Changes in Electroencephalogram Approximate Entropy Reflect Auditory Processing and Functional Complexity in Frogs
Yansu LIU, Yanzhu FAN, Fei XUE, Xizi YUE, Steven E. BRAUTH, Yezhong TANGand Guangzhan FANG,*
1Sichuan Nursing Vocational College. No.173, Longdu Nan Road, Longquan district, Chengdu, Sichuan, China
2College of Life Science, Northwest Normal University. No. 967 Anning East Road, Lanzhou, Gansu, China
3Chengdu Institute of Biology, Chinese Academy of Sciences. No. 9 Section 4, Renmin Nan Road, Chengdu, Sichuan,China
4Department of Psychology, University of Maryland, College Park MD 20742, USA
Changes in Electroencephalogram Approximate Entropy Reflect Auditory Processing and Functional Complexity in Frogs
Yansu LIU1, Yanzhu FAN2, Fei XUE3, Xizi YUE3, Steven E. BRAUTH4, Yezhong TANG3and Guangzhan FANG3,*
1Sichuan Nursing Vocational College. No.173, Longdu Nan Road, Longquan district, Chengdu, Sichuan, China
2College of Life Science, Northwest Normal University. No. 967 Anning East Road, Lanzhou, Gansu, China
3Chengdu Institute of Biology, Chinese Academy of Sciences. No. 9 Section 4, Renmin Nan Road, Chengdu, Sichuan,China
4Department of Psychology, University of Maryland, College Park MD 20742, USA
Brain systems engage in what are generally considered to be among the most complex forms of information processing. In the present study, we investigated the functional complexity of anuran auditory processing using the approximate entropy (ApEn) protocol for electroencephalogram (EEG) recordings from the forebrain and midbrain while male and female music frogs (Babina daunchina) listened to acoustic stimuli whose biological significance varied. The stimuli used were synthesized white noise (reflecting a novel signal), conspecific male advertisement calls with either high or low sexual attractiveness (reflecting sexual selection) and silence (reflecting a baseline). The results showed that 1) ApEn evoked by conspecific calls exceeded ApEn evoked by synthesized white noise in the left mesencephalon indicating this structure plays a critical role in processing acoustic signals with biological significance;2) ApEn in the mesencephalon was significantly higher than for the telencephalon, consistent with the fact that the anuran midbrain contains a large well-organized auditory nucleus (torus semicircularis) while the forebrain does not; 3)for females ApEn in the mesencephalon was significantly different than that of males, suggesting that males and females process biological stimuli related to mate choice differently.
electroencephalogram (EEG), approximate entropy (ApEn), complexity,advertisement call, frog
1. Introduction
The vertebrate brain is one of the most complex systems or network assemblies in the natural world, within which temporally and spatially multiscale structures provide the biophysical basis of perception and cognition(Sporns, 2002; Bassett and Gazzaniga, 2011). High brain complexity presumably evolved to enable organisms to solve difficult problems such as those related to sexual selection. For many species, decision processes related to reproduction involve the most sophisticated processingthey are capable of exceeding the complexity of other decision processes such as hunting prey or predator avoidance. It seems logical to propose therefore that the complexity of the underlying neural network activity will be dynamic, reflecting the demands of information processing. One way to monitor dynamic changes in processing in real time is to use the electroencephalogram(EEG).
Most frogs and songbirds vocalize for mate choices,which provides a chance to compare the dynamics of brain complexity under auditory stimulations with sounds of either biological significance or not. In frogs, malemale competition and female choice, the two main forces driving sexual selection, involve vocal communication(Andersson, 1994). Male frogs can maximize fitness by adjusting vocally competitive strategies to matchfemale preferences (Cotton et al., 2006), at the same time, avoiding the interference of other males’ calls(Wells and Schwartz, 2007). For this reason female frogs can use auditory cues to choose mates on the basis of genetic quality and resources offered to the females or her offspring (Moore and Moore, 1988). Previous studies on animals provide considerable information about the strategies used by males to compete (Patricelli et al.,2002; Cotton et al., 2006; Wells and Schwartz, 2007;Byrne, 2008; Fang et al., 2014a; Jiang et al., 2015) and females to choose mates (Janetos, 1980; Parker, 1983;Wittenberger, 1983; Gibson and Langen, 1996; Alexander et al., 1997; Bateson and Healy, 2005; Kirkpatrick et al., 2006; Leonard and Hedrick, 2009), however, we still know little about the neural underpinnings of sexual selection.
Virtually all neural processes which occur in single neurons, neural circuits or brain areas are nonlinear(Babloyantz, 1986; Jeong, 2004; Stam, 2005). Therefore,many non-linear dynamics protocols have been successfully employed for EEG time-series analyses in order to identify subtle changes in neural dynamics and providing novel interpretations of complex neural functions that could not otherwise be obtained using linear analysis (Babloyantz, 1986; Stam, 2005). Among them, approximate entropy (ApEn) is particularly useful for short, noisy time series because it is capable of providing a robust, model-independent, informationtheoretic estimation of dynamical complexity (Pincus,1991; Pincus, 1995a). Previous studies have shown that EEG-based ApEn can be a sensitive discriminator of various neurophysiological states or different cognitive conditions (Lippé et al., 2009; Talebi et al., 2012; Zarjam et al., 2013). Importantly, EEG entropy increases with information processing demands in visual recognition(Hogan et al., 2012), arithmetic (Zarjam et al., 2013) and acoustic cognitive (Sohn et al., 2007) tasks.
In the present study, changes in ApEn were computed based on EEG to investigate the neural dynamics related to sexual selection in an anuran species, the Emei music frog, Babina daunchina. Babina males produce two types of advertisement calls during breeding seasons:calling within the males’ nest burrows (inside call) and outside the burrows (outside call) (Cui et al., 2012). Females prefer behaviorally the inside calls and males preferentially compete vocally in response to inside calls (Cui et al., 2012; Fang et al., 2014a). Furthermore,different types of conspecific vocalizations but not reproductive status can alter the components of the auditory event-related potential (ERP) (Fang et al., 2015). In anurans the midbrain more than the telencephalon plays a crucial role in the integration of visual and auditory information for signal recognition and localization(Wilczynski and Endepols, 2007). In music frogs sex differences have been found for both behaviors and EEG responses evoked by biologically relevant acoustic stimuli (Cui et al., 2012; Fang et al., 2014a; Yang et al.,2014). Thus we predicted that (1) in general, the EEG ApEn index would be higher in the midbrain than in the forebrain; (2) EEG complexity would not be modulated by reproductive status; and (3) high ApEn would result when the frogs listened to conspecific calls including both inside and outside calls compared to other sounds during the breeding season, which would also exhibit a sexually dimorphic pattern. To test our predictions,we recorded multi-channel electrocorticogram signals from B. daunchina in both the reproductive and nonreproductive stages in response to silence, a white noise(WN) stimulus and the two conspecific calls. Signal processing techniques were used to evaluate EEG dynamical complexity responses to these acoustic stimuli in the telencephalon and mesencephalon.
2. Materials and Methods
2.1 Animals Twenty-four adult frogs (12 males and 12 females) captured from the Emei mountain area of Sichuan, China were used. The subjects were separated by sex and housed in four opaque plastic tanks (45 cm ×35 cm and 30 cm deep, six individuals in each tank)containing mud and water and maintained on a 12:12 light-dark cycle (lights on at 08:00). The tanks were placed in a room under controlled temperature conditions(23 ± 1 °C). The animals were fed fresh live crickets every three days. Mean mass was 10.3 ± 2.2 g (mean ± SD) and length was 4.6 ± 0.3 cm at the time of surgery.
2.2 Surgery All experiments were conducted after September when the reproductive season ended for the frogs. Surgical procedures are described in detail in our previous studies (Fang et al., 2011; Fang et al.,2012a; Fang et al., 2012b; Fang et al., 2014b; Yang et al., 2014). In short, sterile surgery was performed under deep anesthesia induced by intraperitoneal pentobarbital sodium (3 mg/100g). Then, four cortical EEG electrodes,composed of miniature stainless steel screws (φ 0.8 mm),were implanted on the frog skull: the left and right sides of the telencephalon and mesencephalon (R1, R2, R3 and R4), and referenced to the electrode (P) above the cerebellum (Figure 1). Each frog was housed singly for 2 days for recovery before the following experimentswere performed. After the end of the experiments, all frogs were euthanized by overdose of intraperitoneal pentobarbital sodium and electrode localizations were confirmed by injecting hematoxylin dye through the skull holes in which the electrodes were installed previously. All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
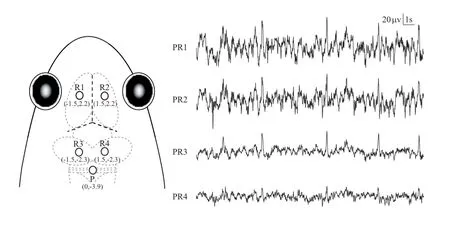
Figure 1 Electrode placements and 20 s of typical EEG tracings for each channel. The intersection of the three dashed lines in bold in the frog head denotes the intersection of suture lines corresponding to lambda.
2.3 Acoustic stimuli Four stimuli were used in the current study: a male call of high sexual attractiveness(HSA, i.e. inside nest), a male call of low sexual attractiveness (LSA, i.e. outside nest), white noise (WN)and silence (Figure 2). The conspecific calls used as playback calls were subject to the following criteria: 1)both HSA and LSA exemplars were recorded from the same individual, both contained five notes and 2) the temporal and frequency parameters of the calls were close to the population averages. Moreover, the call stimuli were one of five pairs used in previous female phonotaxis and male competition studies. WN was constructed as a consecutive “call” and its duration was the average of durations of the HSA and LSA calls (about 1.2 s), with 7.5 ms rise and fall time sinusoidal periods. In addition,silence is also a usual form of acoustic ambience which animals encounter in their natural environment. When given the choice between ethologically relevant sounds(including silence) or other sounds (including music or WN), animals will often prefer the former (Newberry, 1995; Rickard et al., 2005). In other words, silence is also biologically relevant for animals; therefore, it was used as a stimulus in the present study.
2.4 Data acquisition The experiments were performed in a soundproof and electromagnetic shielded chamber in which the background noise was 23.0 ± 1.7 dB (mean ± SD) with an opaque plastic tank (80 cm × 60 cm and 55 cm deep) containing mud and water at the center. Lights and temperature in the chamber were maintained as in the home-cages. A video camera with infrared light source and motion detector was appended centrally about 1 m above the tank for monitoring the subject’s behavior from outside the chamber. In order to eliminate the effects of digestion on the results, the subject was not fed during the experimental period.
The procedure for data collection consisted of electrophysiological and behavioral recordings on four sequential days. On the first day the awake and free-moving subject was placed in the experimental tank and connected to the signal acquisition system(Chengyi RM6280C, Sichuan, China) for habituation. Neurophysiological and behavioral data were collected for the subject in the non-reproductive stage on the second day. The animal was then administered gonadotropin-releasing hormone (GnRH-A6, Sichuan,China; i.p. 1.25μg per frog) on the third day. On the fourth day neurophysiological and behavioral data were acquired for the frog in the reproductive stage. On thesecond and fourth days, the reproductive status of females was determined based on phonotaxis behavior (i.e.,approaching a speaker broadcasting male advertisement calls) and that of males was determined by recording call activity as described previously (Fang et al., 2011; Cui et al., 2012; Fang et al., 2012b; Fang et al., 2014a).
For playbacks, two portable field speakers (SME-AFS,Saul Mineroff Electronics, Elmont, NY, USA) were placed equidistantly from the opposite ends of the tank. The two speakers were set 1.7 m apart, outside of the tank with a rectangular hole (20 cm × 15 cm) at the lower-central wall area of each end. The four stimuli (silence, HSA call, LSA call and WN) were presented to the animals in a randomized sequence, in which each stimulation continued for 10 minutes with 4 s inter-stimulus intervals between each stimulus presentation, approximately equal to the mean of inter-call intervals of B. daunchina (Cui et al., 2012). Each 10 minute stimulation period except for the silence stimulus followed by an hour silent period,and all stimuli except for silence were presented through the two speakers simultaneously at 65 dB SPL (re 20 µPa,C-weighting, fast response; Aihua, AWA6291; Hangzhou,China) measured at the center of the tank, approximately equal to the mean of the natural sound pressure level of male calls (Chen et al., 2011). Under these conditions,the sound level distribution at the bottom of the bank was close to a quasi-free sound field. A trigger pulse was sent to the signal acquisition system at onset of each stimulus type through the parallel port.
B. daunchina remains active throughout the day except for the periods around noon and midnight (Fang et al., 2012a; Yang et al., 2014), with the highest level of activity occurring at dawn and dusk. For this reason the experiments began at 20:30 on the second and fourth days. In this way, the subjects remained awake and motionless at one corner of the tank throughout the experiments, yet produced few movements which could cause EEG artifacts. The signal acquisition system was set to record continuously from the start of the experiments to the end. Bandpass filters, set to 0.16-100 Hz, were used for EEG signals with the notch filter of the amplifiers set to eliminate possible interference at 50 Hz. A sampling frequency of 1000 Hz was used.
2.5 Approximate entropy (ApEn) ApEn is a measure of irregularity or complexity of a dynamical system proposed by Pincus (Pincus, 1991), which is particularly effective for analyzing short and noisy time-series data and which can categorize a wide variety of systems ranging from multi periodic, stochastic to mixed systems(Pincus, 1995a; Pincus, 1995b). The procedure for estimating ApEn is described as follows:
For a given time series u(i), i=1,...,N frommeasurements equally in time, form a sequence of vectors that are defined according to
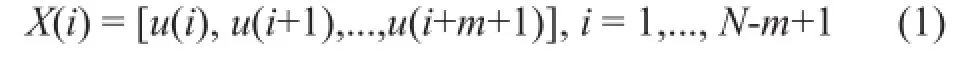
The distance between vectors X(i) and X(j) can be calculated with

For each i ≤ N-m+1, Nm(i) is the number of j in dimension m (the embedding dimension of phase space)such that d[X(i), X(j)] ≤ r, thenis defined as

where r is the tolerance (i.e. previous setting of minimal distance between vectors X(i) and X(j).
Next compute the natural logarithm of each) and average these over i

Then increase the embedding dimension, i.e. from m to m+1. Repeat steps (3)-(4) to obtain) and). Finally ApEn is computed by subtractingfromas

Mathematically, ApEn measures the likelihood that runs of patterns which are close for m observations remain close on the following incremental comparisons(Jaušovec and Jaušovec, 2010). Thus, ApEn is a nonnegative number calculated from a time series using the above protocols. Smaller values of ApEn imply a stronger regularity or persistence in the time series while larger values indicate greater fluctuation or irregularity. The parameter m should be set to 1 or 2 (m = 2 recommended)while r can range from 0.1 to 0.25 times the SD of the original data sequence (Pincus, 1991).
2.6 Data processing Because females usually make a choice in approximately 4 minutes (220 ± 134 s) in phonotaxis experiments, the first 5 minutes of EEG data were analyzed. After band-pass filtering (0.5-45 Hz)and downsampling at 256 Hz, r and N were determined by calculating ApEn (m, r, N) with increasing r from 0.1 to 0.4 SD in 0.05 steps and N from 100 to 2000 in steps of 100 for randomly selected EEG segments under the silence condition while m = 2. ApEn reached its maximum on a plateau when N = 500 and this plateau was stable only when r =0.15 SD. Therefore in the present study, ApEn for five minutes of EEG data was computed using a slide window of N = 500 (approximately 2 s of the EEG signal) overlapping N/2 for each step while r = 0.15 SD, to yield an ApEn vector.
Any epoch with an amplitude extremum beyond ±100 µv was discarded as artifact. The designation of artifact in one channel would result in the removal of data in all other channels in order to ensure the datasets of all channels were derived from the same time periods. Then,ApEn was averaged for the vector over 5 minutes for each stimulus, each channel and each subject based on artifactfree epochs. Because males usually respond vocally to rivals in seconds (Fang et al., 2014a), the first ApEn value(corresponding to about 2 seconds of the EEG signal)and the grand average were subjected to further statistical analyses.
2.7 Statistical analyses The normality of the distribution and homogeneity of variance for ApEn were estimated with the Shapiro-Wilk W test and Levene’s test,respectively. ApEn values were statistically analyzed using a four-way repeated measured ANOVA with the variables of “sex” (male/female), “reproductive status”(non-reproductive/ reproductive stage), “acoustic stimulus” (silence, WN, HSA and LSA) and “brain area”(R1, R2, R3, and R4). Both main effects and interactions were examined. Simple effects analysis was applied when the interaction was significant. For significant ANOVAs,data were further analyzed for multiple comparisons using the least-significant difference (LSD) test. Greenhouse-Geisser epsilon (ε) values were employed when the Greenhouse-Geisser correction was necessary. Estimations of effect size for ANOVAs were determined with partial η2(partial η2= 0.20 is a small effect size, 0.50 is a medium effect size and 0.80 is a large effect size)(Cohen, 1992). SPSS software (release 13.0) was utilized for the statistical analysis. A significance level of p <0.05 was used in all comparisons; p values > 0.05 and <0.1 were considered as marginally significant (Utts and Heckard, 2006).
3. Results
ApEn values computed for the short (2 seconds) and long (5 minutes) time scales for each sex, each acoustic stimulus and each brain area are shown in Figure 3. There were significant differences among sexes, stimuli and brain areas, respectively. High ApEn values were found in response to conspecific calls, for females and in the mesencephalon.
3.1 Significant differences for short time scale (2 seconds) ApEn changes The results of ANOVA revealedthat the main effect was significant for the factor “brain area” (F3,66= 10.478; p = 0.000 < 0.001, ε = 0.651, partial η2= 0.323) but not the factors “reproductive status” (F1,22= 0.048; p = 0.828 > 0.05), “sex” (F1,22= 2.723; p = 0.113 > 0.05) and “acoustic stimulus” (F3,66= 0.513; p = 0.675 > 0.05). The interactions between “brain area”and “sex” (F3,66= 3.403; p = 0.023 < 0.05, partial η2= 0.134) and between “brain area” and “acoustic stimulus”(F9,198= 1.988; p = 0.042 < 0.05, partial η2= 0.083) were significant and, therefore, simple effect analysis was further applied.
For males, there was no significant difference in ApEn between brain areas (Table 1). In contrast for females,ApEn in the telencephalon was significantly lower than that in the mesencephalon while ApEn in the left telencephalon was significantly lower than that in the right telencephalon (p < 0.05, Figure 3A and Table 1). In the mesencephalon, ApEn for females was marginally significantly higher than that for males, suggesting more complex neural processing dynamics for females in response to acoustic stimuli. ApEn in the telencephalon was significantly lower than that in the mesencephalon for all acoustic stimuli except WN, although differences between the telencephalon (each side) and the right mesencephalon did not reach statistical significance for the LSA stimulus (p < 0.05 or p < 0.001, Figure 3A and Table 1). For each sex, ApEn in the left mesencephalon for conspecific calls was higher than that with WN (p< 0.05, Figure 3A) although the difference between HSA calls and WN did not reach statistical significance,implying that the processing of conspecific calls may engender more complex EEG activities.
3.2 Significant differences for long time scale (5 minutes) ApEn changes The main effects were significant for the factors “brain area” (F3,66= 14.068; p= 0.000 < 0.001, ε = 0.535, partial η2= 0.390) and “sex”(F1,22= 5.891; p = 0.024 < 0.05, partial η2= 0.211) but not“reproductive status” (F1,22= 2.877; p = 0.104 > 0.05) and“acoustic stimulus” (F3,66= 0.167; p = 0.918 > 0.05). The interaction between “brain area” and “sex” was significant(F3,66= 5.449; p = 0.002 < 0.05, partial η2= 0.199). Similar to the results for the short time scale, there were no significant differences between brain areas for males(Table 1). For females, ApEn in the telencephalon was significantly lower than that for the mesencephalon while ApEn in the left telencephalon was significantly lower than that in the right telencephalon (p < 0.05, Figure 3B and Table 1). In the mesencephalon, ApEn for females was significantly higher than that of males, consistent with the idea that the acoustic stimuli engender more complex neural dynamics for females.

Table 1 Results of simple effect analysis for ApEn as a function of the factors “sex” and “brain area” for both the 2-s and 5-min segments and the factors “acoustic stimulus” and “brain area” for the 2-s segment.

Figure 3 Means and standard deviations of ApEn for 2-s (A) and 5-min (B) segments respectively. Filled and open stars denote that there were significant differences between the corresponding conditions (P < 0.05 and P < 0.001 respectively), while triangles denote marginally significant differences. Note that the graphs are based on the average over reproductive stages because the main effect for the factor“reproductive status” was not significant for each time segment. LT and RT, the left and right telencephalon; LM and RM, the left and right mesencephalon; M and F, male and female; Sil, HSA, LSA and WN, the four stimuli, i.e. silence, high sexual attractiveness call, low sexual attractiveness call and white noise.
4. Discussion
The present study showed that when four stimuli,consisting of silence, WN, HSA and LSA calls, were presented, 1) ApEn evoked by the conspecific calls was higher than that evoked by the synthesized WN stimulus in the left mesencephalon; 2) ApEn in the mesencephalon was significantly higher than that in the telencephalon,especially for females; 3) ApEn for females wassignificantly different from males. These results are consistent with the hypothesis that the neural processing complexity in the auditory system is dynamic insofar as it is modulated by sex, brain area and the biological significance of acoustic signals.
4.1 Neural processing complexity is modulated by biological significance EEG results from the summed postsynaptic activity of a large number of interacting cortical neurons or neuronal assemblies that spatially distributed while functionally synergized (Anokhin et al.,2006). Accordingly, EEG complexity might reflect the interaction states of a system, in which higher complexity is related to a larger number of separable oscillatory networks (Tononi and Edelman, 1998).
The present results show that ApEn evoked by the conspecific calls is higher than that evoked by WN in the left mesencephalon. Conspecific calls exhibit complex acoustic characteristics which encode information concerning species and individual identity (Fang et al.,2015). In contrast WN has little acoustic structure and is generally perceived by animals as signaling dangerous events (Haff and Magrath, 2010). The perception of conspecific calls therefore involves extracting information about the identity of conspecifics in order to respond appropriately for male competition and mate choice. It seems logical to expect, therefore that increased processing and/or more neural networks would be involved in the perception of conspecific calls compared to WN, and that such increased processing demands would increase ApEn. The present results support this interpretation.
Our results are also consistent with the idea that patterns of brain activity become more intricate during cognition and perception (McIntosh, 1999; Bressler and Kelso, 2001; Varela et al., 2001; Sporns, 2002). For example, measures of the correlation dimension or related complexity increase with the difficulty of cognitive tasks(Bizas et al., 1999; Tomberg, 1999; Micheloyannis et al.,2002). Notably, EEG entropy increases with information processing demands during tasks involving visual recognition (Hogan et al., 2012), arithmetic (Zarjam et al., 2013) and acoustic cognition (Sohn et al., 2007),respectively. Higher values of entropy are believed to arise due to increased neuronal coupling and enhanced connectivity during cognitive processing (Inouye et al.,1991; Inouye et al., 1993).
4.2 Sex differences in EEG complexity The present results showed that ApEn in the anuran mesencephalon for females was significantly higher than that for males. This difference may reflect sex differences in reproductive strategy during the breeding season. Males typically vocalize to compete for territory and mates while females choose potential mates based on assessment of males’sexual displays. In humans ApEn is higher in females reflecting sexual dimorphism in the parieto-occipital area (Jaušovec and Jaušovec, 2010). The measure of dimensional complexity (like ApEn) reflects the complexity of neural generators, i.e. the relative number of concurrently oscillating neuronal assemblies and degrees of freedom in the competitive interaction between them (Anokhin et al., 1999), from which dynamical patterns generated by brain networks emerge which underlie all cognitive and perceptual processes (Sporns,2002). Thus it is clear that for some forms of cognitive processes males and females may differ in local and long range information coding as well as in the excitability dynamics of cortical networks (Jaušovec and Jaušovec,2010).
The structure of brain networks results from epigenetic processes during development which come about not only because of the effects of embryonic neuronal activity but also reflect the adaptive nature of underlying genetic programs which have resulted from natural selection (Sporns, 2002). For this reason it is important to take ultimate causation (i.e. the selection pressures)into account when considering brain functioning(Koch and Laurent, 1999). Pertinent to this point it is well known that sexual selection is a co-evolutionary process between males and females (Cotton et al.,2006). Compared to males, however, females experience stronger selective pressure because females bear more of the cost or investment in reproduction (i.e. greater minimal parental investments). Therefore females who respond to the signals of inferior conspecific males or males of other species will usually waste time, gametes,or produce less viable or less fit offspring (Gerhardt and Bee, 2007). Moreover, moving around to find and assess different males is costly in terms of time and predation risks (Shine, 1980; Ryser, 1989; Grafe, 1997). For these reasons stronger selective pressures usually act on the perceptual systems of females enhancing the ability of females to identify biologically significant differences in conspecific signals.
The situation is even more pronounced in music frogs because males vocalize almost entirely from within hidden mud retuse nests within which amplexus occur. Thus it is the female who must identify the locations and qualities of potential mates. Given these selective pressures, it is reasonable to infer that auditoryinformation processing demands would be greater in females giving rise to greater complexity of the underlying neural networks. Sporns et al. (2000) have pointed out that brain complexity reflects the capacity of neural circuitry to support functional dynamics integrating specialized elements allowing perceptual, cognitive and behavioral states to simultaneously utilize multiple sensory modalities and submodalities.
4.3 Importance of the midbrain in anuran vocal communication The anuran midbrain is relatively well developed, however no direct counterparts to the specific sensory telencephalic areas of amniotes have been identified in the anuran forebrain. The main component of the anuran auditory midbrain is the nucleus torus semicircularis (TS), a large structurally complex nucleus which integrates ascending and descending neural signals essential for processing auditory information related to vocalization (Luksch and Walkowiak, 1998). The TS also has widespread connections linked to motor,endocrine, and a variety of limbic structures (Wilczynski and Endepols, 2007). In this study greater functional brain complexity in females was observed only in the mesencephalon, consistent with the idea that the anuran midbrain plays a key role in mate choice. In fact, the auditory midbrain is the first nucleus within the anuran auditory system to exhibit complex feature detection based on temporal and spectral sound attributes (Narins et al., 2007).
It is likely that information concerning the “what” or sound pattern features of auditory objects and the “where”or location of the sound source are integrated at the level of the TS (Melssen and Epping, 1990; van Stokkum and Meissen, 1991; Gooler et al., 1993; Xu et al., 1994;Gooler et al., 1996; Xu et al., 1996; Zhang et al., 1999;Lin and Feng, 2001, 2003). While lesions of the striatum and superficial and deep thalamic structures may cause disorders in vocal recognition, lesions in TS severely impair phonotactic performance (Endepols et al., 2003),suggesting TS is a key decision point in call recognition. In addition, the TS encodes sound localization for important auditory signals (Rose and Gooler, 2007). Consequently, it may be that purely sensory processes such as stimulus recognition and localization are mostly complete at the level of the auditory midbrain(Wilczynski and Endepols, 2007). Furthermore, the left auditory midbrain receives predominantly contralateral projections from the right ear through which frogs detect preferentially conspecific calls and some neutral sounds but not negative sounds (Fang et al., 2014b; Xue et al.,2015). Such right ear advantage could account for the findings that significant ApEn differences for the acoustic stimuli used in this study were only observed in the left mesencephalon and that ApEn was greater in the left compared to the right mesencephalon although this difference did not reach statistical significance.
The dynamics of EEG complexity as measured by ApEn were not affected by reproductive status in the present study. Although this result may seem surprising in view of the fact that frogs in the reproductive stage would be expected to be more motivated than those in the non-reproductive stage for processing information related to reproduction, it is in agreement with a previous study on ERP showing that auditory ERP components are also not modulated by reproductive status (Fang et al.,2015). Since the complexity of cortical dynamics arises from integrating the activities of functionally segregated neuronal groups linking incoming stimuli with ongoing activity (Sohn et al., 2010), higher cognitive performance may not be a linear function of entropy level, but rather a combination of level and range, or differentiated range of entropy states across the brain (Hogan et al., 2012). In other words the complexity of brain dynamics depends more on how external stimuli activate inherent circuit properties rather than global internal states. Furthermore,variations in brain sizes are obvious among anuran species (Liao et al., 2015), which may reflect the natural selection on cognitive and behavioral demands (Striedter,2005). Hence, further study of EEG ApEn across species would shed light on evolutionary pathways of neural mechanisms for cognitive behaviors, such as mate choice and predation avoidance.
Acknowledgements The authors gratefully acknowledge all the members of the Behavioral Neuroscience Group for their discussion and help for the experiments reported here. This work was supported by the grants from the National Natural Science Foundation of China (No. 31372217 and No. 31672305) to Guangzhan Fang. Animal procedures were approved by the Animal Care and Use Committee of the Chengdu Institute of Biology.
References
Alexander R. D., Marshall D. C., Cooley J. R. 1997. Evolutionary perspectives on insect mating. In J. C. Choe and B. J. Crespi(Eds.), The evolution of mating systems in insects and arachnids. Cambridge (UK): Cambridge University Press, p 4-31
Andersson M. B. 1994. Sexual selection. Princeton, NJ: Princeton University Press
Anokhin A. P., Lutzenberger W., Birbaumer N. 1999. Spatiotemporal organization of brain dynamics and intelligence:an EEG study in adolescents. Int J Psychophysiol, 33: 259-273.
Anokhin A. P., Müller V., Lindenberger U., Heath A. C., Myers E. 2006. Genetic influences on dynamic complexity of brain oscillations. Neurosci Lett, 397: 93-98
Babloyantz A. 1986. Evidence of chaotic dynamics of brain activity during the sleep cycle, Dimensions and entropies in chaotic systems: Springer, p 241-245
Bassett D. S., Gazzaniga M. S. 2011. Understanding complexity in the human brain. Trends Cogn Sci, 15: 200-209
Bateson M., Healy S. D. 2005. Comparative evaluation and its implications for mate choice. Trends Ecol Evol, 20: 659-664.
Bizas E., Simos P., Stam C., Arvanitis S., Terzakis D.,Micheloyannis S. 1999. EEG correlates of cerebral engagement in reading tasks. Brain Topogr, 12: 99-105
Bressler S. L., Kelso J. S. 2001. Cortical coordination dynamics and cognition. Trends Cogn Sci, 5: 26-36
Byrne P. G. 2008. Strategic male calling behavior in an Australian terrestrial toadlet (Pseudophryne bibronii). Copeia, 2008: 57-63
Chen Q., Cui J. G., Fang G. Z., Brauth S. E., Tang Y. Z. 2011. Acoustic Analysis of the Advertisement Calls of the Music Frog,Babina daunchina. J Herpetol, 45: 406-416
Cohen J. 1992. A power primer. Psychol Bull, 112: 155-159
Cotton S., Small J., Pomiankowski A. 2006. Sexual selection and condition-dependent mate preferences. Curr Biol, 16: R755-765
Cui J. G., Tang Y. Z., Narins P. M. 2012. Real estate ads in Emei music frog vocalizations: female preference for calls emanating from burrows. Biol Lett, 8: 337-340
Endepols H., Feng A. S., Gerhardt H. C., Schul J., Walkowiak W. 2003. Roles of the auditory midbrain and thalamus in selective phonotaxis in female gray treefrogs (Hyla versicolor). Behav Brain Res, 145: 63-77
Fang G. Z., Chen Q., Cui J. G., Tang Y. Z. 2012a. Electroencephalogram bands modulated by vigilance states in an anuran species: A factor analytic approach. J Comp Physiol A,198: 119-127
Fang G. Z., Cui J. G., Chen Q., Yang P., Song J., Tang Y. Z. 2011. Changes in electroencephalographic power spectra associated with reproductive status in frog. Adv Neu Net-ISNN,2011: 139-147
Fang G. Z., Jiang F., Yang P., Cui J. G., Brauth S. E., Tang Y. Z. 2014a. Male vocal competition is dynamic and strongly affected by social contexts in music frogs. Anim Cogn, 17: 483-494.
Fang G. Z., Xue F., Yang P., Cui J. G., Brauth S. E., Tang Y. Z. 2014b. Right ear advantage for vocal communication in frogs results from both structural asymmetry and attention modulation. Behav Brain Res, 266: 77-84
Fang G. Z., Yang P., Cui J. G., Yao D. Z., Brauth S. E., Tang Y. Z. 2012b. Mating signals indicating sexual receptiveness induce unique spatio-temporal EEG theta patterns in an anuran species. PLoS One, 7: e52364
Fang G. Z., Yang P., Xue F., Cui J. G., Brauth S. E., Tang Y. Z. 2015. Sound classification and call discrimination are decoded in order as revealed by event-related potential components in frogs. Brain Behav Evol, 86: 232-245
Gerhardt H. C., Bee M. A. 2007. Recognition and localization of acoustic signals. In P. M. Narins, A. S. Feng, R. R. Fay and A. N. Popper (Eds.), Hearing and sound communication in amphibians. New York: Springer, p 113-146
Gibson R. M., Langen T. A. 1996. How do animals choose their mates? Trends Ecol Evol, 11: 468-470
Gooler D. M., Condon C. J., Xu J., Feng A. S. 1993. Sound direction influences the frequency-tuning characteristics of neurons in the frog inferior colliculus. J Neurophysiol, 69:1018-1030
Gooler D. M., Xu J., Feng A. S. 1996. Binaural inhibition is important in shaping the free-field frequency selectivity of single neurons in the inferior colliculus. J Neurophysiol, 76: 2580-2594
Grafe T. U. 1997. Costs and benefits of mate choice in the lekbreeding reed frog, Hyperolius marmoratus. Anim Behav, 53:1103-1117
Haff T., Magrath R. 2010. Vulnerable but not helpless: nestlings are fine-tuned to cues of approaching danger. Anim Behav, 79:487-496
Hogan M. J., Kilmartin L., Keane M., Collins P., Staff R. T.,Kaiser J., Lai R., Upton N. 2012. Electrophysiological entropy in younger adults, older controls and older cognitively declined adults. Brain Res, 1445: 1-10.
Inouye T., Shinosaki K., Iyama A., Matsumoto Y. 1993. Localization of activated areas and directional EEG patterns during mental arithmetic. Electroencephalogr Clin Neurophysiol,86: 224-230
Inouye T., Shinosaki K., Sakamoto H., Toi S., Ukai S., Iyama A.,Katsuda Y., Hirano M. 1991. Quantification of EEG irregularity by use of the entropy of the power spectrum. Electroencephalogr Clin Neurophysiol, 79: 204-210
Janetos A. C. 1980. Strategies of female mate choice: a theoretical analysis. Behav Ecol Sociobiol, 7: 107-112
Jaušovec N., Jaušovec K. 2010. Resting brain activity: differences between genders. Neuropsychologia, 48: 3918-3925
Jeong J. 2004. EEG dynamics in patients with Alzheimer’s disease. Clin Neurophysiol, 115: 1490-1505.
Jiang F., Fang G. Z., Xue F., Cui J. G., Brauth S. E., Tang Y. Z. 2015. Male music frogs compete vocally on the basis of temporal sequence rather than spatial cues of rival calls. Asian Herpetol Res, 6: 305-316
Kirkpatrick M., Rand A. S., Ryan M. J. 2006. Mate choice rules in animals. Anim Behav, 71: 1215-1225
Koch C., Laurent G. 1999. Complexity and the nervous system. Science, 284: 96-98
Leonard A. S., Hedrick A. V. 2009. Male and female crickets use different decision rules in response to mating signals. Behav Ecol, 20: 1175-1184
Liao W., Lou S., Zeng Y., Merilä J. 2015. Evolution of anuran brains: disentangling ecological and phylogenetic sources of variation. J Evol Biol, 28: 1986-1996
Lin W. Y., Feng A. S. 2001. Free-field unmasking response characteristics of frog auditory nerve fibers: comparison with the responses of midbrain auditory neurons. J Comp Physiol A, 187:699-712
Lin W. Y., Feng A. S. 2003. GABA is involved in spatial unmasking in the frog auditory midbrain. J Neurosci, 23: 8143-8151
Lippé S., Kovacevic N., McIntosh A. R. 2009. Differential maturation of brain signal complexity in the human auditory and visual system. Front Hum Neurosci, 3: 48
Luksch H., Walkowiak W. 1998. Morphology and axonal projection patterns of auditory neurons in the midbrain of thepainted frog, Discoglossus pictus. Hear Res, 122: 1-17
McIntosh A. R. 1999. Mapping cognition to the brain through neural interactions. Memory, 7: 523-548
Melssen W. J., Epping W. J. 1990. A combined sensitivity for frequency and interaural intensity difference in neurons in the auditory midbrain of the grassfrog. Hear Res, 44: 35-49.
Micheloyannis S., Papanikolaou E., Bizas E., Stam C. J., Simos P. G. 2002. Ongoing electroencephalographic signal study of simple arithmetic using linear and non-linear measures. Int J Psychophysiol, 44: 231-238
Moore A. J., Moore P. J. 1988. Female strategy during mate choice: threshold assessment. Evolution, 42: 387-391
Narins P. M., Feng A. S., Fay R. R., Popper A. N. 2007. Hearing and sound communication in amphibians. New York: Springer Verlag
Newberry R. C. 1995. Environmental enrichment: increasing the biological relevance of captive environments. Appl Anim Behav Sci, 44: 229-243
Parker G. 1983. Mate quality and mating decisions. In P. Bateson(ed.), Mate choice. Cambridge (UK): Cambridge University Press, p 141-164
Patricelli G. L., Uy J. A. C., Walsh G., Borgia G. 2002. Sexual selection: male displays adjusted to female’s response. Nature,415: 279-280
Pincus S. 1995a. Approximate entropy (ApEn) as a complexity measure. Chaos, 5: 110-117
Pincus S. M. 1991. Approximate entropy as a measure of system complexity. Proc Nat Acad Sci USA, 88: 2297-2301
Pincus S. M. 1995b. Quantifying Complexity and Regularity of Neurobiological Systems. Methods Neurosci, 28: 336-363
Rickard N. S., Toukhsati S. R., Field S. E. 2005. The effect of music on cognitive performance: Insight from neurobiological and animal studies. Behav Cogn Neurosci Rev, 4: 235-261
Rose G. J., Gooler D. M. 2007. Function of the amphibian central auditory system. In P. M. Narins, A. S. Feng, R. R. Fay and A. N. Popper (Eds.), Hearing and Sound Communication in Amphibians. New York: Springer, p 250-290.
Ryser J. 1989. Weight loss, reproductive output, and the cost of reproduction in the common frog, Rana temporaria. Oecologia,78: 264-268
Shine R. 1980. “Costs” of reproduction in reptiles. Oecologia, 46:92-100
Sohn H., Kim I., Lee W., Peterson B. S., Hong H., Chae J.-H.,Hong S., Jeong J. 2010. Linear and non-linear EEG analysis of adolescents with attention-deficit/hyperactivity disorder during a cognitive task. Clin Neurophysiol, 121: 1863-1870
Sohn H., Lee W., Kim I., Jeong J. 2007. Approximate entropy(ApEn) analysis of the EEG in attention-deficit/hyperactivity disorder (AD/HD) during cognitive tasks. In: World Congress on Medical Physics and Biomedical Engineering 2006. p 1083-1086
Sporns O. 2002. Network analysis, complexity, and brain function. Complexity, 8: 56-60
Sporns O., Tononi G., Edelman G. M. 2000. Connectivity and complexity: the relationship between neuroanatomy and brain dynamics. Neural Networks, 13: 909-922
Stam C. J. 2005. Nonlinear dynamical analysis of EEG and MEG:review of an emerging field. Clin Neurophysiol, 116: 2266-2301
Striedter G. F. 2005. Principles of Brain Evolution. Sunderland,MA: Sinauer Associates Inc
Talebi N., Nasrabadi A. M., Curran T. 2012. Investigation of changes in EEG complexity during memory retrieval: the effect of midazolam. Cogn Neurodynamics, 6: 537-546
Tomberg C. 1999. Focal enhancement of chaotic strange attractor dimension in the left semantic (Wernicke) human cortex during reading without concomitant change in vigilance level. Neurosci Lett, 263: 177-180
Tononi G., Edelman G. M. 1998. Consciousness and complexity. Science, 282: 1846-1851
Utts J. M., Heckard R. F. 2006. Statistical ideas and methods. Belmont, CA: Thompson Higher Education
van Stokkum I. H., Meissen W. J. 1991. Measuring and modelling the response of auditory midbrain neurons in the grassfrog to temporally structured binaural stimuli. Hear Res, 52: 113-132
Varela F., Lachaux J.-P., Rodriguez E., Martinerie J. 2001. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci, 2: 229-239
Wells K., Schwartz J. 2007. The behavioral ecology of anuran communication. In P. M. Narins, A. S. Feng, R. R. Fay and A. N. Popper (Eds.), Hearing and Sound Communication in Amphibians. New York: Springer Verlag, p 44-86
Wilczynski W., Endepols H. 2007. Central auditory pathways in anuran amphibians: the anatomical basis of hearing and sound communication. In P. M. Narins, A. S. Feng, R. R. Fay and A. N. Popper (Eds.), Hearing and sound communication in amphibians. New York: Springer, p 221-249
Wittenberger J. F. 1983. Tactics of mate choice. In P. Bateman(ed.), Mate choice. Cambridge: Cambridge University Press, p 435-447
Xu J., Gooler D., Feng A. 1996. Effects of sound direction on the processing of amplitude-modulated signals in the frog inferior colliculus. J Comp Physiol A, 178: 435-445
Xu J., Gooler D. M., Feng A. S. 1994. Single neurons in the frog inferior colliculus exhibit direction-dependent frequency selectivity to isointensity tone bursts. J Acoust Soc Am, 95:2160--2170
Xue F., Fang G. Z., Yang P., Zhao E., Brauth S. E., Tang Y. Z. 2015. The biological significance of acoustic stimuli determines ear preference in the music frog. J Exp Biol, 218: 740-747
Yang P., Fang G. Z., Xue F., Cui J. G., Brauth S. E., Tang Y. Z. 2014. Electroencephalographic signals synchronize with behaviors and are sexually dimorphic during the light-dark cycle in reproductive frogs. J Comp Physiol A, 200: 117-127
Zarjam P., Epps J., Chen F., Lovell N. H. 2013. Estimating cognitive workload using wavelet entropy-based features during an arithmetic task. Comput Biol Med, 43: 2186-2195
Zhang H., Xu J., Feng A. 1999. Effects of GABA-mediated inhibition on direction-dependent frequency tuning in the frog inferior colliculus. J. Comp Physiol A, 184: 85-98
10.16373/j.cnki.ahr.160006
Dr. Guangzhan FANG, from Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, China, with his research focusing on neurobiology and cognition of amphibians.
E-mail: fanggz@cib.ac.cn
27 January 2016 Accepted: 16 August 2016
 Asian Herpetological Research2016年3期
Asian Herpetological Research2016年3期
- Asian Herpetological Research的其它文章
- A New Species of the Genus Rhacophorus (Anura: Rhacophoridae)from Southern China
- Amphibians and Reptiles of Cebu, Philippines: The Poorly Understood Herpetofauna of an Island with Very Little Remaining Natural Habitat
- The Effect of Speed on the Hindlimb Kinematics of the Reeves’Butterfly Lizard, Leiolepis reevesii (Agamidae)
- The Impacts of Urbanization on the Distribution and Body Condition of the Rice-paddy Frog (Fejervarya multistriata) and Gold-striped Pond Frog (Pelophylax plancyi) in Shanghai, China
- The Impact of Phenotypic Characteristics on Thermoregulation in a Cold-Climate Agamid lizard, Phrynocephalus guinanensis
- Sexual Dimorphism of the Jilin Clawed Salamander,Onychodactylus zhangyapingi, (Urodela: Hynobiidae: Onychodactylinae) from Jilin Province, China
