大分子拥挤环境中脂肪酸影响磷脂囊泡相变的差示扫描量热研究
王 娇 杨利军 朱甜甜 汪慎之 陈忠秀
(浙江工商大学食品与生物工程学院,杭州310018)
大分子拥挤环境中脂肪酸影响磷脂囊泡相变的差示扫描量热研究
王娇杨利军*朱甜甜汪慎之陈忠秀*
(浙江工商大学食品与生物工程学院,杭州310018)
脂肪酸诱导的磷脂膜的热力学行为对于认识细胞内复杂的机制有着重要意义,而前人在研究脂肪酸与磷脂膜相互作用时大都在稀溶液中进行;拥挤环境下脂肪酸诱导磷脂膜的相变行为还未见报道。本文以二肉豆蔻酰磷脂酰胆碱(DMPC)构建囊泡模型,采用差示扫描量热法系统地研究了在不同浓度、不同分子量的聚乙二醇(PEG)拥挤环境中不同结构的脂肪酸对DMPC磷脂囊泡相变的影响。研究结果表明,在拥挤环境中,PEG对纯的磷脂囊泡相变的影响与大分子的分子量和浓度相关。对于脂肪酸/磷脂囊泡(FA/DMPC),PEG的存在对囊泡相变产生显著影响。在所考察的分子量和浓度范围内,PEG使FA/DMPC囊泡相变增加。短链饱和脂肪酸、不饱和脂肪酸原本使DPMC囊泡相变降低,但PEG缩小了降低幅度,甚至导致相变增加。进一步的研究表明,在大多数情况下,PEG对FA/DMPC的相变具有协作增强效应,且其影响均与大分子的分子量和浓度相关。另外,随着PEG浓度的升高,磷脂囊泡的协同单位数逐渐降低,表明拥挤环境会影响磷脂双分子层的均一性,使协同发生相变的分子数降低。本文的研究表明,大分子拥挤环境能够对扰动的磷脂双分子层起到一定的修复作用,这一现象在生物膜相关领域不可忽视。
大分子拥挤;磷脂囊泡;脂肪酸;相变;差示扫描量热法
www.whxb.pku.edu.cn
1 Introduction
Fatty acids(FAs)are abundant in biological membranes,mainly as components of phospholipids and cholesterol esters.On the other hand,FAs bind with high affinity to serum albumin as well as to cell membranes and are a key intermediate in lipid metabolism1.Transport of FAs into the cytosol of cells minimally involves adsorption to the plasma membrane,passage through the lipid bilayer,and desorption from the cytosolic face of the membrane2.Among the essential steps which must be understood for the potentially complexed mechanisms that occur in cells, illustrating the thermodynamics and the kinetics of the FAs,free or bound to phospholipids is of great importance because it modulates the lipid membrane behavior.As the lipid membrane may be essential for understanding a variety of membrane-mediated cellular functions altered by FA,extensive research has been undertaken to rationalize the molecular basis for phase transitions using model bilayer membrane3-5.Phospholipid vesicles are often used as models for biological membranes to study the bilayer properties and membrane-mediated processes6.Phase transition of 1,2-dimyristoyl-sn-glycero-3-phosphocholine(DMPC)undergoes transition from gel phase(ordered and less fluid)to liquid crystalline phase(less ordered and fluid)according to the temperature, the hydration grade,the degree of packing,and the chemical environment.The permeability of liposomal membrane is found significantly enhanced at phase transition temperature(Tm)7.Incorporation of FA into liposomal membrane was found to affect the Tmand the membrane fluidity of biological tissues is highly influenced by the hydrocarbon chain length,the π-bond position and the isomeric configuration of FA8.
It is well known that the molecular environment of biological systems is highly crowded because a significant fraction of the intracellular space is occupied by macromolecular species9.The concept of excluded volume and the theory of the effects of excluded volume on the equilibrium and rates of macromolecular reactions in fluid media containing high concentrations of macromolecules(crowded media)has been reviewed10.Among several macromolecules,polyethylene glycol(PEG)can promote phase separation to a greater extent than other inert polymers because of its spherical conformation,which makes it appropriate for mimicking physiological crowding11.Moreover,PEG has long been used to fuse cells,and much attention has been attracted to study the effect of PEG on the phase transition of phospholipids membrane.For example,PEG can enhance the lateral packing of phosphatidylcholine,decrease the surface potential of monolayers of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine(DPPC)and alter the Tm12.It was also found that the increase in main transition temperature of DPPC vesicles caused by PEG was concentrationdependent13.In addition,PEG appears to be excluded from the lipid bilayers,acting on phospholipids through changing water structure14.Several mechanisms have been suggested for the function of PEG on cell fusion.It is now accepted that the PEG influences aggregation of membranes not by surface absorption, cross-linking,solubilization but through volume exclusion and dehydration in areas of contact15.Therefore,the effect of macromolecular crowding may contribute to the phase transition of lipid bilayer and promote the cell fusion.
During FA digestion,it is inevitable that it would interact with other compounds,such as polysaccharides,proteins and so on. These macromolecules create a crowed media,which would affect the interaction of FA with bilayer and the modulation of the lipid membrane.However,previous research on the interaction of fatty acid and lipid membrane or model vesicles was usually performed in diluted solution.To the best of our knowledge,no study has focused on the effects of an external crowding medium on the phase transition of the lipid membrane induced by fatty acid.In this paper,it is of special interest to study systematically the effect of PEG with varied molecular weight and concentration on the phase transition of DMPC mixed with FA.As differential scanning calorimetry(DSC)has become a standard technique for studying the thermally induced transition of phospholipid molecules from an ordered crystalline-like state at low temperature to a liquid crystalline-like state at higher temperature16,the present report is concerned with the interaction of fatty acid with DMPC in the presence of PEG using DSC technique.As the reported studies are not wide enough to fully understand the thermodynamics of FA-induced phase transition of lipid in crowded media,our work may enrich the overall thermodynamics and mechanism of interaction between fatty acid and model phospholipid membranes in macromolecular crowding.
2 Materials and methods
2.1Materials
1,2-dimyristoyl-sn-glycero-3-phosphocholine(DMPC;≥97%), octanoic acid(C8:0;≥98%),trans-2-octenoic acid(C8:1,trans; ≥98%),3-octenoic acid(C8:1;≥95%),decanoic acid(C10:0; ≥98%),cis-9-hexadecenoic acid(C16:1;≥98%),linoleic acid(C18:2;≥97%),γ-linolenic acid(C18:3;≥97%),and elaidic acid(C18:1,trans;≥95%)were bought from Tokyo Chemical Industry.Lauric acid(C12:0;≥99.5%),palmitic acid(C16:0;≥99%),stearic acid(C18:0;≥99%),oleic acid(C18:1;≥99%), PEG200,PEG400,PEG800,PEG2000,PEG6000,PEG10000,and PEG20000 were obtained from Aladdin Industrial Corporation. Phosphate buffered solution(PBS)was prepared by KH2PO4(≥99.5%),Na2HPO4(≥99%),NaCl(≥99.5%),and KCl(≥99.5%).Ethanol and chloroform were used inAR grade.All experiments were performed in deionized water.
2.2Sample preparation
The DMPC vesicles were prepared as follows17:required amount of DMPC was dissolved in chloroform.The solvent was then fully removed by vacuum using a rotary evaporator at 40°C, which is above the main phase transition temperature.PBS buffer at pH 7.4 was added to the film and then followed by sonication at a temperature above the main phase transition temperature to make liposome suspension.The vesicle suspension and PEG solution were prepared separately before mixed together.The concentration of DMPC in the vesicle suspension is 0.4 mmol·L-1and the concentration of PEG ranges from 0 to 0.30 g·mL-1.
Stock solution of fatty acid was prepared by dissolving FA in alcohol and the concentration was 5 mmol·L-1.Required amount of FAwas added into vesicle suspension by micropipette and then mixed together using an incubator shaker.All the solution was prepared in PBS and the ionic strength was kept constant.Because the concentration of FA needed in mixture was 0.04 mmol·L-1, only little amount of alcohol existed in the samples.Control experiment was conducted and the results show that the effect of alcohol could be ignored.
2.3Differential scanning calorimetry measurements
Differential scanning calorimetry measurements were performed using a VP-DSC(MicroCal Inc.,Northampton,USA)in a temperature range of 1 to 40°C.Data analysis was done using the Microcal Origin 7.0 software provided by Microcal.The sample volume was 0.52 mL.The samples were kept at 1°C for 15 min before the scan was started.The DSC thermograms recorded the differential power which was required to maintain the sample and the reference at the same temperature.Reference thermograms were recorded under the same conditions by filling in the sample cell with buffer and the reference cell with water.All heating scans were recorded at a rate of 1.0°C·min-1.Calorimetric enthalpies were calculated by integrating the peak areas after manual baseline adjustment.Under the experimental conditions, the obtained thermal recordings were reproducible.
3 Results and discussion
3.1Phase transition of phospholipid vesicles mixed with PEG with different molecular weights
Before investigating the details of how PEG affects the phase transition of FA/DMPC mixture vesicles,we studied the phase transition of DMPC vesicles alone in the crowded media.PEG of different molecular weight such as PEG200,PEG400,PEG800, PEG2000,PEG6000,PEG10000,and PEG20000 were selected. The concentration of PEG at certain molecular weight ranged from 0 to 0.30 g·mL-1in the mixture.The characteristic thermogram of DMPC consists of a small peak named pre-transition(Tp), which represents the transition of gel phase to rippled gel phase. The sharp peak named main phase transition temperauture(Tm) represents the transition of rippled gel phase to liquid crystalline phase18,19.The changes of the thermotropic properties allow evaluating the interactions of the added substance with the membranes.
The DSC traces and the changes of phase transition temperature are shown in Fig.1 and Fig.2,respectively.At low concentration, PEG has little influence on the thermodynamic property of DMPC vesicle.With the concentration increases,Tmshifts to high temperature,indicating that both the pre-transition temperature and main transition temperature increased.The peak of main phase transition becomes lower and broader suggesting the van′t Hoff enthalpy(ΔHv)decreased.In addition,the intensity at the pretransition peak turns less with the increasing concentration of PEG.These results show that both Tpand Tmincrease with the increasing concentration of PEG regardless of the molecular weight.Similar results were also found in DPPC and 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine(DPPE)vesicles in the presence of PEG by other researchers13,20.
The pre-transition of DMPC is related to the formation of periodic ripples on the membrane surface which depends on the headgroup hydration.For the dehydrated lipid membranes,the pretransition did not occur21.It was also proposed that the pre-transition is linked with the melting process of acyl chain21,22.PEG bound water in aqueous solution23and PEG with low molecule weight bound less water than that with high molecule weight24. Fig.2 shows that the change of Tpreaches to 7°C whereas the maximum change of Tmis just 1.5°C,indicating that the PEG might have more influence on Tpthan Tmat the same concentration.The reason lies in that PEG could change the structure of water at membrane-water interface and dehydrate the DMPC headgroup,which leads to increased Tp.On the other hand,the free motion of the headgroups was limited because of the increased viscosity of the solution and/or the structuring effect of PEG on water24.Another mechanism is the so-called“osmoelastic coupling”proposed by Ito et al.25.In the case of external addition of high-molecular-weight PEG to the preformed neutral phospholipid bilayers,PEG is excluded from the region adjacent to the membranesurface.Anosmolytegradientbetweentheexclusionlayerand thebulkPEGsolutioncausesanosmoticallydrivenwateroutflow andtheshrinkageof the bilayers.Bartucci et al.26demonstrated that the effects that PEG on the neutral lipid bilayers came from both shrinkage and polymer-induced dehydration of the phospholipid polar head-groups,but the polymer-induced shrinkage should be the main reason.
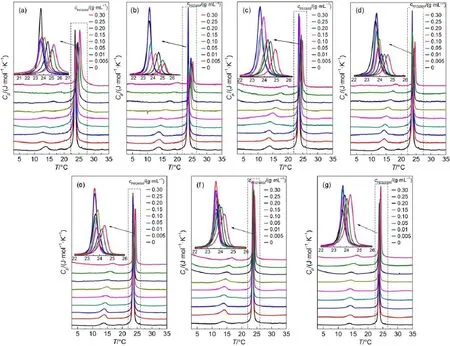
Fig.1 DSC thermograms of DMPC vesicles in the presence of PEG with different molecular weights
Fig.2 also implies that the effect of the PEG on DMPC transition temperature(Tm)is both molecular-weight and concentration dependent.The more PEG added,the higher Tmwas reached.PEG with low molecular weight has low degree of polymerization and the length of hydrocarbon chain could match with DMPC molecule.The low-molecular-weight PEGs might be able to permeate into DMPC vesicles24through hydrophobic interaction,which increases the transition temperature.On the other hand,at the same mass concentration,PEG with lower molecular weight could generate high osmotic pressure,which promoted the increase of Tm.Fig.2(c,d)suggests that at equivalent molar concentration, PEG with high molecular weight results in larger value of ΔTm.According to the accepted idea that the effect of PEG on membrane vesicles mainly comes from the volume exclusion effect, which is related to the change of osmotic pressure,it is reasonable to deduce that the more crowded of the solution,the higher transition temperature of the vesicles.PEG with longer chain has high degree of polymerization,may provide a more crowed media. Therefore,the macromolecular crowding effect contributes to the dependence of both molecule weight and concentration of PEG.
3.2Phase transition of phospholipid vesicles mixed with fatty acid in the presence of PEG
3.2.1Effect of PEG on the phase transition of DMPC
vesicles mixed with saturated fatty acid
Phase transition of DMPC mixed with different saturated fatty acid such as octanoic acid(C8:0),decanoic acid(C10:0),lauric acid(C12:0),palmitic acid(C16:0)and stearic acid(C18:0)(FA/ DMPC vesicles)without PEG is shown in Fig.3.As the pretransition was not found for DMPC in the presence of FA,the following research was mainly concentrated on the main phase transition.When short-chain FA such as octanoic acid(C8:0), decanoic acid(C10:0),lauric acid(C12:0)was added,the curve′s continuity and pattern imply that the thermal behavior involved in all the solutions is similar.However,when long-chain FAsuch as palmitic acid(C16:0)or stearic acid(C18:0)was added into the suspension,a broadening Cp(specific heat capacity)profile is observed.The DSC peak shifts to higher temperature region and gradually collapses.The peak temperature(Tm)for the pure DMPC in the buffer is 23.7°C.FAat C8,C10,and C12 resulted in a slight decrease of Tm,whereas C16 and C18 shifted the Tmto 26.6 and 25.0°C,respectively.Herein the results are consistent with an earlier research in which the long-chain saturated fatty acids increased and broadened the gel-to-liquid crystalline phase transition,whereas fatty acids of C10 or fewer carbons and fatty acidderivatives results in lower and broadened phase transition,all the fatty acids being capable of eliminating the pretransition27,28.
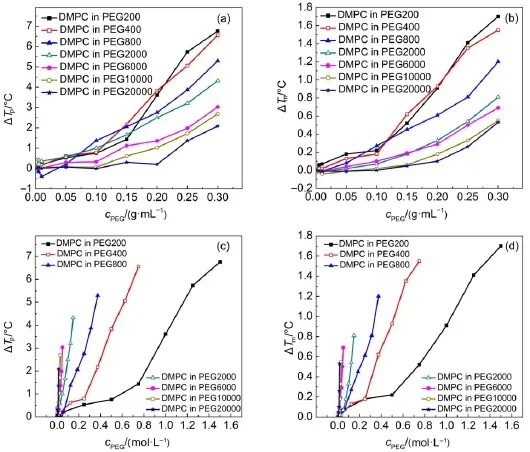
Fig.2 Changes of pre-transition temperature(a,c)and main phase transition temperature(b,d)of DMPC vesicles with PEG
Fig.4 depicts the DSC scans of DMPC vesicles mixed with saturated FAin the presence of PEG200,PEG2000 and PEG20000 at varied concentrations,respectively.It can be seen that the presence of PEG results in significant changes in the phase transition of FA/DMPC vesicles.The original decreased Tmof DMPC by octanoic acid is found increased in the presence of PEG200 or PEG2000 compared with that of DMPC in buffer without PEG.Actually,Tmof FA/DMPC vesicles increased in most of the covered concentration and molecular weight of PEG.
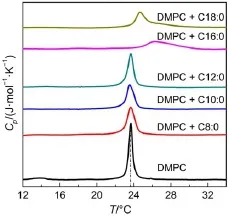
Fig.3 DSC thermograms of DMPC vesicles mixed with fatty acids with different chain lengths
To find the details of the change of Tm,ΔTmwas obtained by substring the transition temperature of pure DMPC in PBS buffer from the Tmshown in Fig.5.For PEG200(Fig.5(a)),increased concentration of PEG induces higher Tm.This concentration dependence is also observed for most FA/DMPC vesicles in PEG2000(Fig.5(b))and PEG20000(Fig.5(c)),which suggests that concentrated PEG enhanced the interaction of DMPC with saturated FA.Compared with FA/DMPC vesicles in diluted solution,the increased ΔTminduced by PEG indicates the collaboration of the macromolecular crowding during the phase transition process.It is noteworthy that PEG200 supports the increase of Tm, resulting in the gradual increase along with the increased concentration(Fig.5(d)).The matchable hydrocarbon chain of PEG200 with the phospholipid might facilitate the interaction, which strengthened the packing of bilayer.
Comparing the results of the phase transition of DMPC vesicles in PEG with or without FAshown in Fig.5,we can see that for C8, C10,and C12,the increased Tmof FA/DMPC vesicles in PEG mainly came from the interaction of DMPC with PEG.However, for the long-chain FA,PEG200 helps strengthen of liquid crystalline phase of DMPC.This collaborative effect results were also found in the case of PEG2000 and PEG20000.
3.2.2Effect of PEG on the phase transition of DMPC vesicles mixed with unsaturated fatty acid
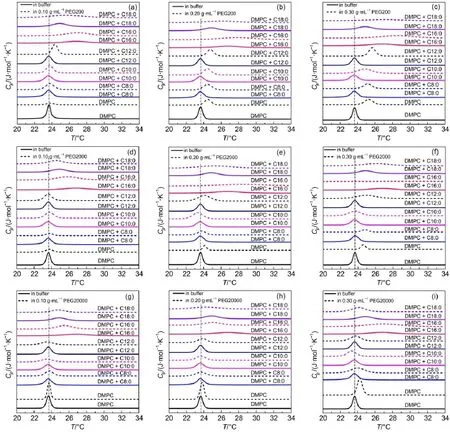
Fig.4 DSC thermograms of DMPC vesicles mixed with saturated FAin the presence of PEG200,PEG2000,and PEG20000 at varied concentrations
As unsaturated FA usually lowers the phase transition temperature and affects the fluidity of the membrane,we extended our research to find the influence of PEG on the vesicles disrupted by unsaturated FA.DMPC vesicles mixed with unsaturated shortchain FA(Fig.6)and long-chain FA(Fig.7)in the presence of different molecular weight and concentration of PEG was subjected to DSC scans.Fig.8 lists the change of Tmof DMPC vesicles mixed with short-chain and long-chain unsaturated FAin the presence of PEG with varied molecular weight and concentration. For pure DMPC vesicles,unsaturated short-chain FA induces a little decrease of the phase transition,resulting in negative ΔTm. But in the presence of PEG200,the ΔTmturns positive,which means that PEG200 repaired the disturbed vesicles and shifted the phase transition to higher temperatures.More concentrated PEG200 causes a higher increase of ΔTm.This concentration dependence was also found for PEG2000.But PEG20000 seems to support the phase transition to occur at lower temperature, displaying more negative ΔTmwhen the concentration was 0.10 and 0.20 g·mL-1.Only at higher concentration(0.30 g·mL-1)of PEG20000,increased Tmis observed.
Unlike the unsaturated short-chain FA,addition of the unsaturated long-chain FA to DMPC vesicles results in significant decrease of Tm(Fig.7)except when the elaidic acid was presented. The addition of PEG could not compensate the decreased Tmenough,which results in the left shift of most of the DSC thermographs.PEG200 could not repair the membrane-distorted vesicles before the concentration reached 0.20 g·mL-1.ForPEG2000 and PEG20000,the discrepancy of changed phase transition temperature became smaller at a higher concentration (0.30 g·mL-1).This implies that the unsaturated long-chain FA generates stronger disturbance than that of short-chain FA. Moreover,PEG could not afford enough repairing effect for the disturbed DMPC vesicles in the case of unsaturated long-chain FA.
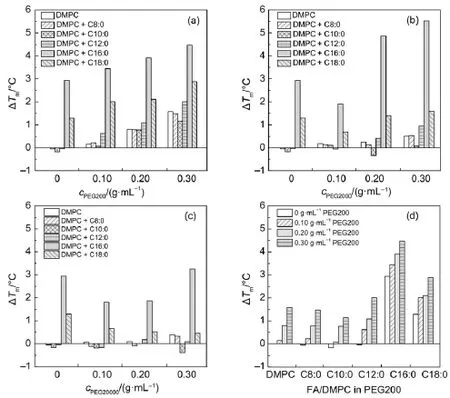
Fig.5 Variation of the differences of the main phase transition temperature(ΔTm)of DMPC vesicles mixed with saturated FAagainst the change of molecular weight and concentration of PEG
It was reported that adding small amounts of saturated FAwith carbon number of 12-18 increased the Tmand trasnsition entrapy (ΔH)of dipalmitoylphosphatidylcholine(DPPC)multilamellar dispersions whereas saturated fatty acid with fewer carbons(<10) and unsaturated fatty acid lowered the Tmand ΔH29.In fact membrane fluidity is highly influenced by π-bond position and configuration in the long chain isomers of phyto-fatty acids. Filippelli et al.8found that onset of Tmof the membrane containing Z-vaccenic acid occurred at a lower temperature than that containing an equal amount of oleic acid.In Fig.8,only a little difference of ΔTmwas observed for trans-2-octenoic acid(C8:1, trans)and 3-octenoic acid(C8:1),whereas a big discrepancy of ΔTmwas found for elaidic acid(C18:1,trans)and oleic acid(C18: 1).These results suggest that the configuration of π-bond in longchain unsaturated FA influenced the membrane fluidity greatly. Values of Tmfor all the membranes that contained the oleic acid are lower than those containing elaidic acids.These results are in agreement with literature that the Tmof phospholipid vesicle that containing trans unsaturated FA is higher than that containing its cis isomers30,31.The mixtures of stearic,arachic,oleic,and linoleic acids with DMPC and distearylphosphatidylcholine have been studied by Ortiz and Gómez-Fernández32.Saturated fatty acids were found to partition preferentially into solid-like domains, while cis-unsaturated fatty acids partition preferentially into fluidlike domains.These effects have been considered to reflect principally the destructive influence of a Z-π bond on the packing of the hydrocarbon chains in the gel state,a less compact arrangement resulting in a lower Tm.The E configuration has a completely different effect on the fluidity of the phospholipid bilayer resulting in more rigidly packed in the membrane.Our results showed that PEG increases the Tmof the DMPC vesicles incorporated by either Z or E-isomers of long-chain unsaturated fatty acids,but could not reduce the differences.
3.3Change of the heterogeneity of DMPC vesicles with or without FA in the presence of PEG
In addition to identifying the temperatures when phase transitions occur and the enthalpy change associated with the transition,DSC thermograms also provide the width at half-height of the transition temperature(ΔT1/2).The shape of the peaks contains useful information on the system and the cooperative nature of the transition.The van′t Hoff enthalpy change(ΔHv)can be calculated from ΔT1/2based on the assumed simple two-state first-order model.For VP-DSC,ΔHvcan be obtained directly by fitting the data with a Non-2-state model(Both ΔH and ΔHvcan be found in the Supporting Information).The cooperative unit(CU),which was defined as the ratio of ΔHv/ΔH,reflects the degree of intermolecular cooperation between phospholipid molecules in a bi-layer33.CU gives valuable information on lipid organization, which represents the number of molecules going through the gelliquid crystalline phase transition simultaneously.A decrease of CU generally indicates that there is an increase in heterogeneity among lipid molecules in the bilayer membranes during the phase transition34.

Fig.6 DSC thermograms of DMPC vesicles mixed with fatty acid with unsaturated short-chain fatty acid in the presence of different molecular weights and concentrations of PEG
Fig.9 shows the change of CU of pure DMPC and that mixed with fatty acid with different chains,saturated or unsaturated in the presence of PEG.It can be seen that for pure DMPC vesicles,the presence of long-chain FAcauses greater change of CU than shortchain FA.The increase in heterogeneity of DMPC may come from more matchable chain length of long-chain FA with the phospholipid molecule.The unsaturation degree of FA influences greatly on the CU in the absence of PEG.The CU of stearic acid (C18:0),oleic acid(C18:1),linoleic acid(C18:2),γ-linolenic acid (C18:3),and elaidic acid(C18:1,trans)increases gradually, suggesting that the more double bonds,the more homogeneity generated in the binary fatty acid-phospholipid system.Moreover, configuration of the double bonds also affects the heterogeneity of the bilayers,which is in agreement of the observed change of Tm.

Fig.7 DSC thermograms of DMPC vesicles mixed with fatty acid with unsaturated long-chain fatty acid in the presence of different molecular weights and concentrations of PEG
In the presence of PEG,great changes of CU are found both in pure DMPC and for FA/DMPC vesicles.The CU of pure DMPC vesicles decreases when the concentration of PEG increases in most cases.For example,in the case of PEG200,The CU of DMPC vesicles first increases then decreases with the concentration increase.But for PEG2000 and PEG20000,the CU of DMPC vesicles decreases gradually with the concentration of macromolecules increases.In addition,the increase of the molecular weight also causes the decrease of CU of DMPC vesicles. Generally,the presence of PEG results in CU decreases of most FA/DMPC systems.These results indicate that fewer molecules were going through the gel-liquid crystalline phase transition simultaneously in a more complexes system.In most cases the enthalpy change of FA/DMPC/PEG is larger than that of FA/ DMPC.The presence of PEG200 and PEG2000 decreased the polymer mobility and resulted in an increase of ΔH.Accordingly, the increase in heterogeneity among polymer molecules in the vesicle membranes during the phase transition contributed to the decrease of CU.It is well accepted that the CU is a measure of the degree of intermolecular cooperation between phospholipid molecules in a bilayer.For a completely cooperative,first-order phase transition of an absolutely pure substance,this ratio should approach infinity,while for a completely non-cooperative process this ratio should approach unity33.FA may be incorporated into membranes and affect phospholipid fluidity and molecular packing.The present study addresses the role of PEG in membrane structure.The determined CU values can be useful in quantitating the degree of cooperativity of lipid phase transitions. The aggregation of FA/DMPC vesicles in the presence of PEG is in a manner consistent with a steric exclusion mechanism.In the crowed media,PEG promoted exchange of lipids between mul-tilameilar vesicles in the liquid-crystalline state.The decreased CU caused by PEG suggests that the crowded media contribute the heterogeneity of the bilayers and that less molecules have cooperatively participated in the phase transition process.
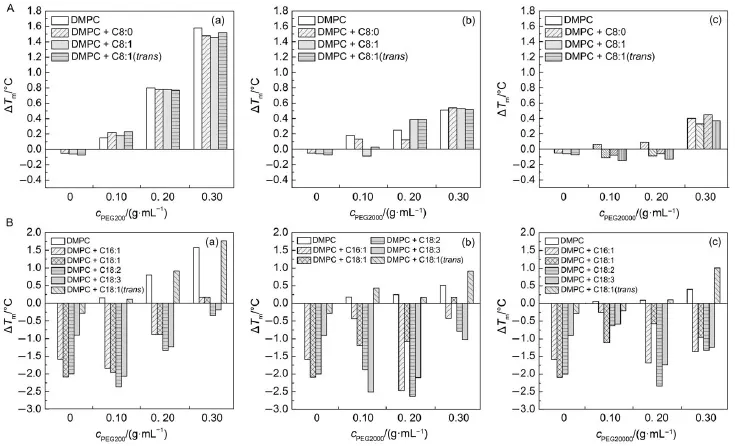
Fig.8 Change of Tmof DMPC vesicles mixed with(A)short-chain and(B)long-chain unsaturated FAin the presence of PEG with varied molecular weight and concentration
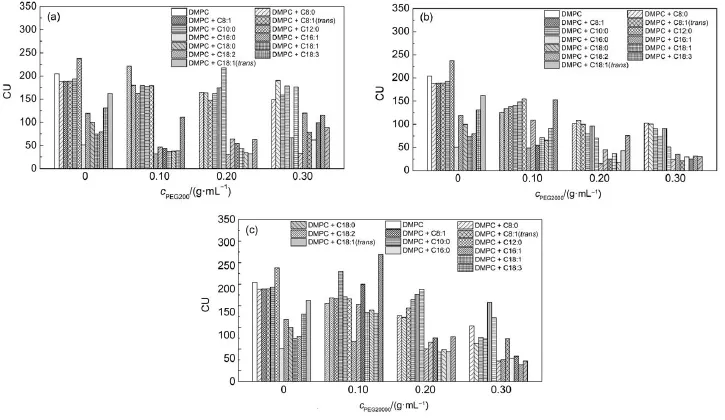
Fig.9 CU for DMPC and FA/DMPC vesicles in the presence of PEG with varied molecular weight and concentration
We also studied the change of micropolarity and fluidity of FA/ DMPC in PEG200 using steady-state fluorescence technique(see Supporting Information).In general,the fluidity of membrane decreased in crowding environment.The short-chain saturated fatty acids made the fluidity increased slightly,but it was not enough to offset the effect of crowding environment.The fluidity of membrane increased in the presence of unsaturated fatty acids. But it cannot offset the effect of PEG.The fluidity of mixed membrane in crowding environment was lower than that of membrane in diluted solution.
4 Conclusions
The effect of PEG with varied molecular weights and different concentrations on the phase transition of DMPC mixed with free fatty acid was investigated systematically using DSC technique. Fatty acids were differently located in DMPC vesicles and underwent different types of modulation movements in the crowed media according to their hydrocarbon chain length and degree of unsaturation.The results showed that the effect of PEG on the phase transition of pure DMPC vesicles was both molecular weight and concentration dependent.The presence of PEG results in significant changes in the phase transition of FA/DMPC vesicles.Tmof FA/DMPC vesicles increased in PEG at most of the covered concentration and molecular weight.The original decreased Tmof DMPC by some FA was found increased in the presence of PEG and the enhancement was concentration-dependent,suggesting a collaborative effect of the molecular crowding existed,especially for PEG200.The matchable hydrocarbon chain of PEG200 with the phospholipid may facilitate the interaction which strengthened the packing of bilayer,resulting in a higher transition temperature.For short-chain fatty acid,the increased Tmof FA/DMPC vesicles in PEG mainly come from the interaction of DMPC with PEG.Unsaturated long-chain FA generated stronger disturbance than that of short-chain FA,resulting in significant decreased of Tm.PEG could not offset the disturbed DMPC vesicles mixed with long-chain unsaturated FA in which the Tmof most FA/DMPC vesicles are still smaller than that of pure DMPC vesicles.Moreover,The presence of PEG results in decreased CU for most of DMPC and FA/DMPC vesicles,suggesting that the crowded media contribute the heterogeneity of the bilayers and that fewer molecules have cooperatively participated in the phase transition process.The class of long chain polyunsaturated fatty acids is believed to be incorporated into membranes and provide some specific function.Our work suggested that the crowded media may repair the disturbed membrane which should not be neglected.To fully understand of the mechanism behind the interaction of fatty acid and phospholipid membranes in macromolecular crowding,further work will be required to elucidate the details about the solid-like or fluidlike domains that different fatty acids preferentially partitioned into and the overall dynamics and thermodynamics involved.
Supporting Information:available free of charge via the internet at http://www.whxb.pku.edu.cn.
References
(1)Glatz,J.F.C.;Luiken,J.J.F.P.Prostag.Leukotr.Ess.2015,92, 57.doi:10.1016/j.plefa.2014.02.007
(2)Zhang,F.;Kamp,F.;Hamilton,J.A.Biochemistry 1996,35, 16055.doi:10.1021/bi961685b
(3)Wu,F.G.;Jia,Q.;Wu,R.G.;Yu,Z.W.J.Phys.Chem.B 2011, 115,8559.doi:10.1021/jp200733y
(4)Wu,F.G.;Sun,H.Y.;Zhou,Y.;Wu,R.G.;Yu,Z.W.RSC Adv. 2014,4,51171.doi:10.1039/C4RA09158B
(5)Wu,F.G.;Sun,H.Y.;Zhou,Y.;Deng,G.;Yu,Z.W.RSC Adv. 2015,5,726.doi:10.1039/C4RA07569B
(6)Wu,R.G.;Chen,L.;Yu,Z.W.Acta Phys.-Chim.Sin.2012,28, 2008.[邬瑞光,陈琳,尉志武.物理化学学报,2012,28, 2008.]doi:10.3866/PKU.WHXB201205171
(7)Chen,J.;Cheng,D.;Li,J.;Wang,Y.;Guo,J.;Chen,Z.;Cai,B.; Yang,T.Drug Dev.Ind.Pharm.2013,39,197.doi:10.3109/ 03639045.2012.668912
(8)Filippelli,L.;Rossi,C.O.;Uccella,N.A.Colloids Surf.B: Biointerfaces 2011,82,13.doi:10.1016/j.colsurfb.2010.07.052
(9)Ellis,R.J.;Minton,A.P.Nature 2003,425,27.doi:10.1038/ 425027a
(10)Hall,D.;Minton,A.P.Biochim.Biophys.Acta-Proteins Proteom 2003,1649,127.doi:10.1016/S1570-9639(03)00167-5
(11)Hassan,M.M.;Martin,A.D.;Thordarson,P.J.Mater.Chem.B 2015,3,9269.doi:10.1039/C5TB02139A
(12)Maggio,B.;Lucy,J.A.Febs Lett.1978,94,301.doi:10.1016/ 0014-5793(78)80962-4
(13)Tilcock,C.P.S.;Fisher,D.Biochim.Biophys.Acta-Biomembranes 1979,557,53.doi:10.1016/0005-2736(79) 90089-0
(14)Arnold,K.;Zschoernig,O.;Barthel,D.;Herold,W.Biochim. Biophys.Acta-Biomembranes 1990,1022,303.doi:10.1016/ 0005-2736(90)90278-V
(15)Lentz,B.R.Eur.Biophys.J.2007,36,315.doi:10.1007/ s00249-006-0097-z
(16)Luo,J.J.;Wu,F.G.;Yu,J.S.;Wang,R.;Yu,Z.W.J.Phys. Chem.B 2011,115,8901.doi:10.1021/jp200296v
(17)Maulucci,G.;De Spirito,M.;Arcovito,G.;Boffi,F.;Castellano, A.C.;Briganti,G.Biophys.J.2005,88,3545.doi:10.1529/ biophysj.104.048876
(18)Needham,D.;Evans,E.Biochemistry 1988,27,8261. doi:10.1021/bi00421a041
(19)Mabrey,S.;Sturtevant,J.M.Proc.Natl.Acad.Sci.U.S.A. 1976,73,3862.doi:10.1073/pnas.73.11.3862
(20)Yamazaki,M.;Ohshika,M.;Kashiwagi,N.;Asano,T.Biophys. Chem.1992,43,29.doi:10.1016/0301-4622(92)80039-8
(21)Heimburg,T.Biophys.J.2000,78,1154.doi:10.1016/S0006-3495(00)76673-2
(22)Riske,K.A.;Barroso,R.P.;Vequi-Suplicy,C.C.;Germano,R.;Henriques,V.B.;Lamy,M.T.Biochim.Biophys.Acta-Biomembranes 2009,1788,954.doi:10.1016/j. bbamem.2009.01.007
(23)Blow,A.M.J.;Botham,G.M.;Fisher,D.;Goodall,A.H.; Tilcock,C.P.S.;Lucy,J.A.Febs Lett.1978,94,305. doi:10.1016/0014-5793(78)80963-6
(24)Tilcock,C.P.S.;Fisher,D.Biochim.Biophys.Acta-Biomembranes 1982,688,645.doi:10.1016/0005-2736(82) 90375-3
(25)Ito,T.;Yamazaki,M.;Ohnishi,S.Biochemistry 1989,28,5626. doi:10.1021/bi00439a043
(26)Bartucci,R.;Montesano,G.;Sportelli,L.Colloids Surf.A: Physicochem.Eng.Asp.1996,115,63.doi:10.1016/0927-7757 (96)03665-5
(27)Eliasz,A.W.;Chapman,D.;Ewing,D.F.Biochim.Biophys. Acta-Biomembranes 1976,448,220.doi:10.1016/0005-2736 (76)90238-8
(28)Schullery,S.E.;Seder,T.A.;Weinstein,D.A.;Bryant,D.A. Biochemistry 1981,20,6818.doi:10.1021/bi00527a012
(29)Kantor,H.L.;Mabrey,S.;Prestegard,J.H.;Sturtevant,J.M. Biochim.Biophys.Acta-Biomembranes 1977,466,402. doi:10.1016/0005-2736(77)90333-9
(30)Roach,C.;Feller,S.E.;Ward,J.A.;Shaikh,S.R.;Zerouga,M.; Stillwell,W.Biochemistry 2004,43,6344.doi:10.1021/ bi049917r
(31)Koynova,R.;Caffrey,M.Biochim.Biophys.Acta-Rev. Biomembranes 1998,1376,91.doi:10.1016/S0304-4157(98) 00006-9
(32)Ortiz,A.;Gómez-Fernández,J.C.Chem.Phys.Lipids 1987,45, 75.doi:10.1016/0009-3084(87)90041-7
(33)Rn,M.E.Chem.Phys.Lipids 1982,30,229.doi:10.1016/0009-3084(82)90053-6
(34)Barenholz,Y.;Bombelli,C.;Bonicelli,M.G.;di Profio,P.; Giansanti,L.;Mancini,G.;Pascale,F.J.Colloid Interface Sci. 2011,356,46.doi:10.1016/j.jcis.2010.11.062
Phase Transition of Phospholipid Vesicles Induced by Fatty Acids in Macromolecular Crowding:a Differential Scanning Calorimetry Study
WANG JiaoYANG Li-Jun*ZHU Tian-TianWANG Shen-ZhiCHEN Zhong-Xiu*
(College of Food&Biology Engineering,Zhejiang Gongshang University,Hangzhou 310018,Zhejiang Province,P.R.China)
Investigation of the thermodynamics of fatty acid(FA)modulation of lipid membrane behavior is important to understand the mechanisms that occur in cells.Previous research of the interaction between FAs and lipid membranes has been performed in dilute solution,and no study has focused on the effect of an external crowding medium on the phase transition of the lipid membrane induced by FAs.In this paper,the effect of various molecular weights and concentrations of polyethylene glycol(PEG)on the phase transition of 1,2-dimyristoyl-sn-glycero-3-phosphocholine(DMPC)vesicles mixed with FA was systematically investigated by differential scanning calorimetry(DSC).The results show that the effect of PEG on the phase transition of pure DMPC vesicles is both molecular weight and concentration dependent.The presence of PEG significantly changes the phase transition of FA/DMPC vesicles.Phase transition temperature(Tm)of FA/DMPC vesicles increased in PEG for most of the considered concentrations and molecular weights.The original Tmof DMPC induced by short-chain saturated FAor unsaturated FAincreased in the presence of PEG.Further investigation revealed that in most cases a collaborative effect of molecular crowding existed and the effect of PEG on Tmwas both molecular weight and concentration dependent.Moreover,the cooperative unit(CU)of pure DMPC vesicles and most FA/DMPC systems decreased with increasing PEG concentration,indicating that the crowded medium contributes to the heterogeneity of the bilayers and that fewer molecules cooperatively participate in the phase transition.The results suggest that crowded media might repair disturbed membranes,which should not be ignored in the FA-modulating membrane related area.
Macromolecular crowding;Phospholipid vesicle;Fatty acid;Phase transition;Differential scanning calorimetry
February 29,2016;Revised:May 3,2016;Published on Web:May 3,2016.
O648
10.3866/PKU.WHXB201605033
*Corresponding authors.CHEN Zhong-Xiu,Email:zhxchen@mail.zjgsu.edu.cn;Tel:+86-11-28008980.
YANG Li-Jun,Email:spxy21019@126.com.
The project was supported by the Zhejiang Provincial Top Key Discipline of Food Science and Biotechnology for Financial Support, China(JYTSP20141012).
浙江省食品科学与生物技术“重中之重”基金(JYTSP20141012)资助项目
©Editorial office ofActa Physico-Chimica Sinica
[Article]

