血红素加氧酶-1过表达对急性坏死性胰腺炎大鼠的保护作用
张飞虎 孔立 董晓斌 韩宁 赵浩 范开亮 毛恩强 汤耀卿 张圣道
血红素加氧酶-1过表达对急性坏死性胰腺炎大鼠的保护作用
张飞虎孔立董晓斌韩宁赵浩范开亮毛恩强汤耀卿张圣道
目的应用基因转染方法上调血红素加氧酶-1(HO-1)基因表达,观察其对急性坏死性胰腺炎(ANP)大鼠胰腺的保护作用,探讨其作用机制。 方法63只SD雄性大鼠采用5%牛磺胆酸钠逆行胰管内注射法制备ANP模型。按数字表法将ANP大鼠随机分为ANP组、腺病毒空载组、腺病毒基因组,每组21只。ANP组大鼠制模后30 min腹腔注射生理盐水1 ml;腺病毒空载组注射Adv-0 2.0×109pfu/ml;腺病毒基因组注射Adv-HO-1 2.0×109pfu/ml。Adv-0和Adv-HO-1由上海东方肝胆外科医院病毒与基因治疗中心完成。每组大鼠15只观察72 h生存情况;6只于制模后20 h处死,取血检测血HO-1、IL-10、TNF-α含量,取胰腺组织常规病理学检查,实时PCR法检测胰腺组织HO-1、IL-10、TNF-α mRNA表达量。 结果制模后20 h,ANP组、腺病毒空载组、腺病毒基因组大鼠血清HO-1含量分别为(0.90±0.09)、(0.89±0.07)、(1.29±0.07)μg/L,IL-10含量为(85.68±5.61)、(81.11±2.48)、(120.93±8.07)ng/L,TNF-α含量为(47.77±6.54)、(50.08±6.60)、(28.07±2.98)ng/L;胰腺组织HO-1 mRNA表达量分别为160.44±11.47、158.23±10.51、237.25±12.09,IL-10 mRNA表达量为33.93±2.56、34.13±3.01、60.02±2.89,TNF-α mRNA表达量为25.23±2.37、25.03±2.07、13.12±1.77。腺病毒基因组HO-1、IL-10含量及mRNA表达量均较腺病毒空载组和ANP组显著升高,TNF-α含量及mRNA表达量较腺病毒空载组、ANP组显著下降,差异均有统计学意义(P值均<0.05);腺病毒空载组与ANP组的差异均无统计学意义。制模后72 h,ANP组、腺病毒空载组、腺病毒基因组大鼠生存率分别为6.67%、0、46.67%,腺病毒基因组较腺病毒空载组及ANP组均显著提高,差异有统计学意义(P值均<0.05);腺病毒空载组与ANP组间的差异无统计学意义。结论应用基因转染方法上调HO-1表达对ANP大鼠胰腺具有保护作用,其机制可能是通过上调IL-10表达和抑制TNF-α表达实现的。
胰腺炎,急性坏死性;血红素加氧酶-1;转染;肿瘤坏死因子-α;白介素-10
Fund program: National Natural Science Foundation of China(81503543); Natural Science Foundation of Shandong Provincial(2015ZRB14327)
随着对细胞因子研究的深入,人们认识到细胞因子过度释放是重症急性胰腺炎(SAP)发病及导致病情加重的主要根源,且过度放大的炎症反应使SAP的发病不仅局限于胰腺本身,往往累及全身多个脏器。在炎症反应早期,中性粒细胞、单核巨噬细胞及局部组织释放的促炎细胞因子,如肿瘤坏死因子-α(TNF-α)、白介素-1(IL-1)等进入血液循环导致全身炎症反应综合征(SIRS)和多器官功能障碍综合征(MODS)[1]。因此,如何在SAP早期有效地控制机体炎症反应的过度放大成为提高SAP整体存活率的重要环节。血红素加氧酶-1(heme oxygenase-1,HO-1)是一种应激诱导的热休克蛋白,是降解血红素为一氧化碳(CO)、胆绿素和亚铁的限速酶。既往研究[2-4]提示,HO-1可通过抗氧化、抗炎症反应和维持微循环正常功能等机制在应激状态下保护细胞。因此,有效维持HO-1高表达对于SAP机体应该具有保护作用。本研究旨在探讨SAP应激状态下,应用基因转染的方法上调急性坏死性胰腺炎(ANP)大鼠胰腺HO-1表达,观察其对胰腺组织病理损伤的影响及调控促炎和抗炎细胞因子的作用。
材料及方法
一、材料与试剂
6~7周龄的SPF级健康雄性SD大鼠63只购于中科院上海实验动物中心,体重220~260 g。应用免疫缺陷型人5腺病毒(Adenoviral destination vector,Adv)作为载体构建插入HO-1基因的腺病毒载体(Adv-HO-1),由上海东方肝胆外科医院病毒与基因治疗中心协助完成。
纯度>95%的牛磺胆酸钠(货号86339)购自Sigma公司,逆转录试剂盒(货号A3500)购自Promega公司,荧光定量PCR Premix试剂盒(货号A2010A0112)购自BioTNT公司,HO-1、IL-10、TNF-α ELISA试剂盒(货号E0548r、E0056r、E0133r)均购自EIAabTM公司。
二、研究方法
1.动物模型建立与实验分组:63只大鼠均采用5%牛磺胆酸钠0.1 ml/100 g体重逆行胰管内注射法制备ANP模型,术后大鼠自由饮水。按数字表法将ANP大鼠随机分为ANP组、腺病毒空载组、腺病毒基因组,每组21只。制模后30 min ANP组大鼠腹腔注射生理盐水1 ml,腺病毒空载组注射Adv-0 2.0×109pfu/ml,腺病毒基因组注射Adv-HO-1 2.0×109pfu/ml。每组15只大鼠用于观察72 h的生存情况。其余6只于制模后20 h应用乙醚进行麻醉,腹主动脉取血,分离血清,置-20℃储存备用;取部分胰腺组织置10%甲醛溶液中固定,部分组织置液氮保存。
2.胰腺组织病理学检查:取固定的胰腺组织,常规脱水、透明、浸蜡、包埋、切片、HE染色,由上海交通大学医学院病理教研室教员读片,并按Schmidt等[5]标准进行胰腺组织病理评分。
3.血清HO-1、TNF-α、IL-10含量检测:采用酶联免疫吸附试验(ELISA)法检测血清HO-1、TNF-α、IL-10含量。
4.胰腺组织HO-1、TNF-α、IL-10 mRNA表达检测:取液氮保存的胰腺组织,研磨成粉状后采用Trizol提取组织总RNA,先逆转录成cDNA,之后应用荧光定量PCR法检测HO-1、TNF-α、IL-10 mRNA表达。引物序列及PCR退火温度见表1。PCR反应条件:95℃ 10 min、退火温度20 s、72℃ 30 s,40个循环。由仪器自带软件获得Ct值,以公式2-ΔΔCt计算mRNA相对表达量。
为了快速定位中压、低压配电网中的故障,本文根据停电事件的触发类型、多源信息之间的关联关系,研究了基于多源数据的停电故障研判方法,并通过停电事件信息池的设计,用以加快停电故障的研判以及在多个应用之间的共享。
三、统计学处理
结 果
一、血清HO-1、IL-10、TNF-α含量变化
制模后20 h,ANP组、腺病毒空载组、腺病毒基因组大鼠血清HO-1含量分别为(0.90±0.09)、(0.89±0.07)、(1.29±0.07)μg/L;IL-10含量为(85.68±5.61)、(81.11±2.48)、(120.93±8.07)ng/L;TNF-α含量为(47.77±6.54)、(50.08±6.60)、(28.07±2.98)ng/L。腺病毒基因组血HO-1、IL-10含量较腺病毒空载组及ANP组均显著升高,TNF-α含量较腺病毒空载组及ANP组显著下降,差异均有统计学意义(t值分别为6.847、6.172;9.112、8.674;-11.375、-10.784;P值均<0.05);腺病毒空载组与ANP组间的差异均无统计学意义。
二、胰腺组织HO-1、IL-10、TNF-α mRNA表达量变化
制模后20 h,ANP组、腺病毒空载组、腺病毒基因组大鼠胰腺组织HO-1 mRNA表达量分别为160.44±11.47、158.23±10.51、237.25±12.09;IL-10 mRNA表达量为33.93±2.56、34.13±3.01、60.02±2.89;TNF-α mRNA表达量为25.23±2.37、25.03±2.07、13.12±1.77。腺病毒基因组HO-1、IL-10 mRNA表达量较腺病毒空载组及ANP组显著升高,TNF-α mRNA表达量较腺病毒空载组及ANP组显著下降,差异均有统计学意义(t值分别为7.746、7.254;10.957、11.382;-10.497、-10.976;P值均<0.05);腺病毒空载组与ANP组间的差异均无统计学意义(t值分别为-0.196,0.231,-0.094,P值均>0.05)。
ANP组大鼠胰腺组织镜下见局部出血、坏死以及中性粒细胞浸润;腺病毒空载组大鼠胰腺病变与ANP组无明显差异;腺病毒基因组大鼠胰腺病理损伤较ANP组减轻,中性粒细胞浸润较少,腺管结构完整(图1)。ANP组、腺病毒空载组、腺病毒基因组大鼠胰腺组织病理评分分别为(11.3±1.4)、(11.8±1.3)、(8.5±0.9)分,腺病毒基因组较腺病毒空载组及ANP组均显著下降,差异有统计学意义(t值分别为-5.495,-5.246,P值均<0.05),而腺病毒空载组与ANP组间差异无统计学意义(t=0.247,P>0.05)。
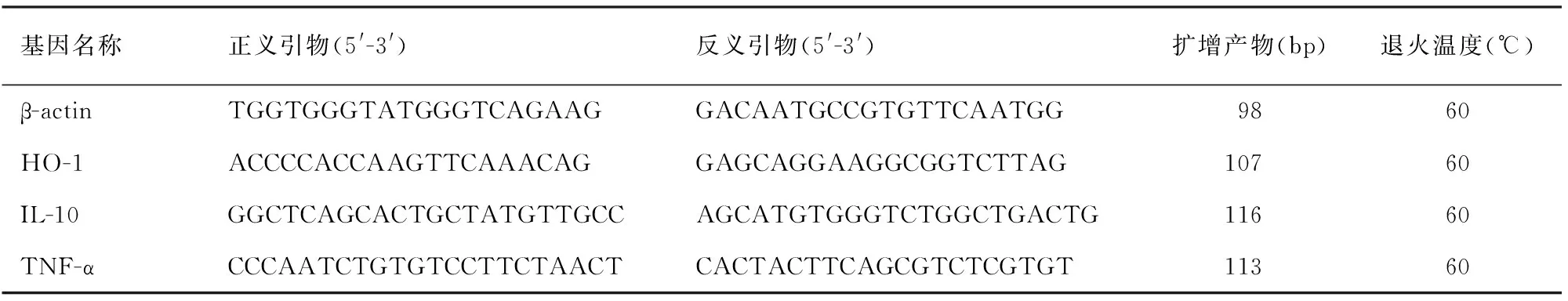
表1 各基因引物序列及退火温度
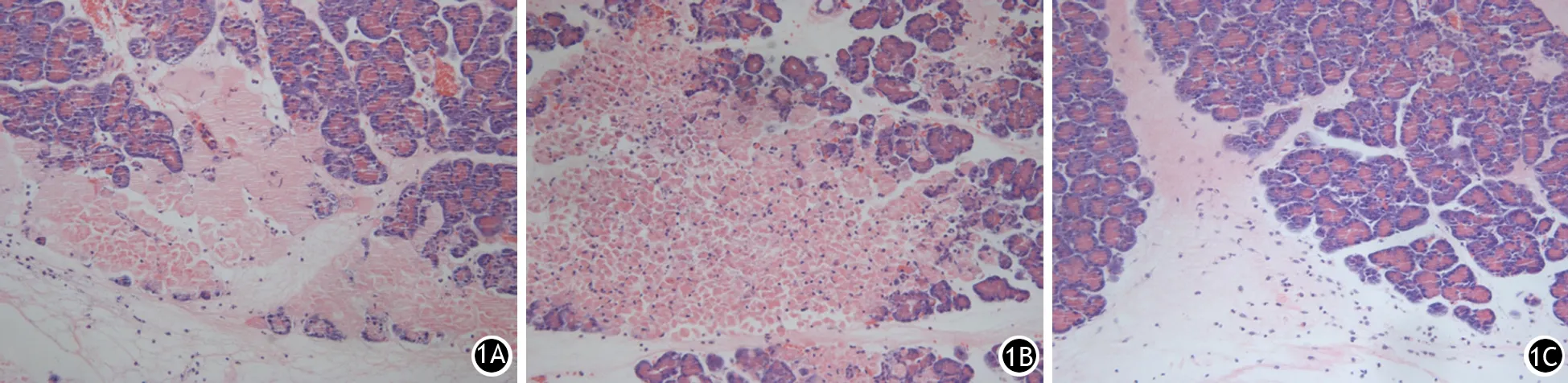
图1 制模后20 h ANP组(1A)、腺病毒空载组(1B)、腺病毒基因组大鼠胰腺病理改变(HE ×200)
四、各组大鼠生存情况
腺病毒基因组制模后24、48、72 h的大鼠生存率分别为86.67%、66.67%、46.67%;腺病毒空载组分别为53.33%、20.00%、0;ANP组分别为60.00%、13.33%、6.67%。腺病毒基因组大鼠生存率较腺病毒空载组及ANP组均显著提高,差异有统计学意义(P值均<0.05);腺病毒空载组与ANP组间的差异无统计学意义(P值均>0.05)。
讨 论
HO-1也称为热休克蛋白-32(heat shock protein-32,HSP-32),是一种应激诱导的蛋白,是降解血红素为胆绿素、CO和亚铁的限速酶。重金属、血红素、一氧化氮(NO)、生长因子、细胞因子、低氧、高氧等均可诱导HO-1表达[6]。既往研究[2-4]提示,应激状态下HO-1通过抗氧化、抗炎症反应及维持微循环正常功能等机制保护细胞。急性胰腺炎发病后,多种应激因素可引起氧化应激反应相关基因表达上调,尤其以HO-1基因表达上调最为显著[7],表明机体具有对抗应激反应的代偿机制。但胰腺局部病变并未因此减轻或停止,原因在于对抗炎症反应的抗炎细胞因子表达难以拮抗和抑制大量释放的促炎细胞因子表达。因此通过外源性措施提高HO-1表达能够降低促炎细胞因子的释放,以达到保护脏器功能的作用。
基因转染的方法可使目的基因长时间表达,从而调节细胞因子的免疫反应,在炎症疾病的治疗中越来越受到重视。腺病毒是一种双链DNA病毒,其基因组为36 000 bp,可有效感染分裂期及非分裂期细胞;且宿主广泛,对胰腺、肝脏、肾脏及肺脏组织均有较强的组织亲嗜性[8-10],并且以腺病毒作为载体可使目的基因有较高水平表达[11]。腹膜表面积大,血管丰富,且无DNA酶的降解作用,是腺病毒感染较好的部位,从而使目的基因可有较长时间的转录和表达,且不影响病变部位的治疗效果[12],所以腹腔内注射是腺病毒感染的常用方法。Abraham等[13]报道,HO-1基因治疗在高血压、心肌病、器官移植、肺脏疾病及内毒素血症等的方面已取得显著成就。
本研究结果显示,应用腺病毒载体使ANP大鼠胰腺组织HO-1高表达,能够减轻胰腺组织病变程度,提高大鼠生存率。说明HO-1对胰腺组织损伤具有保护作用。
SAP发生、发展过程中有多种抗炎和促炎细胞因子的参与,TNF-α和IL-10分别是体内重要的促炎和抗炎细胞因子。TNF-α是由激活的淋巴细胞、巨噬细胞分泌的促炎细胞因子,其在SAP的发生、发展过程中具有重要的作用[14],TNF-α升高可诱导IL-8、IL-6及其自身表达的升高,从而引起级联反应,造成炎症递质的失控性释放[15]。IL-10主要由Th-2细胞产生,具有潜在的抗炎功能,可抑制IL-2、IL-3及TNF-α等促炎细胞因子的合成与释放,对SAP所致的多器官损伤具有改善作用[16-17]。SAP发生后,胰腺内的巨噬细胞首先释放出TNF-α等促炎细胞因子导致胰腺局部病变恶化,同时机体也开始释放IL-10等抗炎细胞因子。但抗炎因子难以阻止全身过度炎症反应和胰腺局部坏死而发生脓毒症休克,从而引发肝脏等远隔器官的细胞因子(TNF-α、IL-1β、IL-6等)表达升高,并进展为MODS。在SAP早期提高IL-10等抗炎细胞因子表达和抑制TNF-α等促炎细胞因子表达可缓解或阻断胰腺的局部病变,提高生存率[18-20]。
本研究结果显示,ANP大鼠血HO-1含量和胰腺组织HO-1 mRNA表达升高,血IL-10含量和胰腺组织IL-10 mRNA表达也明显升高,而血TNF-α含量和胰腺组织TNF-α mRNA表达均显著降低。由此推论,HO-1对ANP大鼠脏器保护作用可能是通过提高抗炎细胞因子IL-10表达和抑制促炎细胞因子TNF-α表达所实现的。
[1]Yasar M, Uysal B, Kaldirim U, et al. Poly(ADP-ribose) polymerase inhibition modulates experimental acute necrotizing pancreatitis-induced oxidative stress, bacterial translocation and neopterin concentrations in rats[J]. Exp Biol Med (Maywood), 2010, 235(9):1126-1133. DOI: 10.1258/ebm.2010.010091.
[2]Scott JR, Cukiemik MA, Ott MC, et al. Low-dose inhaled carbon monoxide attenuates the remote intestinal inflammatory response elicited by hindlimb ischemia-reperfusion[J]. Am J Physiol Gastrointest Liver Physiol, 2009, 296(1):G9-G14. DOI: 10.1152/ajpgi.90243.2008.
[3]Wang CF, Wang ZY, Li JY. Dual protective role of HO-1 in transplanted liver grafts: a review of experimental and clinical studies[J]. World J Gastroenterol, 2011, 17(26):3101-3108. DOI: 10.3748/wjg.v17.i26.3101.
[4]Tamion F, Richard V, Renet S, et al. Protective effects of heme-oxygenase expression against endotoxic shock: inhibition of tumor necrosis factor-alpha and augmentation of interleukin-10[J]. J Trauma, 2006, 61(5):1078-1084. DOI: 10.1097/01.ta.0000239359.41464.ef.
[5]Schmidt J, Rattner DW, Lewandrowshi K, et al. A better model of acute pancreatitis for evaluating therapy[J]. Ann Surg, 1992, 215(1):44-56.
[6]Castilho A, Aveleira CA, Leal EC, et al. Heme oxygenase-1 protects retinal endothelial cells against high glucose-and oxidative/nitrosative stress-induced toxicity[J]. PLoS One, 2012, 7(8):e42428. DOI: 10.1371/journal.pone.0042428.
[7]Saruc M, Yuceyar H, Turkel N, et al. The role of heme in hemolysis-induced acute pancreatitis[J]. Med Sci Monit, 2007, 13(3):BR67-BR72.
[8]Vallabhaneni R, Kaczorowski DJ, Yaakovian MD, et al. Heme oxygenase 1 protects against hepatic hypoxia and injury from hemorrhage via regulation of cellular respiration[J]. Shock, 2010, 33(3):274-281. DOI: 10.1371/journal.pone.0042428.
[9]An L, Liu CT, Yu MJ, et al. Heme oxygenase-1 system, inflammation and ventilator-induced lung injury[J]. Eur Pharmacol, 2012, 677(1-3):1-4. DOI: 10.1016/j.ejphar.2011.12.010.
[10]Van-Assche T, Huygelen V, Crabtree MJ, et al. Gene therapy targeting inflammation in atherosclerosis[J]. Curr Pharm Des, 2011, 17(37):4210-4223.
[11]Mullan B, Dugue C, Moutard V, et al. Robust functional gene validation by adenoviral vectors: one-step Escherichia coli-derived recombinant adenoviral genome construction[J]. Gene Ther, 2004, 11(21):1599-1605. DOI:10.1038/sj.gt.3302333.
[12]Evans JM, Navarro S, Doki T, et al. Gene transfer of heme oxygenase-1 using an adeno-associated virus serotype 6 vector prolongs cardiac allograft survival[J]. J Transplant, 2012, 2012:740653. DOI: 10.1155/2012/740653.
[13]Abraham NG, Asija A, Drummond G, et al. Heme oxygenase-1 gene therapy: recent advances and therapeutic applications[J]. Curr Gene Ther, 2007, 7(2): 89-108. DOI: 10.2174/156652307780363134.
[14]Bishehsari F, Sharma A, Toth C, et al. TNF-alpha gene (TNFA) variants increase risk for muti-origan dysfunction syndrome (MODS) in acute pancreatitis[J]. Pancreatology, 2012, 12(2):113-118. DOI: 10.1016/j.pan.2012.02.014.
[15]Malleo G, Mazzon E, Siriwardena AK, et al. Role of tumor necrosis factor-alpha in avute pancreatitis: from biological basia to clinical evidence[J]. Shock, 2007, 28(2):130-140. DOI: 10.1097/shk.0b013e3180487ba1.
[16]Keceli M, Kucuk C,Sozuer E, et al. The effect of interleukin-10 on acute pancreatitis induced by cerulein in a rat experimental model[J]. J Invest Surg, 2005, 18(1):7-12. DOI:10.1080/08941930590905080.
[17]Chen ZQ, Tang YQ, Zhang Y, et al. Adenoviral transfer of human interleukin-10 gene in lethal pancreatitis[J]. World J Gastroenterol, 2004, 10(20):3021-3025.
[18]Escobar J, Pereda J, Arduini A, et al. Cross-talk between oxidative stress and pro-inflammatory cytokines in acute pancreatitis: a key role for protein phosphatases[J]. Curr Pharm Des, 2009, 15(26): 3027-3042. DOI:10.2174/138161209789058075.
[19]Malmstrom ML, Hansen MB, Andersen AM, et al. Cytokines and organ failure in acute pancreatitis:inflammatory response in acute pancreatitis[J]. Pancreas, 2012, 41(2):271-277. DOI: 10.1097/MPA.0b013e3182240552.
[20]中国医师协会胰腺病学专业委员会. 中国急性胰腺炎多学科(MDT)诊治共识意见(草案)[J]. 中华胰腺病杂志, 2015,15(4):217-224. DOI: 10.3760/cma.j.issn.1674-1935.2015.04.001.
(本文编辑:屠振兴)
Protective effect of heme oxygenase-1 overexpression on acute necrotizing pancreatitis rat model
ZhangFeihu,KongLi,DongXiaobin,HanNing,ZhaoHao,FanKailiang,MaoEnqiang,TangYaoqing,ZhangShengdao.
DepartmentofEmergencyCenter,AffiliatedHospital,ShandongUniversityofTraditionalChineseMedicine,Jinan250011,China
Correspondingauthor:KongLi,Email:kongli_sdszyy@sina.com
ObjectiveTo observe the effect of up-regulating Heme oxygenase-1(HO-1)expression in acute necrotizing pancreatitis(ANP) rats and to investigate its potential mechanism. MethodsA total of 63 male healthy Sprague-Dawley(SD) rats were treated by retrograde injection of 5% sodium taurocholate into pancreatic duct to establish ANP model. ANP rats were randomly divided into ANP group, Adenoviral empty vector(Adv-0) group and Adenoviral carriging HO-1 gene(Adv-Ho-1) group with 21 rats in each group. Adv-0 and Adv-HO-1 were constructed by Virus and Gene Therapy Center, Eastern Hapatobiliary Surhery Hospital.
In ANP group, 1ml normal saline solution per animal was intraperitoneally injected at 30 minutes after the establishment of model; in Adv-0 group, 2.0×109pfu/mL Adv-0 per animal was intraperitoneally injected; in Adv-HO-1 group, 2.0×109pfu/mL Adv-HO-1 per animal was intraperitoneally injected. 15 rats in each group were used to observe the survival rate for 72 hours and the other 6 rats in each group were executed at 20 hours after the establishment of ANP model to detect serum HO-1, IL-10 and TNF-α level. Pancreatic tissue was collected and the histopathological changes were observed. HO-1, IL-10 and TNF-α mRNA level in pancreatic tissue were detected by real-time PCR. ResultsAt 20 hours after ANP model establishment, serum HO-1 level in ANP group, Adv-0 group and Adv-HO-1 gene group was (0.90±0.09),(0.89±0.07) and(1.29±0.07)μg/L; serum IL-10 was (85.68±5.61),(81.11±2.48) and(120.93±8.07)ng/L; serum TNF-α was (47.77±6.54),(50.08±6.60) and (28.07±2.98)ng/L. HO-1 mRNA expression in pancreatic tissue in each group was 160.44±11.47, 158.23±10.51 and 237.25±12.09; IL-10 mRNA expression was 33.93±2.56, 34.13±3.01 and 60.02±2.89; TNF-αmRNA expression was 25.23±2.37, 25.03±2.07 and 13.12±1.77. The serum level and mRNA expression of HO-1 and IL-10 in Adv-HO-1 gene group were both significantly higher than those in ANP group and Adv-0 group, while TNF-αl level and mRNA expression were decreased, indicating that the differences were statistically significant(P<0.05). No statistically significant differences on serum level and mRNA expression of HO-1, IL-10 and TNF-αl were observed between Adv-0 group and ANP group(allP>0.05). At 72 hours after ANP model establishment, the survival rate in ANP group, Adv-0 group and Adv-HO-1 group was 6.67%, 0 and 46.67%. The survival rate of Adenoviral HO-1 gene group was significantly higher than that of ANP group and Adv-0 group, and the difference was statistically significant(P<0.05).No statistically significant difference on the survival rate was found between Adv-0 group and ANP group(P>0.05). ConclusionsAdenoviral transfection of HO-1 gene can up-regulate HO-1 expression, which could exert protective effects on ANP rats. The potential mechanism might be associated with up-regulating IL-10 and down-regulating TNF-α.
Pancreatitis, acute necrotizing;Heme oxygenase-1;Transfection;Tumor necrosis factor-alpho;Interleukin-10
10.3760/cma.j.issn.1674-1935.2016.04.010
250011济南,山东中医药大学附属医院急诊科(张飞虎、孔立、董晓斌、韩宁、赵浩、范开亮);上海交通大学医学院附属瑞金医院重症医学科(毛恩强、汤耀卿、张圣道)
孔立,Email:kongli_sdszyy@sina.com
国家自然科学基金(81503543);山东省自然科学基金(2015ZRB14327)
2015-12-31)

