掺杂钇的铕稀土配合物的荧光绝对量子产率和寿命的研究
费邦忠,陶栋梁,张 宏,崔玉民,3,张 坤,王永忠,3,杨森林,鲁仕梅
(1.安徽华辉塑业科技股份有限公司,安徽 合肥,231300;2.阜阳师范学院 化学与材料工程学院,,安徽 阜阳,236037;3.安徽省环境污染物监测与降解省级重点实验室,安徽 阜阳,236037;4.国家再生有色金属橡塑材料质量监督检验中心,安徽 阜阳,236000)
掺杂钇的铕稀土配合物的荧光绝对量子产率和寿命的研究
费邦忠1,陶栋梁2,3*,张宏2,崔玉民2,3,张坤2,王永忠2,3,杨森林2,鲁仕梅4
(1.安徽华辉塑业科技股份有限公司,安徽 合肥,231300;2.阜阳师范学院 化学与材料工程学院,,安徽 阜阳,236037;3.安徽省环境污染物监测与降解省级重点实验室,安徽 阜阳,236037;4.国家再生有色金属橡塑材料质量监督检验中心,安徽 阜阳,236000)
在无水乙醇中,利用Eu3+和Y3+作为中心离子,α-噻吩甲酰三氟丙酮(TTA)和1,10-邻菲啰啉(phen)作为配体制备了一系列共发光稀土配合物EuxY1-x(TTA)3phen,并对TTA和EuxY1-x(TTA)3phen进行了红外表征。掺杂钇的铕配合物与没掺杂钇相比,荧光绝对量子产率和平均寿命都发生了很大变化,随着钇含量的增大,EuxY1-x(TTA)3phen的荧光绝对量子产率先增大,然后减小,而平均寿命则以波动方式逐渐减小,说明钇的掺杂改变了EuxY1-x(TTA)3phen的分子微观结构,从而改变了EuxY1-x(TTA)3phen的能量传递方式。
稀土配合物;掺杂;荧光;寿命;绝对量子产率
Lanthanide complex as luminescent material has a narrow emission peak and high quantum efficiency.In order to improve the luminescent property of rare earth complex,it is the most effective way to vary the matching degree of energy level between ligand and rare earth ion[1].However,the matching degree of energy level between ligand and rare earth ions will reach the limit and further increasing becomes very difficult.Therefore,it is necessary by the other methods to improve further the luminescence property of rare earth complex.For example,doping other metal ions to form co-luminescent lanthanide complex or coating silica outside lanthanide complex can effectively improve their luminescent property[2-5].Although doping other metal ions(e.g.,La,Y,Gd,Tb,etc.)into europium complex can increase the fluorescence emission intensity in the form of solid powder,the difference of particle size,density and measurement condition of the solid samples will have a significant impact on the fluorescence emission intensity.At the same time,the powder samples have different optical absorption capacity.Therefore,it is difficult to give absolutely accurate judgment only examining fluorescence emission intensity.Therefore,lanthanide complex solid sample is often dissolved in a solvent using a reference to determine their quantum efficiency.Now,quantum efficiency is already a common way to detect fluorescence property.The samples must be detected with the same excitation wavelength,solvent and instrument settings with standard samples.Even so,due to the solvent effect,the sample in a solution still has a larger gap of the fluorescence property with the sample in solid[6].Therefore,it is difficult to show the quantum yield of sample in solid with the sample in liquid reported in literature.
Currently,using integrating sphere to determine the fluorescent absolute quantum efficiency of solid lanthanide complex is more accurate method,since the integrating sphere system can not only comprehensively collect the fluorescence emitted by the sample and determine its absorption,but also accurately calculate the absolute quantum efficiency of lanthanide complex by correction formula of the integrating sphere system[7].Absolute quantum efficiency of the sample is the ratio of number of photons emitted divided by number of photons absorbed.Absolute quantum efficiency of lanthanide complex doped with other metal ions is seldom reported in the current literature.The fluorescence lifetime of lanthanide complex is the required time that the fluorescence intensity is reduced to the original 1/e when the external excitation source is cut off. Fluorescence lifetime can characterize the rate of exciton transitions from the excited state to the ground state of rare earth ions[8].Fluorescence lifetime variation of europium complex doped with Y can characterize the molecular micro-structural change.In this paper,co-luminescent lanthanide complexes EuxY1-x(TTA)3phen were obtained by doping Y into rare earth complex Eu(TTA)3phen.Absolute quantum efficiency and fluorescence lifetime were investigated with the different proportion of Y-doped.
1 Experimental
1.1Reagentsandinstruments
1.1.1Reagents
EuCl3·6H2O or YCl3·6H2O was prepared by dissolvingEu2O3orY2O3(Aladdin,purity≈99.99%)in dilute hydrochloric acid,and the solvent was slowly evaporated to separate out white crystalline EuCl3·6H2O or YCl3·6H2O.The crystal was filtered and placed in a desiccator.
TTA is of Alfa Aesar reagent(purity 99%).Anhydrous ethanol,triethylamine and phen are of analytical grade reagents.
1.1.2Instruments
IR spectra at 400-4 000 cm-1were obtained by the Fourier transform infrared spectrometer(WQFIR 510)with KBr.UV-vis spectra were measured by the Tu-1901 UV-vis spectroscopy.Fluorescence spectra were measured by the FM4 NIR TCSPC fluorescencespectrometer(JobinYvonCompany,France).
The model of integrating sphere system is HORIBA Scientific with 150 mm size and open hole(less than 1.9%).The internal reflection material is suppressed Polytetrafluoroethylene(PTFE,90%reflectivity),and the wavelength range was from 250 nm to 2 500 nm.To determine absolute quantum yield of EuxY1-x(TTA)3phen,the excitation wavelength was set on 366 nm,and both the entrance and exit slits were 1.7 nm.The scattering spectral range of blank and sample was from 356 nm to 376 nm,and the emission spectral range was from 570 nm to 670 nm.
Fluorescence lifetime was measured with 612 nm as the emission wavelength,and 370 nm LED light as an excitation light source.The collected number of photons was 20 000.
1.2SynthesisofEuxY1-x(TTA)3phen
3 mmol ligand TTA and 1 mmol EuCl3·6H2O were dissolved together in 20 mL anhydrous ethanol,and were stirred constantly until they were completely dissolved.3 mmol triethylamine was added dropwise to the above solution and 1 mmol phen ethanol solution was added with constant stirring,then the white precipitate Eu(TTA)3phen generated. The precipitate was filtered and washed several times with ethanol,dried in air and then placed in a desiccator.1 mmol rare earth complex EuxY1-x(TTA)3phen was prepared with x mmol EuCl3·6H2O and(1-x)mmol YCl3·6H2O,which the other steps were same as the method of preparation pure Eu (TTA)3phen.
2 Results and discussions
2.1Infraredspectroscopy
As shown in Figure 1,a broad peak around 3 416 cm-1indicates the presence of adsorbed water in the ligand TTA.The C=O stretching vibration peak of ligand TTA located at 1 664 cm-1is shifted to 1 606 cm-1after the formation of complex.This indicates that the oxygen atoms of C=O in TTA is bonded with rare earth ions[5,9],which weakens C=O bond strength of TTA and causes the absorption peak red shift.

Figure 1 IR spectra of EuxY1-x(TTA)3phen and ligand TTA
The IR spectra of Y-doped and Y-undoped rare earth complexes EuxY1-x(TTA)3phen are similar,which indicates that their molecular structure is similar.However,the ionic radii of Eu3+and Y3+are 0.95 Å and 0.89 Å,respectively.There is much difference between their ionic radii.Therefore,the vibration peaks around 1 606 cm-1for EuxY1-x(TTA)3phen vary from 1 598 cm-1to 1 610 cm-1with Y content changing.This demonstrates that Y-doped plays a certain role on the C=O vibration peak of rare earth complexes.
2.2Fluorescencespectraandabsolute
quantumefficiency
The excitation spectra of EuxY1-x(TTA)3phen are shown in Figure 2 with monitoring wavelength at 612 nm.As can be seen from the Figure 2,the shapes of excitation spectra of the complexes are basically similar,but the fluorescence intensities are different.This indicates that EuxY1-x(TTA)3phen possesses similar coordination environment and different intramolecular energy transfer efficiency.The maximum excitation wavelength of EuxY1-x(TTA)3phen is around 366 nm.In order to determine accurately the variation of fluorescent property of EuxY1-x(TTA)3phen,an integrating sphere quantum efficiency system is used to measure the absolute quantum efficiency of EuxY1-x(TTA)3phen.Figure 3 reveals the curves of absolute quantum efficiency with the different Y content of EuxY1-x(TTA)3phen(λex=366 nm).It can be clearly seen that with the change of Ycontent,the absolute quantum efficiency is accordingly changed.Overall,with Y content increasing,theabsolutequantumefficiencyofEuxY1-x(TTA)3phen firstly increases and then decreases. This shows that Y-doped causes a certain effect on the molecular structure of rare earth complex,and changes the intramolecular energy transfer system,which affects absolute fluorescence quantum yield of rare earth complex.The absolute quantum efficiency of Eu(TTA)3phen is 38.15%.The maximum absolute quantum efficiency is 43.32%with Y content for 40%,which increases 13.55%.The absolute quantum efficiency of Eu0.1Y0.9(TTA)3phen is the lowest(34.03%),with 10.80%decrease.This is not consistent with the reported results in literature that Y-doped europium complexes are always enhanced[3].The reported method in literature only measured the fluorescence emission intensity of solid samples.However,the powder particle size,sample density and test angle will affect the final result. So,it is very important to employ the precise method of integrating sphere to determine the absolute quantum efficiency of lanthanide complex.

Figure 2 Excitation spectra of EuxY1-x(TTA)3phen (lem=612 nm)
2.3Fluorescencelifetime
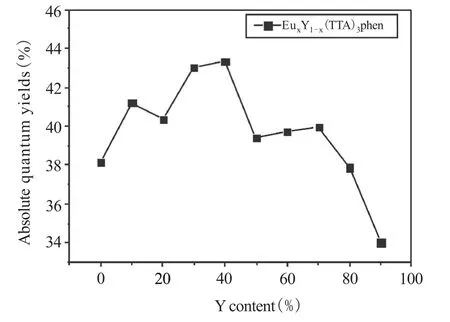
Figure 3 The absolute quantum yields with the different Y content of EuxY1-x(TTA)3phen(lex=366 nm)
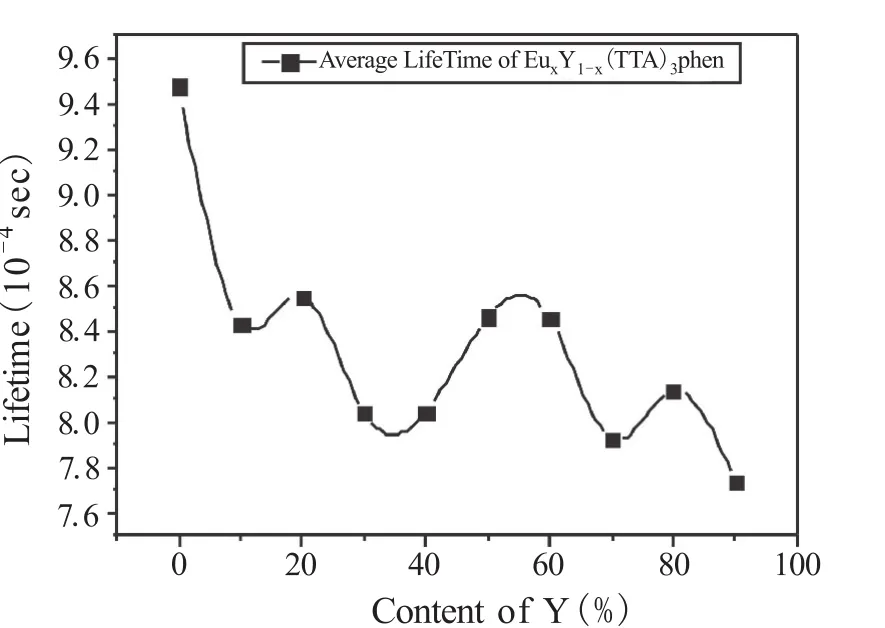
Figure 4 The relationship curves of average fluorescence lifetime and Y content of EuxY1-x(TTA)3phen (λex=370 nm,λem=612 nm)
The fluorescence lifetime of EuxY1-x(TTA)3phen is the required time that the fluorescence intensity is reduced to the original 1/e.Fluorescence lifetime characterizes the average existence time of exciton in excited state of Eu3+.Figure 4 shows the relationship curves between average fluorescence lifetime of EuxY1-x(TTA)3phen and Y content.The fitting method of fluorescence lifetime is double exponential fitting,and the range of goodness of fit is 1.00± 0.10. As can be seen from Figure 4,with the increase of Y content,the average fluorescence lifetimes of EuxY1-x(TTA)3phen decrease overall,which indicates that Y-doped will speed up transition rate of the exciton from the excited state toward ground state of Eu3+.The process of the average fluorescence lifetime reducing is of quasi-periodic trend,which indicates that Y-doped causes the change of molecular structure of EuxY1-x(TTA)3phen in the law of quasi-periodic variation.
3 Conclussiioonnss
Y-doped europium complexes EuxY1-x(TTA)3phen were synthesized in Anhydrous ethanol.IR spectra showed that Y-doped had influence on the micro-structure of EuxY1-x(TTA)3phen.Absolute fluo-rescence quantum efficiency of EuxY1-x(TTA)3phen first increases and then decreases with Y content increasing.This result once again proves that Y-doped has a certain influence on the molecular structure of europium complex,which affects the intramolecular energy transfer of EuxY1-x(TTA)3phen.Y-doped reduces the average fluorescent lifetime of EuxY1-x(TTA)3phen.With Y content increasing the fluorescence lifetime of EuxY1-x(TTA)3phen decreases in a wave downward trend.The experimental results demonstrate that doping Y can indeed increase absolute quantum efficiency of lanthanide complex.The effect doping other metal ions on absolute quantum efficiency of rare earth complex can be further investigated,such as Gd,La and other alkaline earth metal ions.
[1] Tao D L,Cui Y M,Zhang W B,et al.Effect of energy level distribution of ligands on fluorescent properties of lanthanide complexes[J].Journal of the Chinese Rare Earth Society,2010,28(6):693-698.
[2]Shen X F,Yang L Z,Zhang C,et al.Synthesis and Fluorescence Properties of Europium-Yttrium Complexes with p-Phthalic Acid,Chemical Research,2007,18(4):24-26.
[3] Yang F,Song H H,Wang J Y,et al.Synthesis and characterization of the Eu(Gd,Y)-trimesic acid complexes [J].Journal of Hebei Normal University(Natural Science Edition),2008,32(2):204-208.
[4] Tao D L,Cui Y M,Qiao R,et al.Synthesis of SiO2 coated Eu(TTA)3phen and study on its fluorescent property[J],2011,31(3):723-726.
[5] Dai T T,Liu L,Tao D L,et al.Influence of Gd doping on the absolute quantum efficiency and lifetime of EuxGd1-x(TTA)3phens[J],Chinese Chemical Letters,2014,25(6):892-896.
[6] Kong K,Zhang H X,Ma R J,et al.Synthesis,characterization and enhanced luminescence of Terbium complexes with 2-pyrazinecarboxylic acid and butanedioic acid by inert-fluorescent lanthanide ions[J].Journal of Rare Earths,2013,31(1):32-36.
[7] Hitoshi I,Seiji T,Yasuchika H,et al.Recent advances in instrumentation for absolute emission quantum yield measurements,Coordination chemistry reviews,2010,254,2449-2458.
[8] Werts M H V,Jukes R T F,Verhoeven J W.The emission spectrum and the radiative lifetime of Eu3+in luminescent lanthanide complexes[J],Physical Chemistry and Chemical Physics,2002,4(9):1542-1548.
[9] Song Y J,Sun J,Yan L L,et al.Preparation and properties of Eu0.5La0.5(TTA)3/PMMA temperature sensitive paint[J].Journal of the Chinese Society of Rare Earths,2013,31(1):55-59.
Study on fluorescence absolute quantum yield and lifetime of europium complexes by doping yttrium
FEI Bang-zhong1,TAO Dong-liang2,3*,ZHANG Hong2,CUI Yu-min2,3,ZHANG Kun2,WANG Yong-zhong2,3,YANG Sen-lin2,LU Shi-mei4
(1.Anhui Huahui Plastic Polytron Technologies Inc,Hefei Anhui 231300,China;2.School of Chemistry and Materials engineering,Fuyang Normal University,Fuyang Anhui 236037,China;3.Anhui Provincial Key Laboratory for Degradation and Monitoring of Pollution of the Environment,Fuyang Anhui 236037,China4.National Recycled Nonferrous Metals rubber Plastic Materials Quality Supervision and Inspection Center,Fuyang Anhui,236000,China)
A series of Co-luminescence EuxY1-x(TTA)3phen were synthesized in anhydrous ethanol by using Eu3+and Y3+as central ions and 2-Thenoyltrifluoroacetone(TTA)and 1,10-phenanthroline(phen)as ligands.IR spectra of the ligand TTA and EuxY1-x(TTA)3phen were determined.The absolute fluorescence quantum yields and average fluorescence lifetimes of europium complexes undergo great change after the europium complexes are doped Y into.With the Y content increasing,the absolute quantum yields of EuxY1-x(TTA)3phen first increase and then decrease,and the average fluorescence lifetimes of EuxY1-x(TTA)3phen become shorter in a wave-like pattern.These results indicate that Y-doped results in intramolecular microstructure change of EuxY1-x(TTA)3phen,which results in change of intramolecular energy transfer system of EuxY1-x(TTA)3phen.
rare earth complex;doping;fluorescence;lifetime;absolute quantum yield
O643
A
1004-4329(2016)02-041-05
10.14096/j.cnki.cn34-1069/n/1004-4329(2016)02-041-05
2016-01-10
安徽省高校自然科学研究重点项目(KJ2016A550),阜阳师范学院重大科技成果孵化基金项目(kjfh20160 6),安徽省科技攻关计划项目(1301042112),省级科研机构校级委托专项课题(2014HJJC02),安徽高校省级自然科学研究一般项目(2015KJ001)资助。
费邦忠(1971-),男,硕士,工程师,研究方向:稀土发光材料。
陶栋梁(1972-),男,博士,教授,研究方向:精细化学品和稀土发光材料。Email:tdlpku@163.com。
——电视连续剧《栋梁》主题曲

