糖尿病肾病患者血清瘦素水平变化与氧化应激的关系
张学磊 金明花 王 莹 孙韫琪 冯大伟 刘 哲 柴国禄.黑龙江省佳木斯市骨科医院内科,黑龙江佳木斯 54002;2.佳木斯大学第五临床医院内分泌科,黑龙江佳木斯 54002
糖尿病肾病患者血清瘦素水平变化与氧化应激的关系
张学磊1金明花1王 莹1孙韫琪1冯大伟1刘 哲1柴国禄2▲
1.黑龙江省佳木斯市骨科医院内科,黑龙江佳木斯 154002;2.佳木斯大学第五临床医院内分泌科,黑龙江佳木斯 154002
目的 研究血清瘦素(Leptin)水平变化和氧化应激(OS)在糖尿病肾病(DN)发病中的可能作用,DN患者血清Leptin水平变化与OS的关系。 方法 选择2014年6月~2015年6月在佳木斯大学第五临床医院治疗的2型糖尿病(DM)患者90例,依据尿白蛋白排泄率分为3组:正常白蛋白尿组(单纯DM组)、微量白蛋白尿组(早期DN组)、临床白蛋白尿组(临床DN组),每组30例。选取同期我院体检健康者30例为正常对照组(对照组)。检测四组血清Leptin、丙二醛(MDA)、超氧化物歧化酶(SOD)、谷胱甘肽过氧化物酶(GSH-Px)水平。分析DN患者血清Leptin水平与MDA、SOD、GSH-Px水平的相关性。观察给于DN组患者替米沙坦(telmisartan)治疗,80 mg/d,治疗4周患者血清Leptin、MDA、SOD、GSH-Px水平的变化。 结果 临床DN组血清Leptin、MDA[(21.97±5.12)ng/ml、(21.86±3.42)nmol/mL]均明显高于早期DN组[(15.62±4.31)ng/mL、(16.75±2.98)nmol/mL],早期DN组血清Leptin、MDA明显高于单纯DM组[(10.16±3.07)ng/mL、(12.34±2.31)nmol/mL],临床DN组血清SOD、GSH-Px[(60.59±5.06)、(87.34±4.02)U/mL]均明显低于早期DN组[(72.78±6.32)、(103.29±5.75)U/mL],早期DN组SOD、GSH-Px明显低于单纯DM组[(83.46±6.97)、(114.58±6.84)U/mL],差异有高度统计学意义(P<0.01)。DN患者血清Leptin与血清MDA水平呈明显正相关(r=0.685,P<0.01),与SOD、GSH-Px水平呈明显负相关(r=-0.597、-0.656,P<0.01)。DN患者给予Telmisartan治疗后随着血清Leptin水平降低患者血清MDA水平降低,SOD、GSH-Px水平升高(P<0.01)。 结论DM患者血清Leptin水平升高及所致的OS在DN的发病中发挥重要作用。
糖尿病肾病;瘦素;丙二醛;超氧化物歧化酶;谷胱甘肽过氧化物酶
糖尿病肾病(DN)是糖尿病(DM)最常见的微血管并发症,是导致终末期肾衰竭的重要原因。以往的研究认为DN的发病可能是由遗传、糖代谢紊乱、血流动力学改变、细胞因子、炎症等多种因素相互作用引起的[1],近年研究发现脂肪细胞因子瘦素(Leptin)与DN的发病有关[2],氧化应激(OS)与DN的发病也密切相关[3-4]。有关DN患者血清Leptin水平变化与OS关系的研究国内尚少报道。本研究测定DN患者血清Leptin、丙二醛(MDA)、超氧化物歧化酶(SOD)、谷胱甘肽过氧化物酶(GSH-Px)水平,观察替米沙坦(Telmisartan)治疗对其影响,探讨DN患者血清Leptin水平变化与OS的关系及它们在DN发病中的可能作用。
1 资料与方法
1.1 一般资料
按1999年WHO糖尿病诊断标准选择2014年6月~2015年6月在佳木斯大学第五临床医院(以下简称“我院”)治疗的2型DM患者90例,男45例,女45例,年龄32~70岁,平均(51.86±9.35)岁。按Mogenson DN诊断分期标准,依据尿白蛋白排泄率(UAER)分为三组:正常白蛋白尿组UAER<30 mg/24 h(单纯DM组),微量白蛋白尿组UAER 30~300 mg/24 h(早期DN组),临床蛋白尿组UAER>300 mg/24 h(临床DN组),每组各30例。早期DN组与临床DN组合称为DN组。全部病例均除外原发性高血压、肾脏疾病及泌尿系感染。血压正常(收缩压<140 mmHg,舒张压<90 mmHg,1 mmHg=0.133 kPa),均未使用血管紧张素转换酶抑制剂及血管紧张素Ⅱ受体拮抗剂。选择我院同期健康体检健康者30例为正常对照组,男15例,女15例,年龄31~69岁,平均(52.41±8.96)岁。四组性别、年龄、体重指数、血压等一般资料比较,差异无统计学意义(P>0.05),具可比性(表1)。本研究获医院医学伦理委员会批准,研究对象知情同意,并签署知情同意书。
表1 各组一般资料比较(±s)

表1 各组一般资料比较(±s)
注:与对照组比较,*P<0.01;BMI:体重指数;SBP:收缩压;1 mmHg= 0.133 kPa
组别 例数 性别(例,男/女)年龄(岁)BMI (kg/m2)SBP (mmHg)对照组单纯DM组早期DN组临床DN组30 30 30 30 15/15 15/15 16/14 14/16 52.4±8.96 51.86±7.87 52.33±8.24 52.59±9.12 23.87±5.62 24.01±6.45 24.26±6.49 24.73±7.01 120.62±9.87 121.17±10.06 126.54±11.74 13133±10.82
1.2 治疗方法
所有患者均控制饮食,DN患者给优质低蛋白饮食[0.6~1.0 g/(kg·d)]。应用口服降糖药或注射胰岛素使血糖控制在空腹血糖<7.0 mmol/L,餐后2 h血糖<10.0 mmol/L。DN组在常规治疗的基础上于采血后给于Telmisartan(天津华津制药有限公司,国药准字H20051847)治疗,80 mg/d,治疗4周。
1.3 观察指标及检测方法
对照组、单纯DM组、DN组应用Telmisartan治疗前后,清晨采取肘静脉血5 mL,4℃,3000 r/min离心15 min,分离血清,置-70℃冰箱保存,分别用于Leptin、MDA、SOD、GSH-Px测定。血清Leptin测定采用酶联免疫法,试剂盒由上海工硕生物技术有限公司提供;MDA测定采用硫代巴比妥酸法,SOD测定采用黄嘌呤氧化酶法,GSH-Px测定采用二硫代二硝基苯甲酸法,试剂盒均由南京建成生物工程研究所提供。留取24 h尿,放射免疫法测定尿白蛋白,计算UAER,试剂盒有北京华英生物技术研究所提供。所有检测指标均在同次实验,用同批试剂完成。
1.4 统计学方法
采用SPSS 19.0对数据进行分析,正态分布的计量资料以均数±标准差(±s)表示,多组间比较采用方差分析,两两比较采用LSD-t检验。计数资料以率表
示,采用χ2检验。相关性分析采用Pearson直线相关分析。以P<0.05为差异有统计学意义。
2 结果
2.1 四组血清 Leptin、MDA、SOD、GSH-Px水平及UAER比较
血清Leptin、MDA水平临床DN组明显高于早期DN组(P<0.01),早期DN组明显高于单纯DM组(P<0.01),血清Leptin水平单纯DM组与对照组比较,差异无统计学意义(P>0.05),血清MDA水平单纯DM组明显高于对照组(P<0.01)。血清SOD、GSH-Px水平临床DN组明显低于早期DN组 (P<0.01),早期DN组明显低于单纯DM组(P<0.01),单纯DM组明显低于对照组(P<0.01)。UAER临床DN组明显高于早期DN组(P<0.01),早期DN组明显高于单纯DM组(P<0.01),单纯DM组与对照组比较,差异无统计学意义(P>0.05)。见表2。
表2 四组血清Leptin、MDA、SOD、GSH-Px水平比较(±s)
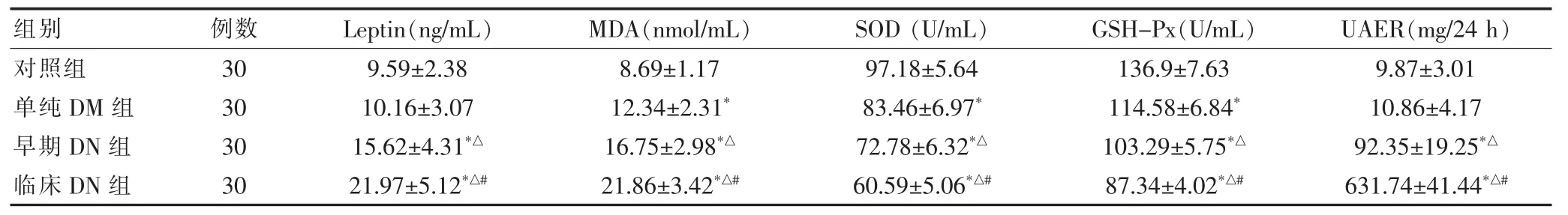
表2 四组血清Leptin、MDA、SOD、GSH-Px水平比较(±s)
注:与对照组比较,*P<0.01;与单纯DM组比较,△P<0.01;与早期DN组比较,#P<0.01;Leptin:瘦素;MDA:丙二醛;SOD:超氧化物歧化酶;GSH-Px:谷胱甘肽过氧化物酶;UAER:尿白蛋白排泄率
组别 例数 Leptin(ng/mL) MDA(nmol/mL) SOD(U/mL) GSH-Px(U/mL) UAER(mg/24 h)对照组单纯DM组早期DN组临床DN组30 30 30 30 9.59±2.38 10.16±3.07 15.62±4.31*△21.97±5.12*△#8.69±1.17 12.34±2.31*16.75±2.98*△21.86±3.42*△#97.18±5.64 83.46±6.97*72.78±6.32*△60.59±5.06*△#136.9±7.63 114.58±6.84*103.29±5.75*△87.34±4.02*△#9.87±3.01 10.86±4.17 92.35±19.25*△631.74±41.44*△#
2.2 DN患者血清Leptin、MDA、SOD、GSH-Px、UAER间相关性
DN病患者血清Leptin与UAER、血清MDA呈明显正相关(r=0.734、0.685,P<0.01),与SOD、GSH-Px呈明显负相关(r=-0.597、-656,P<0.01)。
2.3 DN 患者治疗前后血清 Leptin、MDA、SOD、MSH-Px水平变化
DN患者给予 Telmisartan治疗 4周后,血清Leptin、MDA水平较治疗前明显降低(P<0.01),血清SOD、MSH-Px水平明显升高(P<0.01),UAER明显降低(P<0.01)。见表3。
表3 糖尿病肾病患者治疗前后Leptin、MDA、SOD、GSH-Px水平比较(±s)

表3 糖尿病肾病患者治疗前后Leptin、MDA、SOD、GSH-Px水平比较(±s)
注:与治疗前比较,*P<0.01;Leptin:瘦素;MDA:丙二醛;SOD:超氧化物歧化酶;GSH-Px:谷胱甘肽过氧化物酶;UAER:尿白蛋白排泄率
时间 例数 Leptin(mg/mL) MDA(nmol/mL) SOD(U/mL) GSH-Px(U/mL) UAER(mg/24h)治疗前治疗后60 60 18.75±4.85 11.23±3.16*19.13±3.56 12.07±2.18*66.81±5.91 87.44±6.03*95.69±5.38 115.11±6.14*462.87±40.39 246.53±29.42*
3 讨论
DN的病理特点是肾小球系膜细胞增生,细胞外基质增多,肾小球基底膜增厚和肾小球硬化,临床上早期表现为肾小球高灌注、高滤过,继而出现蛋白尿,晚期表现肾衰竭,其发病机制至今尚不十分清楚。近年研究发现脂肪组织产生的细胞因子在DN发病中发挥重要作用。Leptin就是新发现的由肥胖基因编码,由167个氨基酸组成的蛋白质激素,调节摄食、能量代谢,调节免疫、促进细胞修复及血管形成[5],在糖尿病发病中发挥重要作用[6]。
本研究结果显示DN患者血清Leptin水平升高,并且随着尿蛋白的增加,患者血清Leptin水平升高的越明显,患者血清Leptin水平与UAER呈正相关(P<0.05),提示血清Leptin水平升高参与了DN发病的病理过程。血清Leptinn升高通过激活肾小球内肾素血管紧张素系统(RAS),增强血管紧张素Ⅱ(AngⅡ)活性,导致肾小球内高灌注、高滤过、引起肾小球肥大[7]。血清Leptin水平升高导致高胰岛素血症和胰岛素抵抗 (IR)[8],IR通过增加肾小球滤过率造成肾损伤,高胰岛素血症刺激胰岛素生长因子等细胞因子加重肾小球肥大[9]。Leptin作用于肾小球毛细血管刺激肾小球内皮细胞增殖,促进内皮细胞转化生长因子-β (TFG-β)的合成。使系膜细胞Ⅰ型胶原合成增多,促进肾小球硬化[10]。Leptinn通过肾小球内皮细胞ob-Rb受体激活蛋白质酪氨酸激酶,增加结缔组织生长因子的生成,促进肾间质成纤维细胞的增殖和Ⅰ型胶原蛋白的合成,促进肾小球纤维化[11]。血清Leptin水平升高促进了DN的发生发展。
本研究结果显示DN患者血清MDA水平升高,血清SOD、MSH-Px水平降低,并且随着尿蛋白的增加,血清MDA升高、SOD、MSH-Px降低的更明显。糖尿病肾病患者血清Leptin与UAER、血清MDA呈明显正相关(P<0.01),与SOD、GSH-Px呈明显负相关(P<0.01)。MDA水平反映机体氧化应激损伤的程度[12]。SOD、MSH-Px是机体抗氧化的关键酶,其水
平高低反应机体清除自由基抗氧化的能力[13]。DN患者血清MDA升高,SOD、MSH-Px降低表明DN患者发生了OS,体内活性氧(ROS)产生增多。ROS刺激肾小管上皮细胞分泌AngⅡ增多引起肾小球高灌注[14]。肾小球毛细血管基底膜磷脂在ROS作用下发生脂质过氧化,使肾小球基底膜增厚,通透性增强,同时ROS启动肾小球足细胞凋亡,破坏肾小球基底膜的完整性,导致蛋白尿[15]。ROS介导高血糖诱导的C-Jun端子激酶/核因子kB信号通路的NADPH氧化酶/ROS途径的激活,增强肾小球系膜细胞增殖和纤维连结蛋白过度表达[16],并加重细胞外基质积聚,引起系膜增宽和肾小管间质纤维化发生肾小球硬化[17],OS是导致DN的重要原因。
本研究结果显示DN患者血清Leptin水平与MDA水平呈明显正相关(P<0.01),与SOD、MSH-Px水平呈明显负相关(P<0.01),DN患者应用Telmisartan治疗,随着血清Leptin水平降低,血清MDA降低、SOD、MSH-Px升高,提示血清Leptin水平升高导致了OS。有实验证明Leptin水平升高通过增强线粒体蛋白激酶A介导的脂肪酸氧化,剂量依赖性增加ROS产生[18],并通过降低二乙基对硝基苯磷酸酯酶-1的活性[19],激活PKC途径[20],活化 NADPH氧化酶促进OS[21]。Telmisartan为AngⅡ受体拮抗剂,通过改善胰岛素抵抗使脂肪细胞分泌Leptin减少,血清Leptin水平降低[22],Leptin水平降低使DN患者血清MDA降低,SOD、GSH-Px水平升高,发挥抗OS作用[23],降低UAER。
血清Leptin水平升高及所致的OS是糖尿病肾病发病的重要原因,在临床上抑制Leptin合成、应用Leptin受体拮抗剂,降低OS可能成为防治糖尿病肾病的一条新途径。
[1]赵大鹏,随艳波,栾仲秋,等.糖尿病肾病发病机制的研究进展[J].中国医药导报,2012,9(36):47-48.
[2]Moon HS,Dalamaga M,Kim SY,et al.Leptin's role in lipodystrophic and non-lipodystrophic insulin-resistant and diabetic individuals[J].Endocrine Rev,2013,34(3):377-412.
[3]Naruse R,Suetsugu M,Terasawa T,et al.Oxidative stress and antioxidative potency are closely associated with diabetic retinopathy and nephropathy in patients with type 2 diabetes[J].Saudi Med J,2013,34(2):135-141.
[4]易哗,卢远航,翼倩倩.金水宝对糖尿病肾病血液透析患者氧化应激及微炎症状态的影响[J].中国医药导报,2015,12(7):93-96.
[5]Munzherg H,Morrison CD.Structure,production and signaling of Leptin[J].Mtabolism,2015,64(1):13-23.
[6]史东萍.瘦素在糖尿病发病中的作用[J].中国医药导报,2009,6(30):123-124.
[7]Blanco S,Vaguero M,Gomez-Guerrero C,et al.Potential role of angiotensin-converting enzyme inhibitors and statins on early prodocyte damage in amodel of type 2 diabetes mellitus,obesity and mild hypertension[J].Am J Hypertens,2015,18(4):557-565.
[8]岳伟,王红,李兆雷,等.老年糖尿病合并高血压患者血清瘦素与肾素血管紧张素系统的关系[J].中国医药导报,2014,11(34):47-50.
[9]Gupta A,Gupta V,Agrawal S,et al.Association between circulating Leptin and insulin resistance,the lipid prolife,and metabolic risk factors in North Indian adultwomen[J]. Bio Sci Trends,2010,4(6):325-332.
[10]Han DC,Isono M,Chen S.et al.Leptin stimulates type I Collagen production in db/db mesangial cells glucose uptake and TGF-beta typeⅡ receptor expression[J]. Kidney Int,2001,59(4):1315-1323.
[11]Lee C,Guh JY,Chen HC,et al.Leptin and connective tissue growth factor in advanced glycation end-productinduced effects in NRK-49F cells [J].J Cell Biochem,2004,95(5):940-950.
[12]Gunal SY,Ustunday B,Gunal AI,et al.The assessment of oxidative stress on patients with chronic renal failure at different stagesant on dialysis receiving different hypertensive treatment[J].Indian J Clin Biochem,2013,28 (4):390-395.
[13]Lee Y,Lee JY,Oh JY,et al.Expression of hepatic and ovarian antioxidant enzymes during Estrous cucle in rats[J]. Toxicol lett,2012,212(3):329-336.
[14]Hsieh TJ,Zhang SL,Filep JG,et al.High glucose stimulates angiotensinogen gene expression via reactive oxygen species generation in rat kidney proximal tubular cells[J]. Endocrinology,2002,143(8):2975-2985.
[15]Susztak K,Raff AC,Schiffer M,et al.Glucose-induced reactive oxygen species cause apoptosis and podocyte depletion at the onset of diabetic nephropathy [J].Diabetes,2006,55(1):225-233.
[16]Zhang L,Pang S,Deng B,et al.High glucose induces renal mesangial cell proliferation and fibronect expression through JNK/NF-kB/NADPH oxidase/ROS pathway,which is inhibited by resveratrol[J].Int J Biochem Cell Biol,2012,44(4):629-638.
[17]Lee EA,Seo JY,Jiang Z,et al.Reactive oxygen species mediate high glucose-induced plasminogen activator inhibitor-1 up-regulation in mesangial cells and in diabetic kidney[J].Kidney Int,2005,67(5):1762-1771.[18]Yh R,Jun Q,Scott O,et al.Over expression of Leptin in transgenic mices leads to decreased basal lipolysis,PKA activity,and perilipin levels[J].Biochemical and Biophysical Research Communications,2003,312(4):1165-1170.
[19]Beltowski J,Wjcicka G,Jamroz A.Leptin decreases plasma paraoxinase-1(POW-1)activity and induces oxidative stress:the possible novel mechanism for proatherogenic effect of chronic hyperleptinemia [J].Atherosclecrosis,2003,170(1):21-29.
[20]Maingrette F,Renier G.Leptin increases lipoprotein lipase secretion by macrophages involvement of oxidative stress and protein kinasec[J].Diabetes,2003,52(8):2121-2128.
[21]Ling L,Jean C,Mamput U,et al.Singaling pathways involved in human vascular smooth muscle cells proliferation and matrix metalloproteinase-2 expression induced by leptin inhibitory effect of metformin [J].Diabetes,2005,54(7):2227-2234.
[22]Makino H,Hancda M,Babazono K,et al.Microalbuminura reiduction with talmisartion in normotensive and hypertensive Japanese patients with type 2diabetes:a post-hoc analysis of the incipient to overt:angiotensinⅡ blocker telmisartion on type 2 diabetic nephropathy(INNOVATION)study[J].Hypertens Res,2008,31(1):657-664.
[23]Fujita H,Sakamoto J,Komatsu K,et al.Reducation of circulating superoxide dismutase activity in type 2 diabetic patients with microalbuminuria and its modulation by telmisartion therapy[J].Hypertens Res,2011,34(12):1302-1308.
Relationship between serum Leptin change and oxidative stress in diabetic nephropathy
ZHANG Xuelei1JIN Minghua1WAN Ying1SUN Yunqi1FENG Dawei1LIU Zhe1CHAI Guolu2▲
1.Department of Internal Medcine,Jiamusi Orthopedic Hospital,Heilongjiang Province,Jiamusi 154002,China;2.Department of Endonology,the Fifth Clinical Hospital of Jiamusi University,Heilongjiang Province,Jiamusi 154002,China
Objective To study the effect of serum Leptin change and oxidative stress in the development of diabetic nephropathy and to investigate the relationship between the serum leptin change and oxidative stress in the diabetic nephropathy.Methods 90 patients with type 2 diabetes mellitus were selected from June 2014 to June 2015 in the Fifth Clinical Hospital of Jiamusi University,and they were divided into three group acording to the urinary albumin excretion rat(UAER)∶nomal albumin uria group(simple diabetes mellitus group),microalbumin uria group(early diabetic nephropathy group)and clinical albumin uria group clinical diabetic nephropathy group),each group had 30 cases,were 30 cases of normal individuals with physical examination during the same period were as the control group(control group).The Levels of serum Leptin,malondialdehyde (MDA),superoxide dismulase (SOD)and glutathione peroxidase (GSH-Px)were measured in four groups.The relationship between the serum leptin Level and serum MDA,SOD,GSH-Px levels were analyzed in the patients with diabetic nephrophthy.The changes of serum Leptin,MDA,SOD and GSH-Px levels were investigated in the patients with diabetic nephrophthy after treatment with telmisartan 80 mg/d,for 4 weeks. Results The results showed that the serum Leptin and MDA levels in the clinical diabetic nephropathy group[(21.97± 5.12)ng/mL,(21.86±3.42)nmol/mL]were significantly higher than those in the early diabetic nephropathy group[(15.62± 4.31)ng/mL,(16.75±2.98)nmol/mL],the serum Leptin and MDA levels in the early diabetic nephropathy group were significantly higher than those in the simple diabetes mellitus group [(10.16±3.07)ng/mL,(12.34±2.31)nmol/mL],the
Diabetic nephropathy;Leptin;Malondialdehyde;Superoxide dismulase;Glutathione peroxidase
R587.1
A
1673-7210(2016)06(a)-0054-05
2016-03-02本文编辑:苏 畅)
▲通讯作者
serum SOD and GSH-Px levels in the clinical diabetic nephropathy group[(60.59±5.06)U/mL,(87.34±4.02)U/mL]were significantly lower than those in the early diabetic mellitus group[(72.78±6.32)U/mL,(103.29±5.75)U/mL],the serum SOD and GSH-Px levels in the early diabetic nephropathy group were significantly lower than those in the simple diabetes mellitus group[(83.46±6.97)U/mL,(114.58±6.84)U/mL],the differences were statistically significant(P<0.01). A positive correlation was found between serum Leptin and serum MDA (r=0.685,P<0.01),a negtive correlation was found between serum Leptin and serum SOD,GSH-Px(r=-0.597、-0.656,P<0.01)in the patients with diabetic nephropathy.The levels of serum Leptin and MDA were significantly decreased,the levels of serum SOD and GSH-Px were significantly increased after treatment with telmisartan in the patients with diabetic nephropathy(P<0.01).Conclusion The high serum level of Leptin and the increased oxidative stress might play an important role in the development of diabetic nephropathy.

