A New Species of the Genus Rhacophorus (Anura: Rhacophoridae)from Southern China
Yunming MO, Weicai CHEN, Xiaowen LIAO and Shichu ZHOU
Natural History Museum of Guangxi, Nanning 530012, Guangxi, China
A New Species of the Genus Rhacophorus (Anura: Rhacophoridae)from Southern China
Yunming MO, Weicai CHEN*, Xiaowen LIAO and Shichu ZHOU
Natural History Museum of Guangxi, Nanning 530012, Guangxi, China
A new species of rhacophorid of the genus Rhacophorus is described from the Shiwandashan National Nature Reserve, Guangxi, Southern China. Rhacophorus pinglongensis sp. nov. is compared with congeners from China and other parts of Southeast Asia. The new species is distinguished from its congeners by combination of following characters: (1) small size (adult male, SVL 32.0-38.5 mm); (2) smooth and green dorsum; (3) flanks, axilla, ventral surface of forearms, inguinal, anterior and posterior surfaces of thighs, ventral surface of shank, and dorsal surface of feet covered with black blotches with white spots or white spots with a faint orange tint; (4) ventral surface of feet and webbing tangerine; (5) supratympanic fold weak; (6) outer margin of forearms and feet with low dermal ridges; (7)calcars absent on heels; (8) iris silver, diffusing to ecru laterally with light khaki ring along margin. The new species is closely related to R. dorsoviridis, R. moltrechti, and R. nigropunctatus based on adult morphology. Currently, this species is known only from mid-elevation montane evergreen forest in the Shiwandashan National Nature Reserve,Guangxi, China.
Tree frog, Rhacophorus pinglongensis sp. nov., Rhacophoridae, Southern China DOI: 10.16373/j.cnki.ahr.150070
1. Introduction
Rhacophoridae Hoffman, 1932 with approximately 393 species of frogs is one of the most diverse anuran families (Frost, 2015). The rhacophorid tree frogs are widely distributed across Japan, China, India, Indochina,Sundaland to Sulawesi and the Philippines (Streicher et al., 2014; Hamidy and Kurniati, 2015; Frost, 2015). Among rhacophorid tree frogs, the genus Rhacophorus Kuhl and Van Hasselt, 1822 currently contains approximately 88 species (Frost, 2015). Fei et al. (2012)suggested that twenty-seven species of Rhacophorus occur in China. In the last decade, some new tree frogs are continuously reported in China (Zhao et al., 2005;Rao et al., 2006; Mo et al., 2008; Li et al., 2012a).
Guangxi, locating at Southern China, has the particular geographic location and complicated environment and contains a rich diversity of amphibian. Mt. Shiwandashan,lying in the south of Guangxi, covers 2 600km2, mostof which are natural forest. During field works in the Shiwandashan National Nature Reserve, Guangxi,Southern China (Figure 1 A), from 2009 to 2012, we discovered a small species of tree frog, which are closely related to Rhacophorus dorsoviridis Bourret, 1937, R. moltrechti Boulenger, 1908 and R. nigropunctatus Liu,Hu, and Yang, 1962 based on morphology, but that differs morphologically from Chinese and all Southeast Asian members of Rhacophorus. Herein, we describe these tree frogs as new.
2. Material and Methods

Figure 1 (A) Localities of Rhacophorus pinglongensis sp. nov. (solid circle) and R. dorsoviridis (solid square); (B) Habitat of Rhacophorus pinglongensis sp. nov.
Specimens were deposited at the Natural History Museum of Guangxi. Morphological data were recorded from specimens fixed in 8% formalin and then stored in 70% ethanol. The following morphometric data were taken with digital calipers (to the nearest 0.1 mm): SVL (snoutvent length); HL (head length from tip of snout to rear of jaws); HW (head width at the commissure of the jaws); SNT (snout length from tip of snout to the anterior corner of eye); ED (diameter of the exposed portion of the eyeball); IOD (interorbital distance); TD (horizontal diameter of tympanum); UEW (upper eyelid width,greatest width of upper eyelids); TED (distance from anterior edge of tympanum to posterior corner of the eye);IN (internarial space); EN (distance from front of eye to nostril); TIB (tibia length with the hindlimb flexed);FLL (length of forelimb from tip of disk of finger III to axilla); FTD (maximal diameter of disc of third finger);HLL (length of hindlimb from tip of disk of fourth toe to groin); THL (thigh length, from vent to knee); HTD4(diameter of fourth toe tip, greatest diameter of disc on fourth toe). The webbing formula is given as proposed by Myers and Duellman (1982). Museum acronyms: NHMG for the Natural History Museum of Guangxi, Nanning,Guangxi Province, China; CIB for the Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu,China.
Advertisement calls of three males were recorded in the field using ICD recorder (Sony ICD-TX50). Calls were recorded at a distance of about 0.1-0.3 m, and ambient temperature was recorded using TP-2200 (A-volt). Call recordings were analyzed using Raven Pro 1.3 beta version (http://www.birds.cornell.edu/raven) with default settings.
To display genetic variation and preliminarily determine the generic placement of the new species,we sequenced this species and compared with other rhacophorid tree frogs in the genus Rhacophorus obtained from GenBank. Total genomic DNA was extracted from muscle tissues stored in 100% ethanol, using DNeasy tissue extraction kits (QIAGEN). The primers employed in this study followed Wilkinson et al. (2002) and amplified 12S and 16S fragments including the complete t-RNAvaline. We used Bio-Rad S1000 (USA) to amplify genes following the standard PCR protocols (5 min at 94°C, then 35 cycles at 94°C for 50s, 55°C for 50s, and 72°C for 50s, with a final 10 min extension at 72°C). PCR products were purified using QIAquick Gel Extraction Kit(QIAGEN) according to the manufacturer’s protocols and directly sequenced with an ABI 3730 automated DNA sequencer. New mitochondrial DNA fragments were submitted for BLAST search in GenBank to ensure that the required sequences had been amplified (Altschul et al.,1997). New sequences were deposited in GenBank under the accession numbers KU170683-4. P-distance pairwise sequences divergence between species using a ~530 bp mtDNA 16S fragment was calculated using MEGA version 5 (Tamura et al., 2011). Missing data and indels were removed using Gblocks (Castresana, 2000) with default settings. After removing poorly aligned regions,a 1607 bp matrix was used to reconstruct phylogenetic relationships, using maximum likelihood (ML) and Bayesian inference (BI). BI was implemented in MrBayes 3.1.2 (Ronquist and Huelsenbeck, 2003). Details for BI were given in Li et al. (2012b). ML analyses were performed on the RAxML Web server (http://phylobench. vital-it.ch/raxml-bb/) with 100 rapid bootstrap replicates(Stamatakis et al., 2008). Comparative mtDNA sequences examined in this study were listed in Appendix.
3. Results
Rhacophorus pinglongensis sp. nov.
Holotype NHMG200903002, adult male, from the Shiwandashan National Nature Reserve, Guangxi,China (22.457º N, 107.043º E; alt. 530 m). Collected by Yunming Mo and Shichu Zhou on 23 March 2009(Figure 2 A, B).
Paratypes NHMG200903001, NHMG200903003-004,adult male, from the same site as the holotype, collected by Yunming Mo and Shichu Zhou on 23 March 2009;NHMG201002003-008, NHMG201002010, 201002011,201002013, adult males, from the same site as theholotype, collected by Yunming Mo, Wei Zhang, and Shichu Zhou on 25 February 2010.

Figure 2 Dorsal(A), and lateral (B) views of the holotype NHMG200903002.
Diagnosis The new species is assigned to the genus Rhacophorus by Y-shaped distal end of terminal phalanx,the presence of intercalary cartilage between the terminal and penultimate phalanges of digits, webbed fingers, tips of digits expanded into large disks with circummarginal grooves, vomerine teeth, a supracloacal dermal ridge,eyes large and horizontal pupil (Liem, 1970; Duellman and Trueb, 1986; Brown and Alcala, 1994; Wilkinson and Drewes, 2000; Rowley et al., 2010). The new species distinguishes from its congeners by the combination of the following characters: (1) small size (adult male, SVL 32.0-38.5 mm, n=13; females are unknown); (2) smooth and green dorsum; (3) flanks, axilla, ventral surface of forearms, inguinal, anterior and posterior surfaces of thighs, ventral surface of shank, and dorsal surface of foot covered with black blotches with white spots or white spots with a faint orange tint; (4) ventral surface of feet and webbing tangerine; (5) supratympanic fold weak; (6)outer margin of forearms and feet with low dermal ridges;(7) calcars absent on heels; (8) iris silver, diffusing to ecru laterally with light khaki ring along margin (Figure 2 and Figure 3A, B).
Description of holotype Body moderately robust, SVL 38.2 mm; head width nearly equal to length; snout rounded in lateral profile, protruding; canthus rostralis indistinct, loreal region sloping; interorbital region slightly convex; nostril oval, laterally positioned, slightly protuberant, nearer tip of snout than eye; internarial distance greater than distance from anterior margin of eye to nostril (ID/EN=1.26); pupil horizontal; eye diameter greater than eye to nostril distance (ED/EN=1.21); pineal ocellus invisible; interorbital width greater than upper eyelid width (IOD/UEW=1.28); tympanum barely visible externally, tympanic rim slightly elevated relative to skin of temporal region, diameter less than eye diameter (TD/ ED=0.64); weak supratympanic fold extending to axilla;vomerine ridge present, in two oblique groups, closer to choanae than to each other, separated by a distance about as long as each groups; choanae oval; tongue attached anteriorly, deeply notched posteriorly; external single subgular vocal sac (Figure 2).
Forelimbs relatively robust, relative length of fingers I<II<IV<III; tips of all except first finger with welldeveloped disks with horizontal circummarginal grooves,disks relatively wide compared to finger width, third finger disk width less than tympanum diameter (0.72 mm); fingers moderately webbed, webbing formula: I 1--1-II 1+-1+III 2--2-IV; subarticular tubercles prominent, rounded, formula 1, 1, 2, 2; out palmar tubercle absent(Figure 3C); thenar tubercle absent; prepollex tubercle indistinct; nuptial pads present. Relative length of toes I<II<III<V<IV; tips of toes disks with distinct circummarginal grooves; disks slightly less than those on fingers; toes moderately webbed, webbing formula: I 1+-1-II 2+-2-III 2+-3-IV 3--2+V; subarticular tubercles rounded, distal subarticular tubercles distinct, formula 1,1, 2, 3, 2; inner metatarsal tubercle oval, elongated; outer metatarsal tubercle and additional supernumerary tubercle absent (Figure 3D). Dorsal skin smooth; ventral surface of chest, venter, and thighs coarsely granular; dorsal surface of limbs smooth; ventral surface of forearms smooth; cloaca and posterior surface of thighs granular;tarsal fold present; outer margin of forearms and feet with low dermal ridges.
Color of holotype in life Dorsal surface green; flanks,axilla, ventral surface of forearms, inguinal, anterior and posterior surfaces of thighs, ventral surface of shanks,dorsal surface of feet, and fingers I, II, III covered with black blotches with white spots or white spots with a faint orange tint; ventral surface of feet and webbing tangerine;venter and chest cream; throat white with slightly gray background; groin region and ventral surfaces of thighs cream; iris silver, diffusing to ecru laterally with light khaki ring along margin, pupil horizontal, black (Figure 2 and Figure 3).
Color of holotype in preservative Dorsum dark brown;tangerine of all part of body and limbs faded to cream;color of the iris faded to bluish-gray; color of the throat faded to light gray.
Measurements of holotype (in mm) SVL 38.2, HL 15.2,HW 15.3, SNT 6.9, ED 4.7, IOD 5.5, TD 2.9, UEW 4.3,TED 1.4, IN 4.9, EN 3.9, TIB 17.0, FLL 21.6, FTD 2.1,HLL 55.0, THL 17.1, HTD4 1.8.
Male secondary sexual characters Nuptial pad present;external single subgular vocal sac; linea musculina present beneath belly.
Advertisement call In the field, fifteen advertisement calls from three type specimens were recorded. Ambient air temperature was 18.4 °C. Advertisement calls consisted of six short clicks given at irregular intervals,often with long pauses between bouts of clicking (range 2.5-101.4 s, n=15). The dominant frequency spectrum of the notes lay between 1.6 and 3.0 kHz. Clicks have prominent harmonics at 1.6-3.0 kHz (Figure 4).
Molecular analyses Genetic divergence between R. pinglongensis sp. nov. and all homologous sequences available in Rhacophorus with green dorsum were calculated with P-distance. The results range from 2.2%-12.2 % (Table 1). Among them, R. pinglongensis sp. nov. and R. dorsoviridis (JX219427) (Accession no. of GenBank) have the smallest genetic divergence, at 2.2%,but the genetic divergence between pinglongensis sp. nov. and R. dorsoviridis (JX219423) reaches 5.4%. ML and BI trees have a similar topology and are consistent with Li et al., (2012b). Based on the 1607 bp matrix, ML and BI trees indicated that R. pinglongensis sp. nov. was closely related to R. dorsoviridis (JX219427) with robust values(BP=100; BBP=1.00) (Figure 5).
Variation Measurements of thirteen specimens are shown in Table 2. In life, the coloration of the paratypes was similar to that of the holotype. Irregularly black blotches on flanks are discrete in NHMG200903001,N H M G 2 0 0 9 0 3 0 0 2, N H M G 2 0 1 0 0 2 0 0 6,NHMG201002008, and NHMG201002013. In preservative, NHMG200903001, NHMG200903002,N H M G 2 0 0 9 0 3 0 0 4, N H M G 2 0 1 0 0 2 0 0 3,NHMG201002008, and NHMG201002010 have a dark dorsum, the other specimens have a brown dorsum. NHMG201002004, NHMG201002005,N H M G 2 0 1 0 0 2 0 0 7, N H M G 2 0 1 0 0 2 0 1 0,NHMG201002011, NHMG201002013 have a white pupil in preservative, the rest has a gray pupil. All tangerine is faded in preservative.
Etymology This species is named after the type locality,Mt. Pinglong. The suggested English name is the Pinglong tree frog.
Ecology The holotype and paratypes were found in montane evergreen forest. Females and larval stages of R. pinglongensis sp. nov. remain unknown. Sympatric species include Amolops ricketti Boulenger,1899, Microhyla pulchra Hallowell, 1861, M. fissipes Boulenger, 1884, M. heymonsi Vogt, 1911, Odorrana schmackeri Boettger, 1892, Polypedates megacephalus Hallowell, 1861, P. mutus Smith, 1940, Rhacophorus dennysi Blanford, 1881, Sylvirana guentheri Boulenger,1882 and S. maosonensis Bourret, 1937.

Figure 3 Ventral (A) and lateral (B) view of the holotype NHMG200903002, ventral surface of right hand (C) and right foot (D) of the holotype NHMG200903002.

Figure 4 Oscillogram (top) and corresponding audiospectrogram (bottom) of the two-note advertisement call of a male Rhacophorus pinglongensis sp. nov. (NHMG201003003). Ambient air temperature was 18.4 °C.

Table 1 Uncorrected pairwise distances (P-distance) (in %) with green dorsum tree frogs in Rhacophorus from GenBank available based on mitochondrial 16S rRNA sequences (~530 bp).
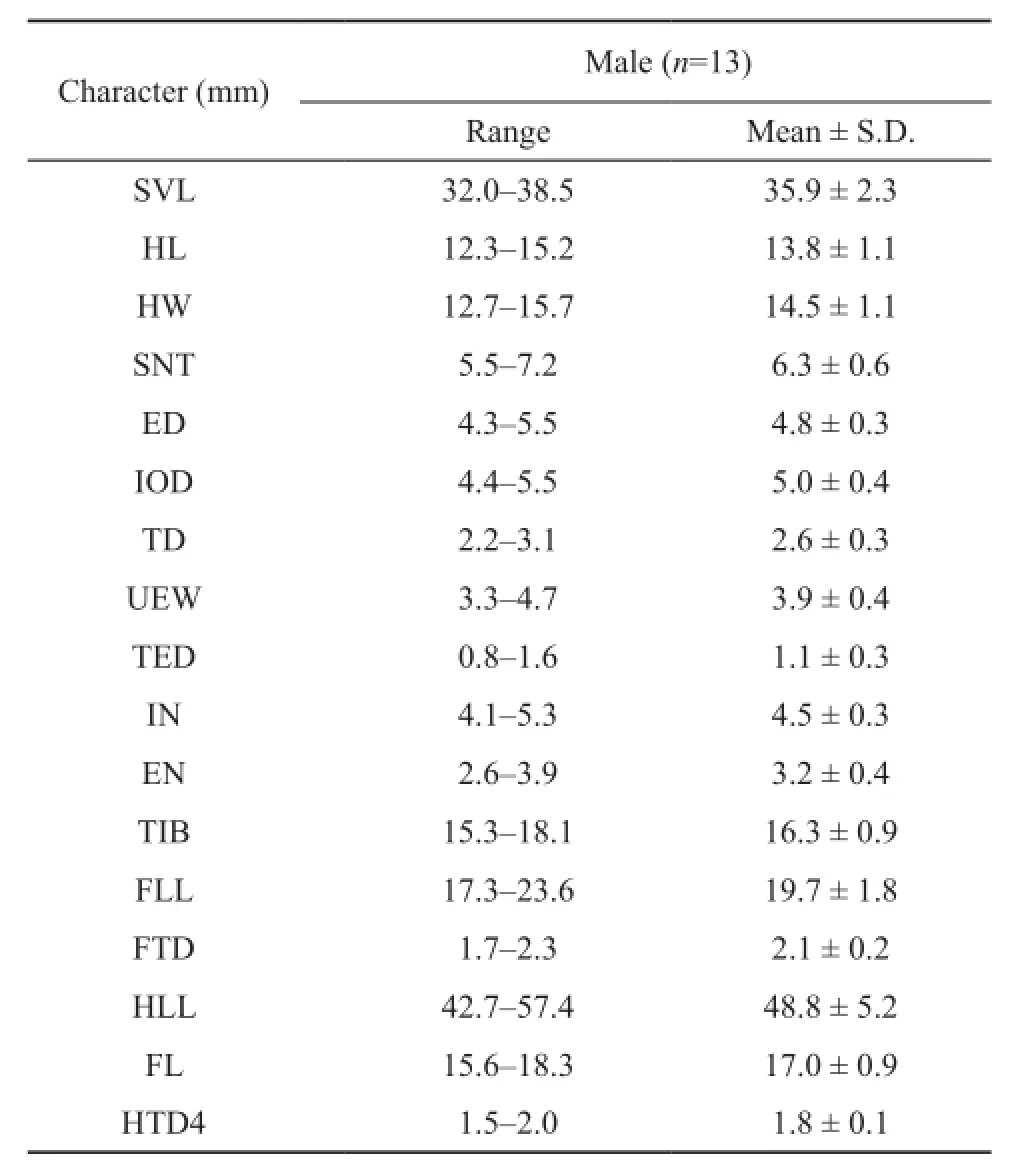
Table 2 Measurements (in mm) of Rhacophorus pinglongensis sp. nov.. Abbreviations defined in the text.
Comparison Smooth and green dorsum, flanks, axilla,ventral surface of forelimbs, inguinal, anterior and posterior surfaces of thighs, ventral surface of shank, and dorsal surface of foot covered with black blotches with white spots or white spots with a faint orange tint, ventral surface of foot and webbing tangerine, supratympanic fold weak, and outer margin of forearm and foot with low dermal ridges distinguish the new species from all Southeast Asian congeners.
The following tree frogs that have brown, grayish brown, reddish brown, or orange red background color on dorsum can be distinguished from this new species(additional distinguishing characters in parentheses):R. annamensis Smith, 1924 (fingers broadly webbed,the membrane between the outer three fingers reaching the discs; toes fully webbed, their discs considerably smaller than those of the fingers; a fold from the eye to the shoulder; dark cross-bars upon the limbs) (Smith,1924); R. calcaneus Smith, 1924 (outer two fingers 3/4 webbed, inner two 1/4 webbed; toes nearly fully webbed;a strong fold from the eye to the shoulder; heel with a well-marked pointed terminal tubercle; a yellow line extending from the tip of the nose along the canthus rostralis and along the outer edges of the upper and lower eyelids (Smith, 1924); R. exechopygus Inger, Orlov, and Darevsky, 1999 (outer fingers fully webbed, forearm and tarsus with crenulated dermal ridge) (Inger et al., 1999);R. cyanopunctatus Manthey and Steiof, 1998 (throat and chest white with brown speckles; snout short and angular with a well-defined ridge running from the eye to the snout; dorsum light brown with medium brown patches)(Manthey and Steiof, 1998); R. hoanglienensis Orlov,Lathrop, Murphy and Ho, 2001 (a pointed projection on heels; irregular black and brown spots on dorsum;loreal region dark brown) (Orlov et al., 2001; Bain and Truong, 2004); R. jarujini Matsui and Panha, 2006 (a narrow darker brown bar crossing upper eyelids; back with irregular, darker brown crossbands) (Matsui and Panha, 2006); R. laoshan Mo, Jiang, Xie and Ohler,2008 (dark brown stripe on limbs) (Mo et al., 2008); R. marmoridorsum Orlov, 2008 (dorsum flesh colored with marble chocolate) (Orlov et al., 2008); R. orlovi Ziegler and Köhler, 2001 (dark brown bar between eyes, loreal region and supratympanic fold dark brown with irregular yellow patches) (Ziegler and Köhler, 2001); R. rhodopus Liu and Hu, 1959 (temporal fold distinct; small rounded tubercles all over the thorax; reddish brown on the head,back and dorsal sides of the limbs; with dark brown mark on the back of the shoulder region and with dark brown transverse bars on the posterior dorsum of the body, some small black spots scattered on the whole back; males with a single internal subgular vocal sac) (Liu and Hu,1959); R. spelaeus Orlov, Gnophanxay, Phimminith and Phomphoumy, 2010 (dorsum grayish brown with dark irregular spots; venter light gray with dark specks) (Orlov et al., 2010); R. translineatus Wu, 1977 (snout with a conspicuous conical dermal protuberance; a triangular skin flap at the outer tibiotarsal region; more than then transverse dark brown bands on the dorsum) (referring to Sichuan Institute of Biology Herpetology Department,1977); R. tuberculatus Anderson, 1871 (fingers broadly webbed, the membrane reaching the disks of the second and fourth fingers; a strong fold from the eye over the tympanum to the shoulder; under surface of thighs granular, with scattered large round tubercles; brownish yellow below with a blackish region round the vent, a short way along the thighs; the tubercles of abdomen and thighs are darker (Anderson, 1871); R. vampyrus Rowley,Le, Thi, Stuart and Hoang, 2010 (pale tan to brick red dorsum; flanks and anterior and posterior surface of thighs black; gray to black webbing between fingers and toes) (Rowley et al., 2010); R. verrucopus Huang, 1983(a pointed projection on heels; forearm and tarsus with tuberculous crenulated dermal ridge) (Huang, 1983).
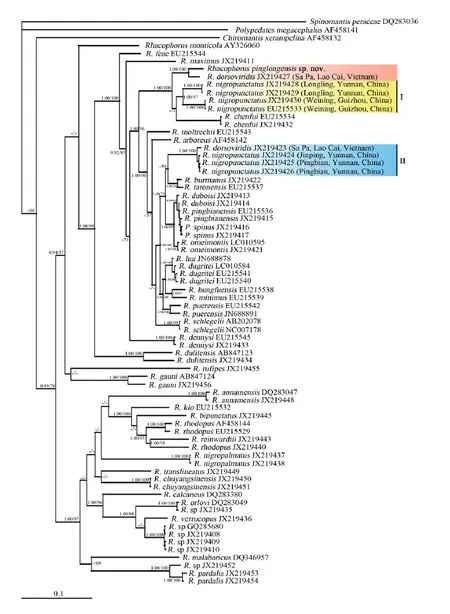
Figure 5 Bayesian inference tree reconstructed from 12S rRNA, tRNAvaland 16S rRNA mitochondrial genes with Spinomantis peraccae and Chiromantis xerampelina as outgroups. Numbers above branches represent bootstrap supports for Bayesian posterior probabilities (BPP) (>0.9 retained)/bootstrap support for maximum likelihood analyses (>50 retained); ‘-’ represents BPP and bootstrap proportions lower than 90% and 50%, respectively.
Rhacophorus burmanus Andersson, 1939, R. dennysi Blanford, 1881, R. duboisi Ohler, Marquis, Swan andGrosjean, 2000, R. feae Boulenger, 1893, R. kio Ohler and Delorme, 2006, R. maximus Günther, 1858, R. omeimontis Stejneger, 1924, R. prasinatus Mou, Risch and Lue, 1983, and R. reinwardtii Schlegel, 1840 have green ground color on dorsum, but the SVL of these species is greater than 50 mm (Ohler et al., 2000; Ohler and Delorme, 2006; Ohler, 2009; Fei et al., 2012). Rhacophorus duboisi, R. dugritei, R. hongchibaenis Li,Liu, Chen, Wu, Murphy, Zhao, Wang and Zhang, 2012,R. omeimontis, R. pingbianensis Kou, Hu and Gao, 2001,and R. zhaojuensis Wu and Zheng, 1994 have black or brown spots or blotches on dorsum (Wu and Zheng, 1994;Ohler et al., 2000; Kou et al., 2000; Fei et al., 2012; Li et al., 2012a). Rhacophorus arvalis Lue, Lai and Chen,1995 lacks black blotches on flanks and anterior and posterior surfaces of thighs, and has a white line on flanks(Lue et al., 1995); R. aurantiventris Lue, Lai and Chen,1994 and R.chenfui Liu, 1945 lack spots on anterior and posterior surfaces of thighs; R. hungfuensis Liu and Hu,1961 lacks spots on flanks and thighs; R. leucofasciatus Liu and Hu, 1962 has a white line running from tip of snout to flanks; R. minimus Rao, Wilkinson and Liu, 2006 has a white line from tip of snout along the upper lip and shoulder to insertion of hind limbs (Rao et al., 2006);R. prasinatus Mou, Risch and Lue, 1983 has a white line extending from tip of snout to flanks, with black spots beneath the line (Fei et al., 2012); R. taipeianus Liang and Wang, 1978 has a yellowish white abdomen and several dark brown spots on inner thighs (Liang and Wang, 1978); R. wui Li, Liu, Chen, Wu, Murphy, Zhao,Wang and Zhang, 2012 has irregularly distributed small tubercles on both dorsum and venter and numerous lightbrown spots on dorsum (Li et al., 2012a); R. yaoshanensis Liu and Hu, 1962 has tangerine on anterior and posterior surface of thighs (Liu and Hu, 1962); R. yinggelingensis Chou, Lau and Chan, 2007 has yellow and red-tinged on front of thighs and red on rear of thighs and inner side of shanks (Chou et al., 2007).
Rhacophorus pinglongensis sp. nov. is closely related to R. dorsoviridis, R. moltrechti, and R. nigropunctatus based on morphology. Firstly, Rhacophorus moltrechti differs from the new species by having large black spots at axillary and lumbar region, flanks of hind limbs bright orange with large black spots, interdigital membrance orange, spotted with black and iris red or reddish brown(Boulenger, 1908). Then, whether R. nigropunctatus and R. dorsoviridis are conspecific is still contraversial (Orlov et al., 2001; Li et al. 2012b; Fei et al., 2012). Herein,we treat them as two valid species, and compare them separately. R. dorsoviridis differs from this new species by lacking naptial pads (vs. present in the new species),having a single median vocal sac that is not distended(vs. exteral single subgular vocal sac); flanks white with variable black spots (vs. flanks black with white spots),venter white with a few small black spots below shank(vs. venter cream in males); feet and hand yellowish white (vs. ventral surface of shanks, dorsal surface of feet, and fingers I, II, III covered with black blotches with white spots or white spots with a faint orange tint); flanks white; but shanks are anteriorly and posteriorly orange(vs. black blotches with white spots); the posterior surface and occasionally the dorsomedial surface of tibiotarsus with large black spots (vs. large white spots), iris red or yellow above, pale below (vs. iris silver, diffusing to ecru laterally with light khaki ring along margin) (Figure 6AD) (Bourret, 1937; Orlov et al., 2001; Zhang et al., 2011;Fei et al., 2012). Finally, Rhacophorus nigropunctatus differs from the new species by having a dark throat;black blotches at flanks and anterior and posterior surfaces of thighs, but lacking white spots; third finger disk width less than tympanum (Liu et al., 1962).
4. Discussion
Preliminary molecular data indicated that R. pinglongensis sp. nov. and R. dorsoviridis (JX219427) are sister species. Although the genetic divergence between R. pinglongensis sp. nov. and R. dorsoviridis (JX219427)is less than 3 % (Table 1), a value usually representing differentiation at the species level in frogs (Vences et al.,2005; Fouquet et al., 2007), morphologically, they can distinguish from each other (Figure 6). Based on Orlov et al. (2001) descriptions, R. dorsoviridis (JX219427,voucher no. ROM 38011, from Sa Pa, Lao Cai, Vietnam,type locality) has a darken single median vocal sac,obviously differing others R. dorsoviridis specimens(males, ROM 38009-38010, 38014-38016, 38018;females, ROM 38006-38008, 38017, from type locality)and this new species. The rest R. dorsoviridis specimens(males, ROM 38009-38010, 38014-38016, 38018;females, ROM 38006-38008, 38017, from type locality)are also different from this new species, such as nuptial pad absent and iris brilliant orange above (vs. nuptial pad present and iris silver) (Orlov et al., 2001). In addition, we reviewed Zhang et al. (2011) descriptions (male, voucher no. KIZ09133-35, from Pingbian, Yunnan, China), those R. dorsoviridis specimens obviously differ from R. pinglongensis sp. nov. by having internal single subgular vocal sac (vs. external single subgular vocal sac) (Zhang et al. 2011). No matter whether R. dorsoviridis came fromthe type locality, Sa Pa, Lao Cai, Vietnam (Figure 6 B;Orlov et al., 2001) or other localities (Figure 6 C, D;Zhang et al., 2011; Fei et al., 2012), R. pinglongensis sp. nov. can distinguish R. dorsoviridis by morphological characters. Ecologically, R. pinglongensis sp. nov. is known from southern Guangxi at ~500 m elevation and montane evergreen forest, but R. dorsoviridis is known from Sa Pa, Lao Cai, Vietnam and Pingbian, Yunnan,China, at 1500-2000 m elevation and evergreen forest(Orlov et al., 2001; Zhang et al., 2011; Fei et al., 2012).

Figure 6 (A) The holotype of Rhacophorus pinglongensis sp. nov., (B) adult male of Rhacophorus dorsoviridis from type locality, Sa Pa, Lao Cai, Vietnam; photo: Nikolai L. Orlov, from Zoological Institute, Russian Academy of Science, (C) adult male of Rhacophorus dorsoviridis, dorsolateral view, and (D) ventral view, from Pingbian County, Yunnan, China; photo: Mian Hou, from Sichuan Normal University.
Being consistent with Yu et al. (2009) and Li et al.(2012b), our results also supported R. nigropunctatus is a species complex. Distinct genetic difference indicated R. nigropunctatus Longling-Weining group and R. nigropunctatus Jinping-Pingbian group are not conspecific. R. dorsoviridis (JX219423) is different from R. dorsoviridis (JX219427) (Orlov et al., 2001),and has seriously lower interspecies genetic distance(at 0.6 %) with R. nigropunctatus Jinping-Pingbian group. R. nigropunctatus ranges from Yunnan, Guizhou,Anhui to Hunan provinces, China (Fei et al., 2012). Similar to Li et al. (2012b) and Yu et al. (2009), our results indicated that R. nigropunctatus group contains a cryptic species, where R. nigropunctatus from the type locality (Weining, Guizhou) and Pingbian, Yunnan form paraphyletic lineages (Figure 5, sublineage I, II). So we suggest R. nigropunctatus Longling-Weining group is R. nigropunctatus (Figure 5, sublineage I), which is similar morphologically with the original descriptions of R. nigropunctatus (Liu et al., 1962; Li et al. 2012b). And R. nigropunctatus Jinping-Pingbian group together R. dorsoviridis (JX219423) (Figure 5, sublineage II) are R. dorsoviridis based on morphology (Bourret, 1937; Orlov et al., 2001). R. dorsoviridis (JX219427), as Orlov et al.(2001) reported, is distinguished from sublineage I and this news species. Although R. dorsoviridis (JX219427)and R. pinglongensis sp. nov. have lower genetic distance, considering their conspicuous morphology, the taxonomic status of R. dorsoviridis (JX219427) need to been investigated to check more material.
So far, females and larval stages are unknown. More breeding ecology of this new species needs to be done to understand them.
Acknowledgements This work was supported by Natural Science Foundation of Guangxi Province, China (Grant No: 2012GXNSFAA053057 and 2016GXNSFAA380007).
References
Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. 1997. Gapped BLAST and PSIBLAST: a new generation of protein database search programs. Nucleic Acids Res, 25: 3389-3402
Anderson J. 1871. A list of the reptilian accession to the Indian Museum, Calcutta from 1865 to 1870, with a description of some new species. J Asiatic Soc Bengal, 40: 12-39
Bain R. H., Nguyen Q. T. 2004. Herpetofaunal diversity of Ha Giang Province in Northeastern Vietnam with descriptions of two new species. Am Mus Novit, 3453: 1-42
Blanford W. T. 1881. On a collection of reptiles and frogs chiefly from Singapore. Proc Zool Soc London, 1881: 215-227
Boulenger G. A. 1882. Catalogue of the Batrachia Salientia s. Ecaudata in the Collection of the British Museum. Sec Ed,London: Taylor and Francis
Boulenger G. A. 1884. Descriptions of new species of reptiles and batrachians in the British Museum. Part. II. Ann Mag Nat Hist,Ser 5, 13: 396-398
Boulenger G. A. 1893. Concluding report on the reptiles and batrachians obtained in Burma by Signor L. Fea dealing with the collection made in Pegu and the Karin Hills in 1887-88. Annali del Museo Civico di Storia Naturale di Genova, Ser 2, 13: 304-347
Boulenger G. A. 1899. On a collection of reptiles and batrachians made by Mr. JD La Touche in NW Fokien, China. Proc Zool Soc London, 11: 159-172
Boulenger G. A. 1908. Descriptions of a new frog and a new snake from Formosa. Ann Mag Nat Hist, Ser 8, 2: 221-222
Bourret R. 1937. Notes herpétologiques sur l’Indochine française. XIV. Les batraciens de la collection du Laboratoire des Sciences Naturelles de l’Université. Descriptions de quinze especes ou variétés nouvelles. Ann Bull Gén Instr Publique, Hanoi, 1937:5-56
Brown W. C., Alcala A. C. 1994. Philippine frogs of the family Rhacophoridae. Proc Calif Acad Sci, 48: 185-220
Castresana J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol,17: 540-552
Chou W. H., Lau M. W. N., Chan B. P. 2007. A new tree frog of the genus Rhacophorus (Anura: Rhacophoridae) from Hainan Island, China. Raffles Bull Zool, 55(1): 157-165
Duellman W. E., Trueb L. 1986. Biology of Amphibians. The John Hopkins University Press Baltimore and London. 670 pp
Fei L., Ye C. Y., Jiang J. P. 2012. Colored Atlas of Chinese Amphibian. Sichuan China: Sichuan Publishing Group (In Chinese)
Fouquet A., Gilles A., Vences M., Marty C., Blanc M., Gemmell N. J. 2007. Underestimation of species richness in Neotropical frogs revealed by mtDNA analyses. PLoS one, 2(10): e1109
Frost D. R. 2015. Amphibian Species of the World: an Online Reference. Version 6.0 (accessed on 15 November 2015). Electronic Database accessible at http://research.amnh.org/ herpetology/amphibia/ index.html. American Museum of Natural History, New York, USA
Günther A. C. L. G. 1858. Neue Batrachier in der Sammlung des britischen Museums. Archiv für Naturgeschichte, Berlin, 24:319-328
Hamidy A., Kurniati H. 2015. A new species of tree frog genus Rhacophorus from Sumatra, Indonesia (Amphibia Anura). Zootaxa, 3947(1): 49-66
Hallowell E. 1861 “1860”. Report upon the Reptilia of the North Pacific Exploring Expedition, under command of Capt. John Rogers, U. S. N. Proc Acad Nat Sciof Philadelphia, 12: 480-510
Hoffman A. C. 1932. Researches relating to the validity of the South African Polypedatidae (Rhacophoridae) as an autonomous family of the Anura. S Afr J Sci, 29: 562-583
Huang Y. Z. 1983. A new species of flying frog from Xizang Rhacophorus verrucopus. Acta Herpetol Sinica, 2 (4): 63-65
Inger R. F., Orlov N., Darevsky I. 1999. Frogs of Vietnam: A report on new collections. Fieldiana Zool, 92: 1-46
Kou Z., Hu J., Gao C. 2000. A new species of the genus Polypedates from Yunnan, China (Amphibian: Rhacophoridae). Acta Zootaxonomica Sin, 26(2): 229-233
Kuhl H., Van Hasselt J. C. 1822. Uittreksels uit breieven van de Heeren Kuhl en van Hasselt, aan de Heeren CJ Temminck, Th. van Swinderen en W. de Haan. Algemeene Konst-en Letter-Bode, 7: 99-104
Li J. T., Liu J., Chen Y. Y., Wu J. W., Murphy R. W., Zhao E. M., Zhang Y. P. 2012a. Molecular phylogeny of treefrogs in the Rhacophorus dugritei species complex (Anura: Rhacophoridae),with descriptions of two new species. Zool J Linn Soc, 165(1):143-162
Li J. T., Li Y., Murphy R. W., Rao D. Q., Zhang Y. P. 2012b. Phylogenetic resolution and systematics of the Asian tree frogs,Rhacophorus (Rhacophoridae Amphibia). Zool Scr, 41(6): 557-570
Liang Y. S., Wang C. S. 1978. A new tree frog Rhacophorus taipeianus (Anura: Rhacophoridae) from Taiwan (Formosa). Quart J Taiwan Museum, 31: 185-202
Liem S. S. 1970. The morphology systematic and evolution of the old world treefrogs (Rhacophoridae and Hyperoliidae). Fieldiana Zool, 57: 1-145
Liu C. C. 1945. New frogs from west China. J West China Border Res, Soc, 15: 28-43
Liu C. C., Hu S. Q. 1959. Preliminary report of Amphibia from southern Yunnan. Acta Zool Sinica, 11(4): 530-533
Liu C. C., Hu S. Q. 1961. Tailless amphibians of China. Shanghai:Science Press
Liu C. C., Hu S. Q., Yang F. H. 1962. Preliminary report of amphibians from Western Kweichow. Acta Zool Sinca, 14(3):381-392
Lue K. Y., Lai J. S., Chen S. L. 1994. A new species of Rhacophorus (Anura: Rhacophoridae) from Taiwan. Herpetologica, 50(3): 303-308
Lue K. Y., Lai J. S., Chen S. L. 1995. A new species of Rhacophorus (Anura: Rhacophoridae) from Taiwan. J Herpetol,29: 338-345
Manthey U., Steiof C. 1998. Rhacophorus cyanopunctatus sp. n. (Anura: Rhacophoridae), ein neuer Flugfrosch von derMalaiischen Hablinsel Sumatra und Borneo. Sauria, 20: 37-42
Matsui M., Panha S. 2006. A new species of Rhacophorus from eastern Thailand (Anura: Rhacophoridae). Zool Sci, 23(5): 477-481
Mo Y. M., Jiang J. P., Xie F., Ohler A. 2008. A new species of Rhacophorus (Anura: Ranidae) from China. Asiat Herpetol Res,1: 85-92
Mou Y. P., Risch J. P., Lue K. Y. 1983. Rhacophorus prasinatus,a new tree frog from Taiwan, China (Amphibia, Anura,Rhacophoridae). Alytes, 2: 154-162
Myers C. W., Duellman W. E. 1982. A new species of Hyla from Cerro Colorado and other tree frog records and geographical notes from western Panama. Am Mus Novit, 275: 32.
Ohler A., Marquis O., Swan S. R., Grosjean S. 2000. Amphibian biodiversity of Hoang Lien Nature Reserve (Lao Cai Province northern Vietnam) with description of two new species. Herpetozoa, 13: 71-87
Ohler A., Delorme M. 2006. Well known does not mean well studied: morphological and molecular support for existence of sibling species in the Javanese gliding frog Rhacophorus reinwardtii (Amphibia Anura). C R Biol, 329: 86-97
Ohler A. 2009. Rhacophorus burmanus (Anderson 1939. the valid nomen for Rhacophorus taroensis Smith, 1940 and Rhacophorus gongshanensis Yang and Su, 1984. Herpetozoa, 21: 179-182
Orlov N. L., Lathrop A., Murphy R. W., Ho T. C. 2001. Frogs of the family Rhacophoridae (Anura: Amphibia) in the northern Hoang Lien Mountains (Mount Fan Si Pan Sa Pa District Lao Cai Province), Vietnam. Russ J Herpetol, 8: 17-44
Orlov N. L., Nguyen N. S., Ho T. C. 2008. Description of a new species and new records of Rhacophorus genus (Amphibia:Anura: Rhacophoridae) with the review of amphibians and reptiles diversity of Chu Yang Sin National Park (Dac Lac Province Vietnam). Russ J Herpetol, 15: 67-84
Orlov N. L., Gnophanxay S., Phimminith T., Phomphoumy K. 2010 “2009”. A new species of Rhacophorus genus (Amphibia:Anura: Rhacophoridae: Rhacophorinae) from Khammouan Province, Lao PDR. Russ J Herpetol, 16: 295-303
Rao D. Q., Wilkinson J. A., Liu H. N. 2006. A new species of Rhacophorus (Anura: Rhacophoridae) from Guangxi Province,China. Zootaxa, 1258: 17-31
Ronquist F. R., Huelsenbeck J. P. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19:1572-1574
Rowley J. J. L., Duong L. T. T., Dao T. T. A., Stuart B. L., Huy H. D. 2010. A new tree frog of the genus Rhacophorus (Anura:Rhacophoridae) from southern Vietnam. Zootaxa, 2727: 45-55
Schlegel H. 1840. Abbildungen neuer oder unvollständig bekannter Amphibien, nach der Natur oder dem Leben entworfen,herausgegeben und mit einem erläuternden. Atlas, Düsseldorf:Arnz & Co.
Sichuan Institute of Biology Herpetology Department. 1977. A survey of amphibians in Xizang (Tibet). Acta Zool Sinica, 23:54-63
Smith M. A. 1924. New tree-frogs from Indo-China and the Malay Peninsula. Proc Zool Soc London, 94(1): 225-234
Smith M. A. 1940. The amphibians and reptiles obtained by Mr. Ronald Kaulback in Upper Burma. Rec Ind Mus, 42(3): 465-486
Stamatakis A., Hoover P., Rougemont J. 2008. A Rapid Bootstrap Algorithm for the RaxML Web Servers. Syst Biol, 75: 758-771
Streicher J. W., Hamidy A., Harvey M. B., Andres B., Shaney K. J., Kurniawan N., Smith E. N. 2014. Mitochondrial DNA reveals a new species of parachuting frog (Rhacophoridae:Rhacophorus) from Sumatra. Zootaxa, 3878: 351-356
Stejneger L. 1924. Herpetological novelties from China. Occasional Papers Boston Soc Nat Hist, 5: 119-121
Tamura K., Peterson D., Peterson N., Stecher G., Nei M.,Kumar S. 2011. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood evolutionary distance and maximum parsimony methods. Mol Biol Evol, 28: 2731-2739
Vences M., Thomas M., Bonett R. M., Vieites D. R. 2005. Deciphering amphibian diversity through DNA barcoding:chances and challenges. Philos Trans R Soc Lond A Math Phys Sci, 360: 1859-1868
Vogt T. 1911. Beitrag zur Amphibien-fauna der Insel Formosa. Sitzungsberichte der Gesellschaft Naturforschender Freunde zu Berlin, 1911: 179-184
Wilkinson J. A., Drewes R. C. 2000. Character assessment genus level boundaries and phylogenetic analyses of the family Rhacophoridae: A review and present day status. Contemp Herpetol, 2: 1-19
Wilkinson J. A., Drewes R. C., Tatum O. L. 2002. A molecular phylogenetic analysis of the family Rhacophoridae with an emphasis on the Asian and African genera. Mol Phylogenet Evol, 24: 265-273
Wu G. F., Zheng X. M. 1994. The karyotypic differentiation of Polypedates dugritei with description of a superspecies(Rhacophoridae Anura). Sichuan J Zool, 13(4): 156-161
Yu G., Rao D., Zhang M., Yang J. 2009. Re-examination of the phylogeny of Rhacophoridae (Anura) based on mitochondrial and nuclear DNA. Mol Phylogenet Evol, 50(3): 571-579
Ziegler T., Köhler J. 2001. Rhacophorus orlovi sp. n., ein neuer Ruderfrosch aus Vietnam (Amphibia: Anura: Rhacophoridae). Sauria, 23: 37-46
Zhang J., Jiang K., Hou M. 2011. Rhacophorus dorsoviridis Bourret,a new record of family Rhacophoiridae to China. Acta Zootaxonomica Sin, 36 (4): 986-989
Zhao E. M., Wang L. J., Shi H. T., Wu G. F., Zhao H. 2005. Chinese rhacophorid frogs and description of a new species of Rhacophorus. Sichuan J Zool, 24(3): 297-300
Prof. Weicai CHEN, from Natural History Museum of Guangxi, Nanning, China, with his research focusing on taxonomy, population genetics and molecular ecology of amphibian.
E-mail: chenweicai2003@126.com
9 December 2015 Accepted: 11 April 2016
 Asian Herpetological Research2016年3期
Asian Herpetological Research2016年3期
- Asian Herpetological Research的其它文章
- Isolation and Characterization of Nine Microsatellite Markers for Red-backed Ratsnake, Elaphe rufodorsata
- Sexual Dimorphism of the Jilin Clawed Salamander,Onychodactylus zhangyapingi, (Urodela: Hynobiidae: Onychodactylinae) from Jilin Province, China
- The Impact of Phenotypic Characteristics on Thermoregulation in a Cold-Climate Agamid lizard, Phrynocephalus guinanensis
- The Impacts of Urbanization on the Distribution and Body Condition of the Rice-paddy Frog (Fejervarya multistriata) and Gold-striped Pond Frog (Pelophylax plancyi) in Shanghai, China
- The Effect of Speed on the Hindlimb Kinematics of the Reeves’Butterfly Lizard, Leiolepis reevesii (Agamidae)
- Changes in Electroencephalogram Approximate Entropy Reflect Auditory Processing and Functional Complexity in Frogs
