丛枝菌根真菌参与下植物-土壤系统的养分交流及调控
韦莉莉, 卢昌熠, 丁 晶, 俞 慎
1 中国科学院城市环境研究所,厦门 361021 2 中国科学院大学,北京 100049
丛枝菌根真菌参与下植物-土壤系统的养分交流及调控
韦莉莉1,*, 卢昌熠1,2, 丁晶1,2, 俞慎1
1 中国科学院城市环境研究所,厦门3610212 中国科学院大学,北京100049
近几年随着有机农业的发展,丛枝菌根的作用受到特别关注。丛枝菌根是由植物根系与丛枝菌根真菌(AMF)形成的一种共生体。在植物-AMF-土壤系统中,AMF为植物提供N、P等营养的同时从根系得到所需的C。概述了植物-AMF-土壤系统中C、N、P等营养物质的交流以及AMF与土壤微生物的互作关系。丛枝菌根的形成可显著提高植物对P的吸收,且在高P条件下多余的P可储存于AMF中。AMF对土壤N循环的影响相当复杂,可能参与调控N循环的多个过程,如硝化作用、反硝化作用和氨氧化作用等。在有机质丰富的土壤中AMF菌丝可快速扩增并吸收其中的N,主要供菌丝自身所需,只有一小部分传递给植物。AMF对土壤C库的影响尚存争议,可能存在时间尺度的差异。短期内可活化土壤C,而在长期尺度上可能有利于土壤C的储存。AMF能够通过改变土壤微生物群落结构而影响植物-土壤体系的物质交流。AMF与解磷菌、根瘤菌和放线菌的协同增效作用可促进土壤有机质的降解或增强其固氮能力;AMF对氨氧化菌的抑制作用可降低氨的氧化减少N2O的释放。AMF与外生共生真菌EMF共存时,表现出协同增效作用,但EMF的优先定殖会限制AMF的侵染。AMF不同类群之间则主要表现为竞争和拮抗关系。AMF与土壤微生物之间的互作关系受土壤无机环境的影响,在养分亏缺条件下微生物之间往往表现为竞争关系。因植物、AMF与土壤微生物之间存在复杂的互作关系,为此AMF并不总是表现出其对植物营养的促进作用。目前关于AMF的作用机理仍以假说为主,需要进一步的实验验证。在植物-AMF-土壤系统中N与C的交流和P与C的交流并未表现出一致性,对N、P循环相互关系的进一步探讨有助于深入理解植物-土壤体系中的养分循环。植物、AMF和土壤微生物的养分来源及其对养分的相对需求强度和吸收效率尚未可知,因此无法深入理解AMF在植物-土壤体系中养分交流和转化的作用。在方法上,传统的土壤学方法在养分动态研究中存在局限性,现代分子生物学手段和化学计量学的结合值得尝试。
丛枝菌根;根际;土壤微生物;养分循环;植物-AMF-土壤系统
菌根是植物根系与菌根真菌形成的共生体,广泛存在于自然界中,约80%的陆地植物能够形成菌根[1]。其中,丛枝菌根(Arbuscularmycorrhiza)是最古老且分布最为广泛的一类菌根[2],是由丛枝菌根真菌(Arbuscularmycorrhizalfungi,AMFs)在植物根细胞内形成的一种分枝共生结构。丛枝菌根真菌不仅存在于自然生态系统中,还可与大部分农作物的根系形成菌根[1]。AMF为宿主植物提供N、P等无机养分,同时从宿主植物获得C源。植物固定的含碳化合物近20%为AMF所利用[3-4]。AMF作为养分交换的通道,能够促进生态系统地上和地下部分进行养分交流,因而在生态系统养分循环中起重要的调节作用[5-7]。但过去关注的焦点在于AMF对植物营养方面的贡献,很少从植物-AMF-土壤系统,整体考察物质循环及其之间的平衡关系。本文重点阐述植物-AMF-土壤体系的碳氮磷物质流及AMF与土壤微生物的互作关系,旨在为今后开展植物养分利用率的协同调控研究和生产实践提供参考。
1 丛枝菌根促进植物-土壤系统的物质交流
1.1对植物-土壤系统磷的调控
磷素极易被固定于土壤中而使其有效性降低[8],尤其在C∶P 比值较高(>300)的土壤中。因此土壤中有效P的水平通常很低,一般只占总P的2%—3%。加之植物对P的吸收往往使根际处于低P状态,根细胞中的P含量远高于根际土壤,使植物对P的进一步吸收更加困难,因此P的吸收过程需要高亲和转运蛋白(PiTs)的协助,同时消耗代谢释放的能量[9]。植物在长期进化过程中形成了特定的适应机制协助植物从土壤中摄取P。其中,菌根的形成可有效地促进植物对土壤P的吸收。在形成丛枝菌根的植物体中,AMF可为植物提供高达100%的P[10]。
AMF菌丝可直接从土壤中吸收无机P,大大扩展了植物吸收P的范围。AMF菌丝的长度远超过植物的根长,在热带森林中,菌丝长度可达根长的13倍[11],覆盖范围可超过根系的700倍。同时,AMF的侵染可引起一系列土壤理化性质的改变,这些变化有可能促进有机P的矿化和难溶态P的解离。比如,植物对P的吸收与菌丝分泌的磷酸酶含量呈正相关,接种AMF后的土壤根际磷酸酶活性增加[12]。但根系和菌丝体的分泌物存在一定差异,致使根际和菌丝圈的理化性质也有所区别,如根际的水解酶活性较高,而菌丝圈的磷酸酶活性较高[13]。另外,AMF分泌的有机酸可降低土壤pH值,也可能活化土壤P[14]。
根系和AMF两种吸收途径对植物P的相对贡献随P含量的变化而不同。在低P条件下,植物主要依赖于菌丝对P的吸收[10]。通常菌丝吸收的P远超过根系直接吸收的量。模型模拟的结果显示,菌丝吸收P的速率比根的吸收速率高一个数量级[14],根对P的吸收甚至可能因接种AMF而完全被抑制[15]。研究表明,虽然接种和未接种作物P的吸收总量没有显著差异,但接种作物吸收的P大部分来自菌丝[10]。AMF对根系吸收的限制可能由两者的竞争所致,也可能由于转运蛋白的减少[16]。因根的吸收过程需要消耗能量,因此推测AMF对根吸收的抑制作用可能在一定程度上能够减少能量的消耗。
在高P条件下,AMF的生物量显著减少[17]。Balzergue 等观察到在P含量高达750 mM条件下,豌豆(Pisumsativum)根系与AMF的物质交流几乎完全停止[17]。最新的研究表明,在高P条件下AMF限制P向地上部传递。随P施入量的增加,未接种的豆科植物地上部的P含量增加了143%,而接种植物地上部分的P含量仅增加了53%,地下部分P的增加幅度没有显著差异[18]。可见,在高P条件下多余的P可能储存在菌丝里。AMF在植物P吸收方面的研究相对较多,但仍然未能解答土壤高P对AMF抑制作用的机理。虽然有学者认为独脚金内酯可能参与了调节过程,但在高P条件下植物产生的独脚金内酯也随之减少,因而很可能存在其他信号物质参与调节AMF的定殖[18]。
1.2对植物-土壤系统氮循环的调节
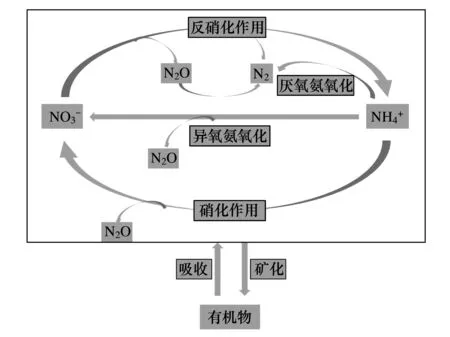
图1 丛枝菌根参与调控的土壤氮循环过程示意图Fig.1 Nitrogen processes regulated by AMF

最新的研究结果预示,AMF可能在温室气体控制方面具有重要的调控作用。Storer[27]通过一系列实验证明,接种AMF能够降低土壤N2O的释放。AMF对N2O的调控主要是通过抑制硝化菌来完成的。因为接种丛枝菌根的植物对N的吸收量增加,因而减少了硝化作用的底物,这可能也是N2O排放量减少的原因之一。另外,N2O也可能被反硝化细菌所消耗,该过程发生在反硝化作用的最后阶段,并在氧化亚氮还原酶的参与下完成[28]。因水分条件直接影响氮循环过程,因此推测丛枝菌根对N2O 的调控可能还与丛枝菌根对植物水分状态的调节有关[29]。
近年来的研究发现,丛枝菌根可直接吸收有机质中的N[19-23]。同位素示踪实验显示,在有机质斑块中AMF菌丝迅速扩增[19-23],菌丝中高达31%的15N来自有机质斑块,并将一小部分吸收的15N传递给宿主植物,但菌丝中的13C并未显著增加[19]。可见,菌丝吸收的N来自有机质降解后的无机含氮化合物。不过目前的研究只观察到Glomeraceae类AMF具有吸收有机质N源的特性,而接种Gigasporaceae的菌根对植物N吸收的贡献较小[24-25]。AMF菌丝并不具有降解有机质的功能[26],可能通过菌丝分泌物刺激特定微生物群落的生长,加速对有机质的降解,间接影响菌丝对N的吸收[19-25]。而且,AMF从有机质中吸收的N主要用于菌丝自身的生长[19]。虽然磷脂脂肪酸图谱分析的结果显示,微生物群落结构并未因菌丝的存在而发生改变,但13C磷脂脂肪酸和DNA的分析结果表明,AMF菌丝的介入改变了土壤微生物的组成[28-30]。Toljander 等验证了菌丝分泌物对土壤微生物组成的改变,并鉴定出菌丝分泌物包含如蔗糖和有机酸等小分子化合物,以及一些尚未鉴别的大分子聚合物[30]。但上述实验并未探明菌丝分泌物是否促进特定微生物群落,如具有降解有机质功能的微生物群落的生长。
1.3影响土壤碳库
虽然已有研究观察到丛枝菌根的形成能够加速有机质的降解,但其生态意义并未得到关注。2012年Cheng等在Science上发文指出,由于菌根促进表层土新鲜凋落物和土壤有机质的降解,可能影响到土壤碳库的储量[11]。该文引发了激烈的讨论。Kowalchuk[31]在同期Science上以“Bad news for carbon sequestration?”为题,强调了这一研究结果的生态意义,即丛枝菌根通过促进有机质的降解可能使土壤CO2排放量增加,同时减少土壤C库的存量。2013年,德国和澳大利亚学者联合发表了一篇论述,分析了由丛枝菌根引起的表层有机质降解对生态系统土壤C库的短期和长期效应,指出短期内丛枝菌根的存在会引起土壤C库的暂时性减少,但从长期来看,多种因素的综合作用反而可能增加土壤C的储存[32]。比如,有机质降解后产生的难降解物质的输入、菌丝的输入、菌丝对土壤团聚体的稳定作用(保护有机质不被降解)、以及植物凋落物输入的增加等,都可能引起土壤有机碳的增加(图2)[33-36]。
1.4丛枝菌根调节下植物-土壤系统中C、N、P的交互作用
AMF与宿主植物之间的物质交流处于动态平衡中,随植物-AMF-土壤系统中能量和养分状态而进行调整。AMF与根系的相互识别和物质交流依赖于信号物质的调控。根系分泌的独脚金内酯能够促进AMF的代谢和分枝,AMF则分泌信号物质刺激根系做出响应[3]。在缺P条件下,根系选择性地为传递较多养分的菌丝提供较多的C。比如,向贫瘠土壤中添加少量或适量的P,菌根根际的水溶性C含量增加[16];但当植物体内P含量较高时,根向AMF提供的C则减少[18]。根系提供的C是AMF的唯一碳源,因此植物的生长状况直接影响AMF可获得的碳量。遮阴处理后植物供给AMF的C减少,但在根际添加蔗糖后,根系和AMF吸收利用的C量均随之增加[19]。Konvalinková等[37]观察到,即使是短期的遮荫处理对AMF的功能也会产生较大的影响,AMF传递给植物的P显著减少,虽然定殖率和菌丝的生长未出现显著变化。离体实验的结果显示,当外源C的供给减半,AMF(Glomusintraradices)的孢子和菌丝里能累积高达7倍的P,占真菌干重的4%[38]。在低C条件下,菌丝未减弱自身的生长但限制了养分向植物的传递。储存在AMF里的P主要以复合磷酸盐的形式存在,植物在缺P条件下,复合磷酸盐能够被碱性磷酸酶解聚成为植物可利用的形式。因而,植物中的C、P含量跟AMF中的C、P含量处于动态平衡中(图3)。
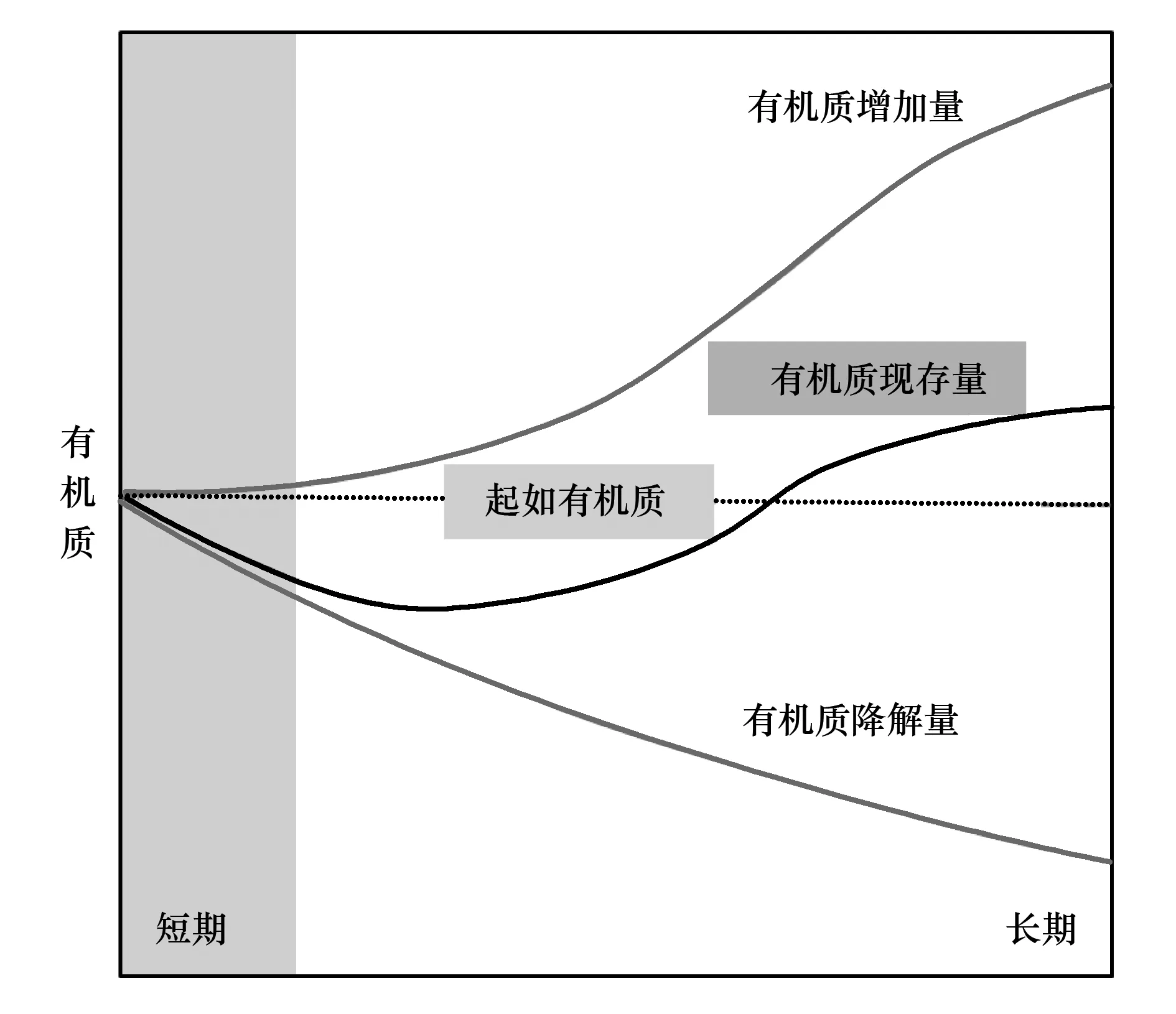
图2 土壤有机质的长期和短期动态概念图Fig.2 Conceptual short-term vs long-term dynamics of organic matter 根据Verbruggen[32-36]修正
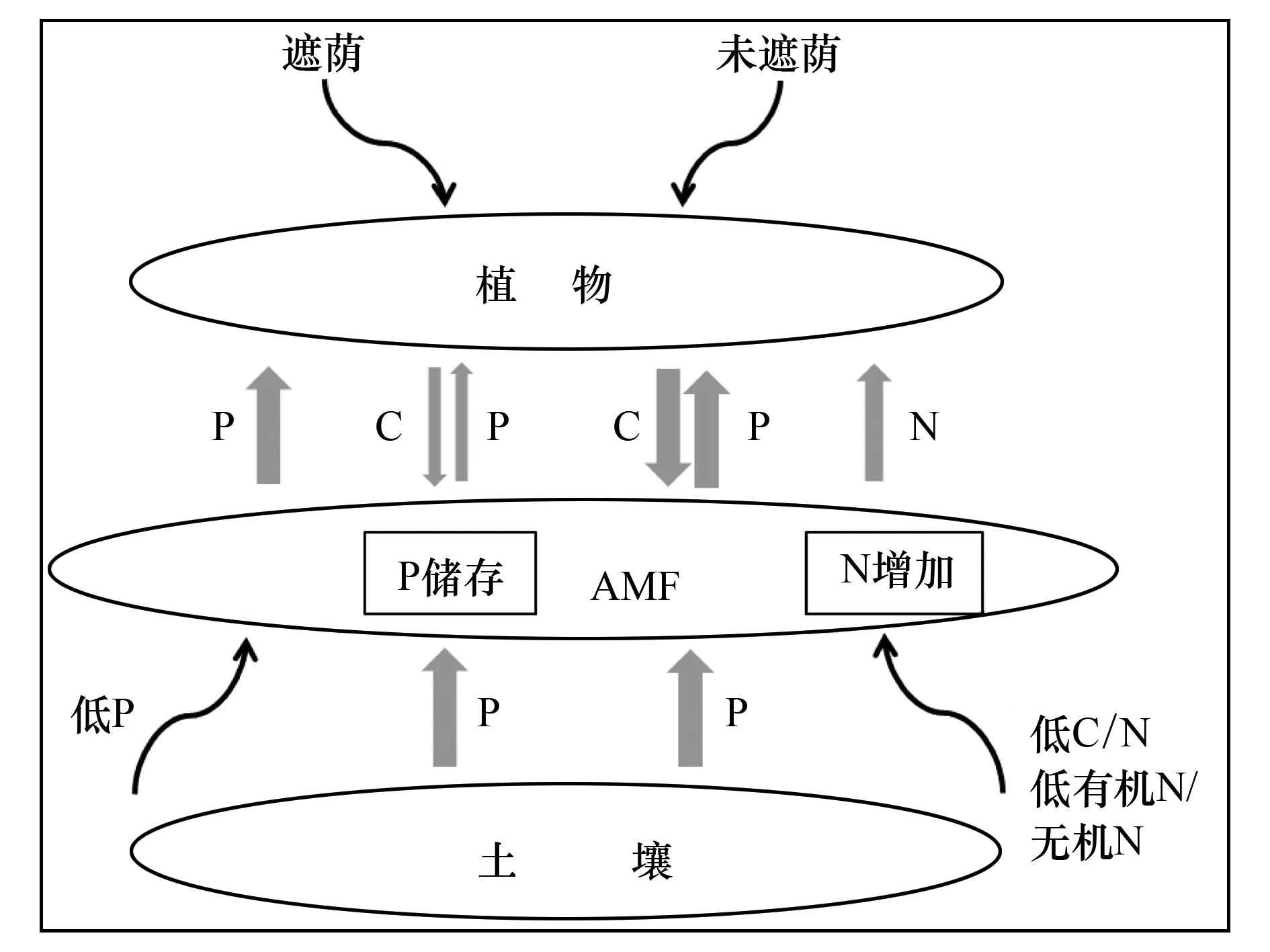
图3 植物-AMF-土壤系统中C-N-P的动态平衡示意图Fig.3 Trade-offs between C-N-P in the Plant-AMF-soil system灰色的箭头粗细代表元素流的量
过去曾推测AMF与植物进行着类似的C-N交换,即AMF为能够供给较多C的根系传递较多的N。但最近的研究表明,AMF菌丝对N的吸收和向植物的传递不受植物供给C量的影响[19]。菌丝的定殖和扩增受土壤有机质C∶N比的调控。AMF菌丝往往定殖于C∶N比较低的有机质土壤中,随着C∶N比的增加,N矿化速率和N2O通量随之降低(图3)[39]。AMF的定殖还与土壤中有机氮和无机氮的比例有关。丛枝菌根常形成于有机氮含量较低(有机氮∶无机氮比较低)的土壤中(图3),但AMF菌丝在富含有机氮的土壤中可快速扩增[19,23]。可见,AMF对土壤N和P的吸收以及向植物的传递及其与植物C源的交流并未表现出一致性。
2 丛枝菌根与土壤微生物的相互作用
对AMF与土壤中特定微生物之间相互作用的认识,有助于我们对土壤养分循环的深入理解,同时也为土壤温室气体排放的调控提供理论支持[40]。目前对AMF与其他土壤微生物的相互作用还知之甚少。土壤是非常复杂的系统,且处于极端动态的过程中,要对土壤进行微观尺度的观察和研究具有一定的难度[41]。近年来,以DNA为基础的分析技术和方法得以快速发展。DNA量化分析的结果表明,AMF与土壤微生物具有很强的相互作用[42-45]。
Marschner等[46]证明了菌根植物和非菌根植物根际具有不同的细菌群落。Miransari M等证明,AMF的侵染可直接或间接地影响根际微生物群落的数量和结构,使其发生变化并达到新的平衡[45]。在大多数陆生植物中,菌根真菌与土壤微生物存在着互惠互利的关系[46]。AMF接种后,菌丝分泌的磷酸酶能够提高土壤可利用P含量,球囊霉素可改变土壤结构、抑制病原菌、促进有益微生物的生长等。土壤微生物可能通过产生植物激素、改善土壤养分状况和土壤结构、控制病原菌和影响植物生长等途径,促进AMF的生长。但土壤微生物也可能因竞争养分资源对AMF产生抑制作用[47]。
通常,丛枝菌根的形成可显著增加根表面细菌、放线菌和固氮菌的数量,而对真菌的影响很小。但亦有少数实验并未观察到菌根对根际微生物产生促进作用。不同类群的微生物对AMF的响应不同,比如何氏球囊霉(Glomushoi)表现为正响应效应,而放线菌和丛毛单胞菌则显示负响应效应。AMF还可能通过影响微生物酶活性而改变微生物群落结构。其他土壤微生物对AMF的影响表现为多元化,既可能促进,也可能不产生任何影响,甚至可能抑制AMF的生长。
2.1AMF与非共生土壤微生物
2.1.1AMF与非共生细菌
AMF与土壤细菌可协同促进植物对P的吸收。Zhang等[48]利用尼龙网隔实验证明了AMF (Rhizophagusirregularis)和解磷菌(Pseudomonasalcaligenes)具有协同增效作用。单独接种AMF或解磷菌均能刺激土壤中酸性磷酸酶的活性。相对而言,单独接种AMF的土壤中酸性磷酸酶活性较高,而同时接种AMF和解磷菌土壤中酸性磷酸酶活性更高。但如果土壤P很低,AMF与土壤微生物可能因竞争有限的养分而发生相互抑制作用[13,39]。

2.1.2AMF与非共生真菌
关于AMF与非共生真菌相互作用的研究相对较少。AMF可能会抑制其他真菌或者与特定真菌表现为协同效应。例如,油松幼苗的丛枝菌根抑制了根际土壤中一部分真菌的生长,使油松幼苗根际土壤中真菌的数量和种类减少[53];AMF(Glomusmosseae)与黄海葵附生真菌(Penicilliunmthomii)能够协同促进英国薄荷(Menthapiperita)的生长,提高植株的营养水平[54]。AMF的侵染可显著改变腐生真菌的群落结构,尤其是降低对葡萄糖敏感的菌群生物量[55]。
2.2AMF与其他共生菌
2.2.1AMF与根瘤菌
AMF和根瘤菌往往同时存在于豆科植物的根系。AMF定殖于根的皮质,固氮菌则一般定殖于根瘤。但在胞外菌丝上也可能着生固氮菌[56]。最近的实验还观察到AMF直接定殖于根瘤上[16,57]。丛枝菌根能够促使根瘤菌的形成并增强固氮作用[58],但根瘤菌在根部的侵染抑制了AMF的定殖。丛枝菌根可能为根瘤提供P和其他营养元素如铜和锌,从而增强根瘤的固氮作用[59-60]。而Larimer等和Kaschuk等对大量实验结果的Meta分析表明,虽然单独接种AMF和根瘤菌对植物N营养都有促进作用,但同时接种两种菌类并未起到协同增效作用[61-62]。
低P是根瘤形成的主要限制因子,AMF与根瘤菌的相互作用取决于土壤P含量[63]。AMF为固氮菌提供其所需要的P,加强其固氮能力,进而为植物提供更多的N[64-65]。此外,接种AMF促进宿主植物的生长发育,为根瘤菌提供充足的能量,最终将促进植物固N。在豆科植物中,AMF与根瘤菌在根中的定殖过程享有一条共同的信号传导途径,这对AMF与根瘤菌的相互作用非常重要。土壤N的水平也影响AMF与根瘤菌的相互作用。施N可降低根瘤的形成,但丛枝菌根的形成能够在一定程度上抵消因N增加对根瘤产生的抑制作用[58]。在只有根瘤菌存在的根系中,如果不施肥则会形成较多的根瘤,但如果同时接种AMF,则施肥对根瘤不产生显著影响。
2.2.2AMF与放线菌
AMF与放线菌的相互作用能够有效的提高宿主植物的固N能力,改善植物的营养状况从而促进宿主植物的生长发育。弗兰克氏放线菌(Frankia)与非豆科植物共生形成根瘤[66]。放线根瘤与丛枝菌根同时接种于黑桤木(Alnusglutinosa)幼苗6个月后,表现出显著地协同增效作用,接种放线菌还促进了AMF菌丝的伸长生长[67]。AMF对放线菌也有促进作用,如辣椒根系中的放线菌数量因接种AMF而增加。同时接种AMF和放线菌,对植物的生长具有更明显的促进作用,植物吸收更多的养分元素,形成的根瘤数量更多、更大;但相对而言,单独接种AMF对植物生长的促进作用更加显著[66-68]。放线菌的侵染可促进AMF的定殖和植物养分的吸收。混合接种AMF和弗兰克放线菌的西藏沙棘,植株的固N水平和生物量明显高于单独接种AMF或单独接种弗兰克放线菌的植株[69]。但AMF与放线菌并非始终保持协同增效作用。在一定条件下,AMF与根际放线菌之间会产生拮抗作用,并对宿主植物的生长有抑制作用[70]。
2.2.3AMF与EMF
有些植物如木麻黄属植物能够同时与AMF和外生菌根真菌(EMF)形成共生体[1]。双重接种AMF和EMF能促进植物的生长和植物对P的吸收,同时也能促进N向其他植物的传递[71-73]。自然条件下AMF比EMF先定殖,AMF的定殖对EMF没有影响。但如果EMF先定殖,可通过形成包膜隔绝AMF在根表面定殖。有实验观察到AMF与EMF发生拮抗,表现为EMF具有较高的定殖率,而AMF定殖率降低[74]。
2.3AMF的种间竞争
长期以来,关于AMF与植物相互关系的研究通常在土壤灭菌条件下进行,忽略了AMF之间相互作用的影响。然而最新的研究表明,AMF存在种间竞争[75-76]。Janoušková等[75]观察到,增加AMF侵染密度并未促进植物的生长,甚至因AMF的种间竞争而抑制植物的生长。相对于根际,AMF之间的竞争在根内表现更加剧烈[76]。AMF之间的竞争强度还与宿主提供的C量有关[77]。荫蔽处理(向AMF传输的C减少)的菌根共生体中,竞争力相对较弱的真菌(如Rhizophagusirregularis)其生长将被完全抑制[78]。Werner和Kiers发现[79],AMF之间的相互作用还与定殖顺序有关。先定殖的AMF对后定殖的AMF产生抑制作用,且随定殖时间间隔的延长,抑制作用加强,但后定殖的AMF并未对先定殖的AMF产生显著影响。土壤养分含量可在一定程度上调控AMF的种间相关性。施肥可改变AMF的群落结构,施入少量的P肥使Glomus spp.类AMF成为优势种,且有新的AMF定殖。当施入大量P肥,只观察到其中的两种AMF存在于土壤中[57]。
3 问题与展望
丛枝菌根在改善土壤结构、增加土壤养分、促进植物对养分的吸收以及减少温室气体排放等方面的作用,对整个生态系统的养分循环和养分平衡的维持具有重要意义。更好地理解AMF与其他土壤微生物的互作关系对于可持续农业管理也是必要的。
相对P而言,丛枝菌根对植物-土壤系统N循环的研究起步较晚,存在疑问较多。与P不同的是,AMF对N的吸收与植物提供的C似乎无关,而与有机质含量密切相关。AMF可在有机质斑块中快速扩增,使有机质降解加快,吸收其中的N供自身生长所需,并传递其中的一小部分N给植物,同时将来自有机质的C传递给其他土壤微生物[7]。有大量实验结果显示,接种AMF的植物其N含量并没有显著增加[4,25,80]。因此,丛枝菌根在N循环和植物N营养方面的生态意义尚存在较大争议。Reynolds等[24]提出,只有在缺N条件下菌根对植物N的吸收才有意义。Hodge和Fitter[19]以及Veresoglou[25]则认为即使在高N水平下,AMF仍然对植物的N营养发挥作用,并认为其意义可能与减少代谢消耗有关。因为相对于根系而言,菌丝的生长需要消耗的能量较少[81]。
植物与丛枝菌根真菌之间的物质交流是有选择的维持着动态平衡。土壤C、N、P之间存在复杂的联系。对这些错综复杂关系的深入理解将有利于有效利用丛枝菌根调控土壤养分循环。在植物-AMF-土壤系统中,AMF的作用是从土壤中吸收N和P,在满足自身需求的前提下将养分输送给植物和土壤微生物,同时将来自植物和有机质中的C传输给土壤微生物。那么,在植物-AMF-土壤系统中C、N、P究竟如何分配,C、N、P循环存在怎样的耦合关系目前尚不清楚。最新研究表明,在剧烈气候变化条件下,由于控制这些元素生物地球化学过程的不同步,C、N、P循环的平衡关系可能被打破[82]。据此推测,人为干扰下造成的AMF多样性的改变将引起生态系统中C、N、P循环的解耦合。因此,变化环境条件下AMF与C、N、P循环的关系值得特别关注。
AMF增效作用的发挥受多种环境因素的调控。一方面受土壤环境中无机养分含量的影响,另一方面受植物生长和土壤微生物群落结构的调控。虽然目前尚未开展相关的研究,但依据生态学种间关系的原理,AMF、植物和土壤微生物的C、N和P来源及三者对养分的相对需求强度在植物-AMF-土壤微生物的相互关系中起决定性作用。随着土壤可供养分的增加,三者之间的关系可由竞争转为互利。已有实验表明,在养分亏缺条件下,AMF向植物和土壤微生物传输的N、P显著减少, AMF与植物和土壤微生物表现为竞争关系。随着养分的增加,AMF与土壤微生物对植物养分的吸收表现为协同增效作用。因此,进一步探讨其相互关系的前提首先是确定AMF、植物和土壤微生物的养分来源。AMF的C源来自植物,土壤微生物的C则一方面来自植物,另一方面来自土壤有机质。有趣的是虽然来自植物的C是AMF的唯一碳源,但对热带森林菌根的研究发现,无论在根际还是AMF的菌丝圈植物分泌的C都优先供给土壤微生物[13]。那么这一现象是否普遍存在于菌根土壤中值得进一步探讨。AMF、植物和土壤微生物的N和P均来自土壤,那么由菌丝吸收的养分优先供给自身所用还是优先供给土壤微生物或宿主植物尚不可知。虽然,目前已知菌丝从有机质中吸收的N可能首先用于自身的扩增,但其在菌丝体N营养所占比例如何也还不清楚。
目前的研究集中于根际范围,而AMF菌丝远远超越了根系分布的范围,菌丝圈(即围绕菌丝的区域)也是土壤微生物活跃的区域[23,69],需要寻找有效的方法开展菌丝圈的研究。另外,将丛枝菌根真菌与传统的有机肥和化学肥料相结合,大规模的推广运用到现实农业中,对农业生产和生态环境都具有重要意义。如何将丛枝菌根真菌运用到现实农业生产中应成为今后研究的重点。
[1]Wang B, Qiu Y L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza, 2006, 16(5): 299-363.
[2]Harrison M J. Signaling in the arbuscular mycorrhizal symbiosis. Annual Review of Microbiology, 2005, 59: 19-42.
[3]Parniske M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nature Reviews Microbiology, 2008, 6(10): 763-775.
[4]Smith F A, Smith S E. What is the significance of the arbuscular mycorrhizal colonisation of many economically important crop plants? Plant and Soil, 2011, 348(1/2): 63-79.
[5]Cheng L, Booker F L, Tu C, Burkey K O, Zhou L S, Shew H D, Rufty T W, Hu S J. Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science, 2012, 337(6098): 1084-1087.
[6]Bissett A, Brown M V, Siciliano S D, Thrall P H. Microbial community responses to anthropogenically induced environmental change: Towards a systems approach. Ecology Letters, 2013, 16(S1): 128-139.
[7]Johnson N C, Angelard C, Sanders I R, Kiers E T. Predicting community and ecosystem outcomes of mycorrhizal responses to global change. Ecology Letters, 2013, 16(S1): 140-153.
[8]Querejeta J I, Allen M F, Caravaca F, Roldán A. Differential modulation of host plant δ13C and δ18O by native and nonnative arbuscular mycorrhizal fungi in a semiarid environment. New Phytologist, 2006, 169(2): 379-387.
[9]Bucher K, Belli S, Wunderli-Allenspach H, Krämer S D. P-glycoprotein in proteoliposomes with low residual detergent: The effects of cholesterol. Pharmaceutical Research, 2007, 24(11): 1993-2004.
[10]Smith S E, Smith F A, Jakobsen I. Functional diversity in arbuscular mycorrhizal (AM) symbioses: The contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytologist, 2004, 162(2): 511-524.
[11]Camenzind T, Rillig M C. Extraradical arbuscular mycorrhizal fungal hyphae in an organic tropical montane forest soil. Soil Biology and Biochemistry, 2013, 64: 96-102.
[12]刘进法, 夏仁学, 王明元, 王鹏, 冉青青, 罗园. 接种丛枝菌根真菌对枳吸收利用磷酸铝的影响. 应用生态学报, 2008, 19(10): 2155-2160.
[13]Nottingham A T, Turner B L, Winter K, Chamberlain P M, Stott A, Tanner E V J. Root and arbuscular mycorrhizal mycelial interactions with soil microorganisms in lowland tropical forest. FEMS Microbiology Ecology, 2013, 85(1): 37-50.
[14]Schnepf A, Jones D, Roose T. Modelling nutrient uptake by individual hyphae of arbuscular mycorrhizal fungi: Temporal and spatial scales for an experimental design. Bulletin of Mathematical Biology, 2011, 73(9): 2175-2200.
[15]Duan T Y, Facelli E, Smith S E, Smith F A, Nan Z B. Differential effects of soil disturbance and plant residue retention on function of arbuscular mycorrhizal (AM) symbiosis are not reflected in colonization of roots or hyphal development in soil. Soil Biology and Biochemistry, 2011, 43(3): 571-578.
[16]Alguacil M D M, Lozano Z, Campoy M J, Roldán A. Phosphorus fertilisation management modifies the biodiversity of AM fungi in a tropical savanna forage system. Soil Biology and Biochemistry, 2010, 42(7): 1114-1122.
[17]Balzergue C, Puech-Pagès V, Bécard G, Rochange S F. The regulation of arbuscular mycorrhizal symbiosis by phosphate in pea involves early and systemic signalling events. Journal of Experimental Botany, 2011, 62(3): 1049-1060.
[18]Nazeri N K, Lambers H, Tibbett M, Ryan M H. Do arbuscular mycorrhizas or heterotrophic soil microbes contribute toward plant acquisition of a pulse of mineral phosphate? Plant and Soil, 2013, 373(1/2): 699-710.
[19]Hodge A, Fitter A H. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(31): 13754-13759.
[20]Tanaka Y, Yano K. Nitrogen delivery to maize via mycorrhizal hyphae depends on the form of N supplied. Plant, Cell & Environment, 2005, 28(10): 1247-1254.
[21]Bago B, Vierheilig H, Piché Y, Azcón-Aguilar C. Nitrate depletion and pH changes induced by the extraradical mycelium of the arbuscular mycorrhizal fungusGlomusintraradicesgrown in monoxenic culture. New Phytologist, 1996, 133(2): 273-280.
[22]Barrett G, Campbell C D, Fitter A H, Hodge A. The arbuscular mycorrhizal fungusGlomushoican capture and transfer nitrogen from organic patches to its associated host plant at low temperature. Applied Soil Ecology, 2011, 48(1): 102-105.
[23]Hodge A, Campbell C D, Fitter A H. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature, 2001, 413(6853): 297-299.
[24]Reynolds H L, Hartley A E, Vogelsang K M, Bever J D, Schultz P A. Arbuscular mycorrhizal fungi do not enhance nitrogen acquisition and growth of old-field perennials under low nitrogen supply in glasshouse culture. New Phytologist, 2005, 167(3): 869-880.
[25]Veresoglou S D, Sen R, Mamolos A P, Veresoglou D S. Plant species identity and arbuscular mycorrhizal status modulate potential nitrification rates in nitrogen-limited grassland soils. Journal of Ecology, 2011, 99(6): 1339-1349.
[26]Smith S E, Read D J. Mycorrhizal Symbiosis. San Diego, CA, USA: Academic Press, 2008.
[27]Storer K E. Interactions between arbuscular mycorrhizal fungi and soil greenhouse gas fluxes. PhD thesis, University of York, 2013.
[28] Herman D J, Firestone M K, Nuccio E, Hodge A. Interactions between an arbuscular mycorrhizal fungus and a soil microbial community mediating litter decomposition. FEMS Microbiology Ecology, 2012, 80(1): 236-247.
[29]Nuccio E E, Hodge A, Pett-Ridge J, Herman D J, Weber P K, Firestone M K. An arbuscular mycorrhizal fungus significantly modifies the soil bacterial community and nitrogen cycling during litter decomposition. Environmental Microbiology, 2013, 15(6): 1870-1881.
[30]Toljander J F, Lindahl B D, Paul L R, Elfstrand M, Finlay R D. Influence of arbuscular mycorrhizal mycelial exudates on soil bacterial growth and community structure. FEMS Microbiology Ecology, 2007, 61(2): 295-304.
[31]Kowalchuk G A. Bad news for soil carbon sequestration? Science, 2012, 337(6098): 1049-1050.
[32]Verbruggen E, Veresoglou S D, Anderson I C, Caruso T, Hammer E C, Kohler J, Rillig M C. Arbuscular mycorrhizal fungi—short-term liability but long-term benefits for soil carbon storage? New Phytologist, 2013, 197(2): 366-368.
[33]Zhu Y G, Miller R M. Carbon cycling by arbuscular mycorrhizal fungi in soil-plant systems. Trends in Plant Science, 2003, 8(9): 407-409.
[34]Rillig M C. Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecology Letters, 2004, 7(8): 740-754.
[35]Wilson G W T, Rice C W, Rillig M C, Springer A, Hartnett D C. Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: Results from long-term field experiments. Ecology Letters, 2009, 12(5): 452-461.
[36]Hoeksema J D, Chaudhary V B, Gehring C A, Johnson N C, Karst J, Koide R T, Pringle A, Zabinski C, Bever J D, Moore J C, Wilson G W T, Klironomos J N, Umbanhowar J. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecology Letters, 2010, 13(3): 394-407.
[37]Konvalinková T, Püschel D, Janoušková M, Gryndler M, Jansa J. Duration and intensity of shade differentially affects mycorrhizal growth-and phosphorus uptake responses ofMedicagotruncatula. Frontiers in Plant Science, 2015, 6: 65-65.
[38]Hammer E C, Pallon J, Wallander H, Olsson P A. Tit for tat? A mycorrhizal fungus accumulates phosphorus under low plant carbon availability. FEMS Microbiology Ecology, 2011, 76(2): 236-244.
[39]Leigh J, Hodge A, Fitter A H. Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytologist, 2009, 181(1): 199-207.
[40]Butterbach-Bahl K, Baggs E M, Dannenmann M, Kiese R, Zechmeister-Boltenstern S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philosophical Transactions of the Royal Society B: Biological Sciences, 2013, 368(1621): 20130122.
[41]Nazaries L, Murrell J C, Millard P, Baggs L, Singh B K. Methane, microbes and models: Fundamental understanding of the soil methane cycle for future predictions. Environmental Microbiology, 2013, 15(9): 2395-2417.
[42]Dumbrell A J, Nelson M, Helgason T, Dytham C, Fitter A H. Idiosyncrasy and overdominance in the structure of natural communities of arbuscular mycorrhizal fungi: Is there a role for stochastic processes? Journal of Ecology, 2010, 98(2): 419-428.
[43]Welc M, Ravnskov S, Kieliszewska-Rokicka B, Larsen J. Suppression of other soil microorganisms by mycelium of arbuscular mycorrhizal fungi in root-free soil. Soil Biology and Biochemistry, 2010, 42(9): 1534-1540.
[44]Scheublin T R, Sanders I R, Keel C, van der Meer J R. Characterisation of microbial communities colonising the hyphal surfaces of arbuscular mycorrhizal fungi. The ISME Journal, 2010, 4(6): 752-763.
[45]Miransari M. Interactions between arbuscular mycorrhizal fungi and soil bacteria. Applied Microbiology and Biotechnology, 2011, 89(4): 917-930.
[46]Marschner P, Crowley D E, Lieberei R. Arbuscular mycorrhizal infection changes the bacterial 16 S rDNA community composition in the rhizosphere of maize. Mycorrhiza, 2001, 11(6): 297-302.
[47] 宰学明, 郝振萍, 赵辉, 钦佩. 丛枝菌根化滨梅苗的根际微生态环境. 林业科学, 2014, 50(1): 41-48.
[48]Zhang L, Fan J Q, Ding X D, He X H, Zhang F S, Feng G. Hyphosphere interactions between an arbuscular mycorrhizal fungus and a phosphate solubilizing bacterium promote phytate mineralization in soil. Soil Biology and Biochemistry, 2014, 74: 177-183.
[49]Bollmann A, Bär-Gilissen M J, Laanbroek H J. Growth at low ammonium concentrations and starvation response as potential factors involved in niche differentiation among ammonia-oxidizing bacteria. Applied and Environmental Microbiology, 2002, 68(10): 4751-4757.
[50]Bender S F, Plantenga F, Neftel A, Jocher M, Oberholzer H R, Köhl L, Giles M, Daniell T J, van der Heijden M G A. Symbiotic relationships between soil fungi and plants reduce N2O emissions from soil. The ISME Journal, 2014, 8(6): 1336-1345.
[51]Chen Y L, Chen B D, Hu Y J, Li T, Zhang X, Hao Z P, Wang Y S. Direct and indirect influence of arbuscular mycorrhizal fungi on abundance and community structure of ammonia oxidizing bacteria and archaea in soil microcosms. Pedobiologia, 2013, 56(4/6): 205-212.
[52]Bürgmann H, Meier S, Bunge M, Widmer F, Zeyer J. Effects of model root exudates on structure and activity of a soil diazotroph community. Environmental Microbiology, 2005, 7(11): 1711-1724.
[53]樊永军, 闫伟, 王黎元. 油松外生菌根真菌对其幼苗根际其它真菌的影响. 北方园艺, 2009, (2): 32-35.
[54]Cabello M, Irrazabal G, Bucsinszky A M, Saparrat M, Schalamuk S. Effect of an arbuscular mycorrhizal fungus, Glomus mosseae, and a rock-phosphate-solubilizing fungus,Penicilliumthomii, onMenthapiperitagrowth in a soilless medium. Journal of Basic Microbiology, 2005, 45(3): 182-189.
[55]Tiunov A V, Scheu S. Arbuscular mycorrhiza and Collembola interact in affecting community composition of saprotrophic microfungi. Oecologia, 2005, 142(4): 636-642.
[56]Minerdi D, Fani R, Gallo R, Boarino A, Bonfante P. Nitrogen fixation genes in an endosymbioticBurkholderiastrain. Applied and Environmental Microbiology, 2001, 67(2): 725-732.
[57]Scheublin T R, Ridgway K P, Young J P W, van der Heijden M G A. Nonlegumes, legumes, and root nodules harbor different arbuscular mycorrhizal fungal communities. Applied and Environmental Microbiology, 2004, 70(10): 6240-6246.
[58]Larimer A L, Clay K, Bever J D. Synergism and context dependency of interactions between arbuscular mycorrhizal fungi and rhizobia with a prairie legume. Ecology, 2014, 95(4): 1045-1054.
[59]Kothari S K, Marschner H, Römheld V. Contribution of the VA mycorrhizal hyphae in acquisition of phosphorus and zinc by maize grown in a calcareous soil. Plant and Soil, 1991, 131(2): 177-185.
[60]Li X L, Marschner H, George E. Acquisition of phosphorus and copper by VA-mycorrhizal hyphae and root-to-shoot transport in white clover. Plant and Soil, 1991, 136(1): 49-57.
[61]Larimer A L, Bever J D, Clay K. The interactive effects of plant microbial symbionts: A review and meta-analysis. Symbiosis, 2010, 51(2): 139-148.
[62]Kaschuk G, Leffelaar P A, Giller K E, Alberton O, Hungria M, Kuyper T W. Responses of legumes to rhizobia and arbuscular mycorrhizal fungi: A meta-analysis of potential photosynthate limitation of symbioses. Soil Biology and Biochemistry, 2010, 42(1): 125-127.
[63]Wang X R, Pan Q, Chen F X, Yan X L, Liao H. Effects of co-inoculation with arbuscular mycorrhizal fungi and rhizobia on soybean growth as related to root architecture and availability of N and P. Mycorrhiza, 2011, 21(3): 173-181.
[64]Artursson V, Finlay R D, Jansson J K. Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth. Environmental Microbiology, 2006, 8(1): 1-10.
[65]Tajini F, Trabelsi M, Drevon J J. Co-inoculation withGlomusintraradicesandRhizobiumtropiciCIAT899 increases P use efficiency for N2fixation in the common bean (PhaseolusvulgarisL.) under P deficiency in hydroaeroponic culture. Symbiosis, 2011, 53(3): 123-129.
[66]Muthukumar T, Udaiyan K. Growth response and nutrient utilization ofCasuarinaequisetifoliaseedlings inoculated with bioinoculants under tropical nursery conditions. New Forests, 2010, 40(1): 101-118.
[67]Oliveira R S, Castro P M L, Dodd J C, Vosátka M. Synergistic effect ofGlomusintraradicesandFrankiaspp. on the growth and stress recovery ofAlnusglutinosain an alkaline anthropogenic sediment. Chemosphere, 2005, 60(10): 1462-1470.
[68]Orfanoudakis M, Wheeler C T, Hooker J E. Both the arbuscular mycorrhizal fungusGigasporaroseaandFrankiaincrease root system branching and reduce root hair frequency inAlnusglutinosa. Mycorrhiza, 2010, 20(2): 117-126.
[69]郑舜怡, 郭世荣, 张钰, 宋夏夏, 房晨, 张杰, 孙锦. 丛枝菌根真菌对辣椒光合特性及根际微生物多样性和酶活性的影响. 西北植物学报, 2014, 34(4): 800-809.
[70]盛江梅, 吴小芹. 菌根真菌与植物根际微生物互作关系研究. 西北林学院学报, 2007, 22(5): 104-108.
[71]Elumalai S, Raaman N.Invitrosynthesis ofFrankiaand mycorrhiza withCasuarinaequisetifoliaand ultrastructure of root system. Indian Journal of Experimental Biology, 2009, 47(4): 289-297.
[72]Gianinazzi S, Gollotte A, Binet M N, van Tuinen D, Redecker D, Wipf D. Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza, 2010, 20(8): 519-530.
[73]Diagne N, Diouf D, Svistoonoff S, Kane A, Noba K, Franche C, Bogusz D, Duponnois R.Casuarinain Africa: Distribution, role and importance of arbuscular mycorrhizal, ectomycorrhizal fungi andFrankiaon plant development. Journal of Environmental Management, 2013, 128: 204-209.
[74]Duponnois R, Plenchette C. A mycorrhiza helper bacterium enhances ectomycorrhizal and endomycorrhizal symbiosis of AustralianAcaciaspecies. Mycorrhiza, 2003, 13(2): 85-91.
[75]Janoušková M, Krak K, Wagg C,torchová H, Caklová P, Vosatka M. Effects of inoculum additions in the presence of a preestablished arbuscular mycorrhizal fungal community. Applied and Environmental Microbiology, 2013, 79(20): 6507-6515.
[76]Garcia J R, Gerardo N M. The symbiont side of symbiosis: do microbes really benefit? Frontiers in Microbiology, 2014, 5: 510.
[77]Fellbaum C R, Mensah J A, Cloos A J, Strahan G E, Pfeffer P E, Kiers E T, Bücking H. Fungal nutrient allocation in common mycorrhizal networks is regulated by the carbon source strength of individual host plants. New Phytologist, 2014, 203(2): 646-656.
[78]Konvalinková T, Püschel D, Janoušková M, Gryndler M, Jansa J. Duration and intensity of shade differentially affects mycorrhizal growth-and phosphorus uptake responses ofMedicagotruncatula. Frontiers in Plant Science, 2015, 6: 65.
[79]Werner G D A, Kiers E T. Order of arrival structures arbuscular mycorrhizal colonization of plants. New Phytologist, 2015, 205(4): 1515-1524.
[80] Atul-Nayyar A, Hamel C, Hanson K, Germida J. The arbuscular mycorrhizal symbiosis links N mineralization to plant demand. Mycorrhiza, 2009, 19(4): 239-246.
[81]Staddon P L, Ramsey C B, Ostle N, Ineson P, Fitter A H. Rapid turnover of hyphae of mycorrhizal fungi determined by AMS microanalysis of14C. Science, 2003, 300(5622): 1138-1140.
[82]Delgado-Baquerizo M, Maestre F T, Gallardol A, Bowker M A, Wallenstein M D, Quero J L, Ochoa V, Gozalo B, Garcia-Gómez M, Soliveres S, García-Palacios P, Berdugo M, Valencia E, Escolar C, Arredondol T, Barraza-Zepeda C, Bran D, Carreiral J A, Chaiebll M, Conceição A A, Derak M, Eldridge D L, Escudero A, Espinosa C I, Gaitán J, Gatica M G, Gómez-González S, Guzman E, Gutiérrez J R, Florentino A, Hepper E, Hernández R M, Huber-Sannwald E, Jankju M, Liu J S, Mau R L, Miriti M, Monerris J, Naseri K, Noumi Z, Polo V, Prina A, Pucheta E, Ramírez E, Ramírez-Collantes D A, Romão R, Tighe M, Torres D, Torres-Díaz C, Ungar E D, Val J, Wamiti W, Wang D L, Zaady E. Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature, 2013, 502(7473): 672-676.
Functional relationships between arbuscular mycorrhizal symbionts and nutrient dynamics in plant-soil-microbe system
WEI Lili1,*, LU Changyi1,2, DING Jing1,2, YU Shen1
1InstituteofUrbanEnvironment,ChineseAcademyofScience,Xiamen361021,China2UniversityofChineseAcademyofSciences,Beijing100049,China
Arbuscular mycorrhizal symbioses (AM symbioses), formed between Arbuscular mycorrhizal fungi (AMFs) and the majority (ca. 80%) of terrestrial plants, play an important part in the regulation of nutrient cycling in plant-soil systems. Owing to their potentially promising role in sustainable agriculture, AM symbioses have attracted increasing interest in the last decade. This review emphasized the functional interrelations among AM symbioses, soil free-living microbes, and the dynamics of carbon (C), nitrogen (N), and phosphorus (P) in plant-soil systems. The contribution of AM symbioses to plant P has become central to our understanding of AM symbiotic function over the past few decades. There is accumulating evidence that plant P uptake is bidirectionally regulated by AM symbioses. More specifically, plant P uptake is enhanced by AMF infection when the soil is P deficient, but when there is excessive soil P, its transfer to the plant is restricted and excessive P accumulates in hyphae, spores, or vessels. The ability of plants to take in P has been correlated with the volume of soil that their roots can explore. However, in the presence of AMF, mycorrhizal P uptake becomes the dominant pathway, even though plant growth or total P uptake may not be enhanced by the interaction. A benefit of AMF infection to plant P uptake is associated with carboxylate exudation produced by hyphae, which promote the mineralization and disaggregation of organic matter through enhancing the activities of phosphate-solubilizing bacteria. Comparatively, the effects of AMFs on N cycling are particularly complex since fungi are likely involved in all N processes. Arbuscular mycorrhizal fungi can take up both inorganic N and low-molecular-weight organic N from soil organic matter, which is primarily used by the fungus, with only a small amount being transferred to the roots. Arbuscular mycorrhizal fungi can also reduce N loss by regulating the trade-off between nitrification and denitrification, through reducing the concentrations of soil mineral N due to AMF uptake, improving rhizosphere aggregate stability, and decreasing the pH of soil subjected to AMF inoculation. Nitrogen loss as N2O is reduced as well from the soil inoculation with exogenous AMF. The reduction of N2O emissions is related to the shift of microbial community composition with the decrease of the microbial community responsible for N2O production and the increase of those microbial groups responsible for N2O consumption. Other soil microorganisms, including ammonia-oxidizing bacteria, can be suppressed by AMF infection, which also contributes to reduced N2O production. Arbuscular mycorrhizal fungi can also be associated with other root symbionts such as root nodules. While the exact mechanisms remain unclear, it is generally believed that AMFs deliver nutrients (such as P) for N fixation in nodules or by enhancing the activity of rhizobia. Because of increasing concerns regarding global climate change, AMF contribution to soil C storage has attracted considerable attention in recent years. Whether AMFs facilitate soil C sequestration or induce soil C loss remains under debate. One proposed explanation is that if AMFs promote soil C storage, then this becomes a short-term liability through the stimulation of organic matter decomposition and acceleration of litter degradation, while the long-term benefits include the incorporation of organic matter into soil aggregates and increased litter production due to enhanced plant growth. The flux of nutrient elements in plant-AMF-soil systems are associated with the interactions between AMF and pertinent soil microbes. Arbuscular mycorrhizal fungi generally facilitate the growth of phosphate-solubilizing bacteria, rhizobia, actinomycetes, and ectomycorrhizal fungi (EMF), and the inoculation of actinomycetes also promotes the growth of AMF. However, rhizobia and EMF appear to suppress the colonization and growth of AMF when they arrive before AMF. The interactions between AMF and soil microbes can also be regulated by soil nutrient level, such as the case of low soil nutrient conditions, in which AMFs compete for soil nutrients with free-living soil microbes. Competition can also occur among different AMF taxa. Indeed, the biogeochemical cycles of C, N, and P are interlinked in plant-soil systems, where there is an interaction between free-living soil microorganisms and AMFs, but it is unknown to date how the interactions among soil organisms regulate biogeochemical cycles of soil macro elements. A combined technique that uses both microbiological and stoichiometry methods may be needed to explore this “mystical territory”.
arbuscular mycorrhiza symbosis; rhizosphere; soil microorganism; nutrient dynamic; plant-soil system
中国科学院战略性先导科技专项(B类)“土壤-生物系统功能及其调控”(XDB15030301);国家自然科学基金项目(31070463)
2014-12-04; 网络出版日期:2015-11-02
Corresponding author.E-mail: llwei@iue.ac.cn
10.5846/stxb201412042407
韦莉莉, 卢昌熠, 丁晶, 俞慎.丛枝菌根真菌参与下植物-土壤系统的养分交流及调控.生态学报,2016,36(14):4233-4243.
Wei L L, Lu C Y, Ding J, Yu S.Functional relationships between arbuscular mycorrhizal symbionts and nutrient dynamics in plant-soil-microbe system.Acta Ecologica Sinica,2016,36(14):4233-4243.

