomal brain tissues[J]. Neurol Res, 2011, 33(3):300-306.
[21]Wu G, Wang L, Hong Z, et al. Effects of minimally invasive techniques for evacuation of hematoma in basal ganglia on cortical spinal tract from patients with spontaneous hemorrhage: observed by diffusion tensor imaging[J]. Neurol Res, 2010, 32(10):1103-1109.
[22]Wu G, Wang L, Liu J, et al. Minimally invasive procedures reduced the damages to motor function in patients with thalamic hematoma: observed by motor evoked potential and diffusion tensor imaging[J]. J Stroke Cerebrovasc Dis, 2013, 22(3):232-240.
[23]Wu G, Zhong W. Effect of minimally invasive surgery for cerebral hematoma evacuation in different stages on motor evoked potential and thrombin in dog model of intracranial hemorrhage[J]. Neurol Res, 2009, 32(2):127-133.
[24]Nguyen T, Huang HC, Pickett CB. Transcriptional regulation of the antioxidant response element.Activation by Nrf2 and repression by MafK[J]. J Biol Chem, 2000, 275(20):15466-15473.
[25]Ishii T, Itoh K, Ruiz E, et al. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal[J]. Circ Res, 2004, 94(5):609-616.
[26]Kwak MK, Itoh K, Yamamoto M, et al. Role of transcription factor Nrf2 in the induction of hepatic phase 2 and antioxidative enzymesinvivoby the cancer chemoprotective agent, 3H-1, 2-dithiole-3-thione[J]. Mol Med, 2001, 7(2):135-145.
[27]Girnun GD, Domann FE, Moore SA, et al. Identification of a functional peroxisome proliferator-activated receptor response element in the rat catalase promoter[J]. Mol Endocrinol, 2003, 16(12):2793-2801.
[28]Shih AY, Imbeault S, Barakauskas V, et al. Induction of the Nrf2-driven antioxidant response confers neuroprotection during mitochondrial stressinvivo[J]. J Biol Chem, 2005, 280(24):22925-22936.
[29]Cho HY, Gladwell W, Wang X, et al. Nrf2-regulated PPARγ expression is critical to protection against acute lung injury in mice[J]. Am J Respir Crit Care Med , 2010, 182(2):170-182.
[30]Park EY, Cho IJ, Kim SG. Transactivation of the PPAR-responsive enhancer module in chemopreventive glutathioneS-transferase gene by the peroxisome proliferator-activated receptor-γ and retinoid X receptor heterodimer[J]. Cancer Res, 2004, 64(10):3701-3713.
[31]Yin XP, Chen ZY, Zhou J, et al. Mechanisms under-lying the perifocal neuroprotective effect of the Nrf2-ARE signaling pathway after intracranial hemorrhage[J]. Drug Des Dev Ther, 2015, 9:5973-5986.
[32]de Bittencourt Pasquali MA, de Ramos VM, Albanus RD, et al. Gene expression profile of NF-κB, Nrf2, glycoly-tic, and p53 pathways during the SH-SY5Y neuronal differentiation mediated by retinoic acid[J]. Mol Neurobiol, 2016, 53(1):423-435.
(责任编辑: 卢萍, 罗森)
Rosiglitazone pretreatment influences expression of PPARγ, Nrf2 and HO-1 in thrombin-activated microglia
HANG Hang, WANG Li-kun, WU Guo-feng, CHEN Xing-yu
(DepartmentofEmergencyMedicine,TheAffiliatedHospitalofGuizhouMedicalUniversity,Guiyang550004,China.E-mail:wuguofeng3013@sina.com)
AIM: To observe the effect of rosiglitazone (RGZ) pretreatment on the expression of peroxisome proliferator-activated receptor γ (PPARγ), nuclear factor E2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) in the microglia cells activated by thrombin. METHODS: Microglia cells were obtained from the brain tissues of the newborn rats and were primarily culturedinvitro. After cultured for 14 d, the microglia cells were used in the experiment. The isolated microglia cells were randomly divided into normal control group, thrombin stimulation group (TH group), rosiglitazone intervention group (RGZ+TH group) and retinoic acid intervention group (RA+TH group). The expression of PPARγ, Nrf2 and HO-1 was observed by immunocytochemistry, real-time PCR and Western blot.RESULTS: The number of positive staining cells of PPARγ, Nrf2 and HO-1 in TH group, RGZ+TH group and RA+TH group were increased remarkably as compared with control group. The significant increases in PPARγ, Nrf2 and HO-1 were observed in RGZ+TH group compared with other groups. The mRNA expression of PPARγ, Nrf2 and HO-1 in RGZ+TH group was increased significantly as compared with TH group, control group or RA+TH group (P<0.01), Besides, the mRNA expression of Nrf2 and HO-1 in RA+TH group was decreased as compared with TH group or RGZ+TH group (P<0.01). The protein levels of PPARγ, Nrf2 and HO-1 in RGZ+TH group were significantly increased as compared with TH group, control group or RA+TH group (P<0.01). The protein expression of Nrf2 and HO-1 in RA+TH group was decreased as compared with TH group or RGZ+TH group (P<0.01).CONCLUSION: Rosiglitazone pretreatment might increase the expression of PPARγ, Nrf2 and HO-1 in the microglia cells activated by thrombin. By inhibiting the expression of Nrf2 after RA pretreatment, the expression of the downstream geneHO-1 is also influenced. The anti-oxidative stress effects of rosiglitazone might be achieved partly by modulating Nrf2 to control the downstream geneHO-1.
Microglia; Cerebral hemorrhage; Rosiglitazone; Peroxisome proliferator-activated receptor γ; Nuclear factor E2-related factor 2; Heme oxygenase-1
1000- 4718(2016)04- 0671- 09
2015- 11- 03
2016- 01- 28
国家自然科学基金资助项目(No. 81460185/H09106);贵州省科技基金资助项目(No. 2013-2043)
Tel: 0851-86752545; E-mail: wuguofeng3013@sina.com
R459.7
A
10.3969/j.issn.1000- 4718.2016.04.015
杂志网址: http://www.cjpp.net

