Photocatalytic degradation of ammonia via graphene oxide-nickel ferrite hybrid catalyst under visible light irradiation
ZHOU Shanshan,XIAO Bo,LIU Chengbao,LIU Shouqing
(Jiangsu Key Laboratory of Environmental Functional Materials,Suzhou 215009,China;School of Chemistry,Biology and Material Engineering,SUST,Suzhou 215009,China)
Photocatalytic degradation of ammonia via graphene oxide-nickel ferrite hybrid catalyst under visible light irradiation
ZHOU Shanshan,XIAO Bo,LIU Chengbao,LIU Shouqing*
(Jiangsu Key Laboratory of Environmental Functional Materials,Suzhou 215009,China;School of Chemistry,Biology and Material Engineering,SUST,Suzhou 215009,China)
Nickel ferrite was modified with graphene oxide (GO),in which graphene oxide-nickel ferrite (GO-NiFe2O4)hybrid catalyst was yielded.GO activated the photocatalytic activity of nickel ferrite to hydrogen peroxide,which resulted in the photo-Fenton degradation of ammonia in alkaline solution using the hybrid photocatalyst under visible light irradiation.The effects of pH,GO content,H2O2concentration on the degradation ratio were explored.The results show that A GO-NiFe2O4catalyst with the mass concentration of 4.0%GO in NiFe2O4produces an optimal degradation ratio of 90.5% at 8 h for the ammonia abatement in pH 10.5 solution with 1.0 mmol·L-1H2O2under visible light irradiation.The stability tests show that the degradation ratio is almost constant for eight runs,which indicates the GO-NiFe2O4catalyst is very stable.The material can be used to deal with wastewater polluted with ammonia.
nickel ferrite;graphene oxide;photo-fenton;ammonia;degradation
Iron oxides and iron-based oxide semiconductors are attracting attention due to their environmentally safe,abundance,low cost and nontoxic nature.Beltrán et al.reported that iron oxide degraded organics in water,and examined the effects of pH and light source on degradation rate[1].Madras studied the degradation of dyes via transition metal oxide loaded MCM catalysts[2].Sharma reviewed combination of ferrites(iron-based oxide)with H2O2either under light irradiation or dark conditions,a type of Fenton-like catalytic systemes is raised,which produces hydroxyl radicals to enhance the degradation processes[3].Among ferrites,Nickel ferrite(NiFe2O4)has been paid much attention because of its practical applications,such as a high-performance supercapacitor[4],gas sensor[5],and photocatalyst[6].However,the presence of nickels in the NiFe2O4crystal lattice played a role of inhibition to Fenton catalytic reaction of hydrogen peroxide[7].Our previous research showed the combination of graphene oxide(GO)with NiFe2O4(denoted as GO-NiFe2O4)enables this material to make response to visible light irradiation and degrade organic dyes in the presence of oxalic acid[8].Ammonia(NH3/NH)is a more recalcitrant pollutant in waters to be degraded,compared with organic dyes,for example,methylene blue was degraded to final species ammonia,instead of dinitrogen[9].We now present the degradation of ammonia using GO-NiFe2O4as the photocatalyst and hydrogen peroxide as the oxidant under visible light irradiation.
1 Experimental
1.1 Chemicals
Graphite powder(average particle size,30 μm)was purchased from Shanghai Colloid Chemical Plan (Shanghai,China).Ferric chloride hexahydrate(FeCl3·6H2O)and sodium hydroxide(NaOH)were purchased from Tianjin Damao Chemical Factory,China.Ni(II)sulfate hexahydrate(NiSO4·6H2O),ammonium sulfate((NH4)2SO4),and ammonium chloride(NH4Cl)were obtained from Nanjing Chemical Reagent Co.,Ltd.All reagents used were of analytical grade and applied without further purification.All solutions were prepared with 18.2 MΩ·cm deionized Milli-Q water.
1.2 Synthesis of GO-NiFe2O4and structure characterization
The GO-NiFe2O4catalyst was synthesized on the basis of the procedures described in reference[8].Typically,the mass ratio of GO to NiFe2O4is 4.0%in the GO-NiFe2O4hybrid catalyst.The structure of the catalyst is identified with X-ray diffraction spectroscopy,Raman spectroscopy,Fourier-transform infrared spectroscopy,transmission electron microscopy.
1.3 Degradation of NH3
Photocatalytic experiments for NH3degradation were conducted under visible light irradiation (λ>400 nm).A 300 W UV-visible lamp(OSRAM,Germany)was used as a light source.Photo-Fenton degradation of 50.0 mL of NH3solution was performed in a 100 mL beaker at room temperature (25±2℃).The distance between the lamp and test solution was approximately 10 cm,and the wall of the beaker was shielded from surrounding light by aluminum foil.Visible light was allowed to pass through a λ>400 nm cut-off filter covering the window of the beaker;this filter absorbed UV light and allowed visible light of λ>400 nm to pass through.In a typical photo-Fenton experiment,50 mL of test solution was used.The NH3solutions were prepared according to the desired concentrations,and 0.20 g catalyst was used for the photocatalytic experiments.NaHCO3-Na2CO3buffer was used to control the pH of the test solutions.
2 Results and discussion
2.1 XRD characterization and TEM observation
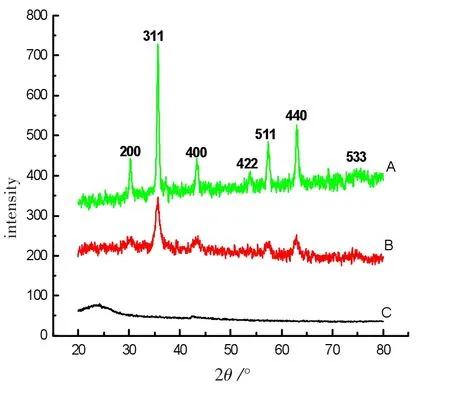
Figure 1 XRD patterns of the NiFe2O4-GO(A),NiFe2O4(B)and GO(C)samples
The XRD patterns of the as-synthesized GO-NiFe2O4,NiFe2O4and GO samples are presented in Figure 1.The diffraction angle positions of the characteristic peaks (2θ)from the GO-NiFe2O4and NiFe2O4samples agree well with the data in the JCPDS card (No.74-2081)of NiFe2O4species.Thus,the crystal structure of the as-synthesized GO-NiFe2O4is identical to that of NiFe2O4.It is a cubic spinel ferrite,crystallizing in the cubic spinel type structure(a=0.8 433 nm),space group Fd3m.The broad peaks of the GO-NiFe2O4and NiFe2O4samples indicated that the size of the GO-NiFe2O4particles is very small.The average diameter(D)of the GO-NiFe2O4particles was calculated as 9.7 nm using the Debye-Scherrer equation D=Kλ/(Wcosθ)at a diffraction angle of 35.59°(2θ),and one of the NiFe2O4particles was 9.4 nm.W is the breadth of the observed diffraction peak at its half height,K is the socalled shape factor(usually approximately 0.9),and λ is the wavelength of the X-ray source used(0.154 nm by our measurement).The calculated diameter of NiFe2O4particles on GO sheets based on the Debye-Scherrer equation is very close to that obtained from the TEM observations as shown in Figure 2.
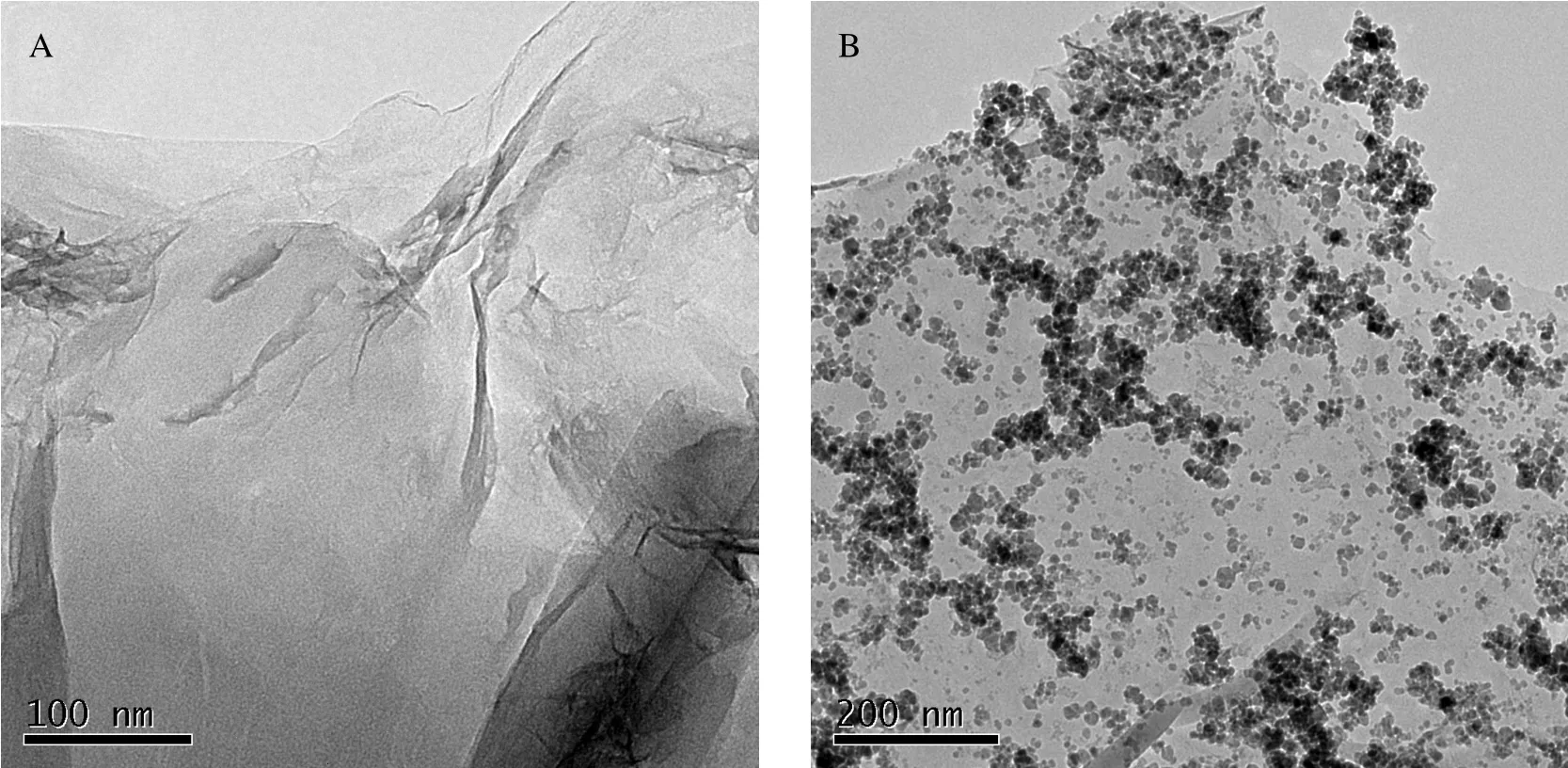
Figure 2 TEM images of GO and GO-NiFe2O4samples
As seen,NiFe2O4particles are grown on GO film to form surface-bound particles.Measurements showed that the diameter of NiFe2O4particles is approximately 10 nm,which confirmed the calculated diameter based on the Debye-Scherrer equation.
2.2 Photo-Fenton degradation of ammonia in the presence of H2O2
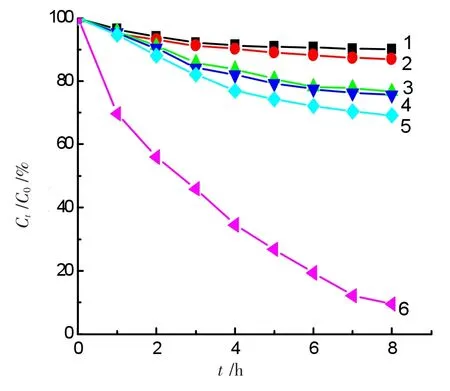
Figure 3 Photo-Fenton degradation of ammonia in a 50.0 mL solution containing 50.0 mg·L-1ammonia-nitrogen
GO-NiFe2O4was also characterized by Fourier-transform infrared and Raman spectroscopy,the results are consistent with those[8].The GO-NiFe2O4sample was utilized as a heterogeneous photo-Fenton catalyst.Figure 3 presented the degradation curves of ammonia using GO-NiFe2O4and NiFe2O4as catalysts in the presence or absence of H2O2under visible light irradiation or in dark.The initial conditions are as follows:(1)The solution+0.20 g GO-NiFe2O4catalyst in dark;(2)The solution+0.20 g GONiFe2O4+1.0 mmol·L-1H2O2in dark;(3)The solution+0.20 g GO-NiFe2O4+visible light irradiation without H2O2;(4)The solution+1.0 mmol·L-1H2O2+visiblelight irradiation without the photocatalyst;(5)The solution+0.20 g NiFe2O4+1.0 mmol·L-1H2O2+visible irradiation;(6)The solution+0.20 g GO-NiFe2O4+1.0 mmol·L-1H2O2+visible irradiation.Curve 6 showed an 90.5%degradation ratio for ammonia using GO-NiFe2O4as the heterogeneous photo-Fenton catalyst in the presence of 1.0 mmol·L-1H2O2under visible light irradiation,while only a 30.0%abatement ratio(curve 5)for ammonia was achieved using unmodified NiFe2O4as the heterogeneous photo-Fenton catalyst under similar conditions.Compared curve 6 with curves 1-5,the GO-NiFe2O4heterogeneous catalyst displayed the photocatalytic degradation property for ammonia in the presence of hydrogen peroxide and the graphene oxide played a very vital role in absorbing visible light.
2.3 Effects of GO content
A series of GO-NiFe2O4hybrid materials containing various mass ratios of GO from 0.0%to 8.0%were synthesized,the as-synthesized products were utilized as the photo-Fenton catalysts for the degradation of ammonia.The degradation curves were shown in Figure 4.A 50.0 mL solution at pH 10.5 containing 50.0 mg·L-1ammonia-nitrogen+1.0 mmol·L-1H2O2+a series of 0.20 g GO-NiFe2O4catalysts containing various mass concentrations ofGO.The results showed that the degradation ratio increases as the mass ratio of GO rises in the composite at the initial stage,and the ratio reaches a top of 90.5%when the GO content is equal to 4.0%.Then,the degradation ratio starts to decrease when the GO content continues to increase.Both 6.0%and 8.0%contents of GO do not result in any expected increase of degradation ratio.Contrary to what it was expected,the degradation ratio decreases slightly.Thus,an optimal mass concentration of GO is equal to 4.0%in the composite catalyst for the degradation of ammonia.The reason for the fluctuation could involve the adsorption of ammonia on nickel ferrite.On the one hand,the increase of GO content in the composite catalyst can improve the absorption of the catalyst to visible light,resulting in promoting the degradation rate for ammonia.On the other hand,the overload of GO could inhibit the adsorption of ammonia on the surface of nickel ferrite catalyst because GO decreased the concentration of transition metal atoms exposed on the surface of the composite catalyst.
2.4 Effects of pH
The pH value in solutions affects significantly the reaction rate for Fenton catalytic reactions.Generally,acidic solutions facilitate Fenton reaction rate[10-12].However,alkaline solutions promote the degradation rate of ammonia.The degradation curves of ammonia were shown in Figure 5 in various pH media.50.0 mL solutions containing 50.0 mg·L-1ammonia-nitrogen+0.20 g GO-NiFe2O4+1.0 mmol·L-1H2O2.The pH value was adjusted with 0.1 mol·L-1NaHCO3/Na2CO3.The results indicated that the solutions with pH value less than 9.0 have very low degradation ratios.A pH 7.0 solution has only 15.0%of the degradation ratio for ammonia for irradiation 8 h,another pH 8.0 solution responds to a 23.0%of degradation ratio under similar conditions.And the degradation ratio approached higher and higher as pH value rose.Finally,it achieved 90.5%in 10.5 solution whereas it was only 15.0%in pH 7.0 solution.This trend of the ratio degradation is increasing with pH rise,as opposed to traditional one.The pH effect of solutions on the degradation ratio is attributed to the protonation of ammonia[13].The pKa of NHis equal to 9.3,so it is predominantly protonated in pH<9.3 solutions.The protonated ammonia is charged positively,which is not easy to coordinate to the nickel ferrite surface.The higher pH values of solutions favor to adsorption of ammonia on the catalyst.Thus,the degradation rate is faster and faster with the increase of pH value in solutions.In addition,the hydroxyl radicals(·OH)·can oxidize NH3but not NH,the more concentration of NH3led to the faster degradation rate in a higher pH solution[14].
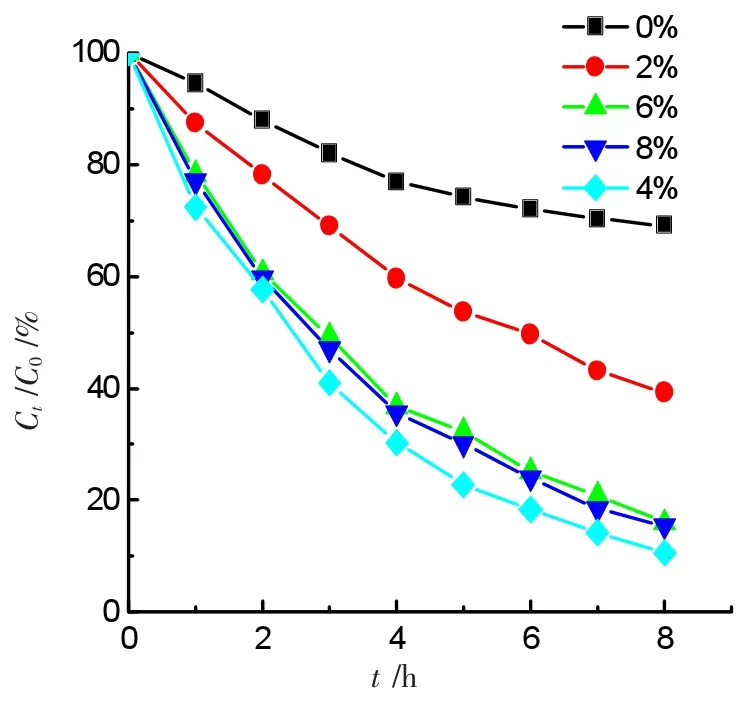
Figure 4 Effects of the GO content on degradation ratio of ammonia under visible light irradiation
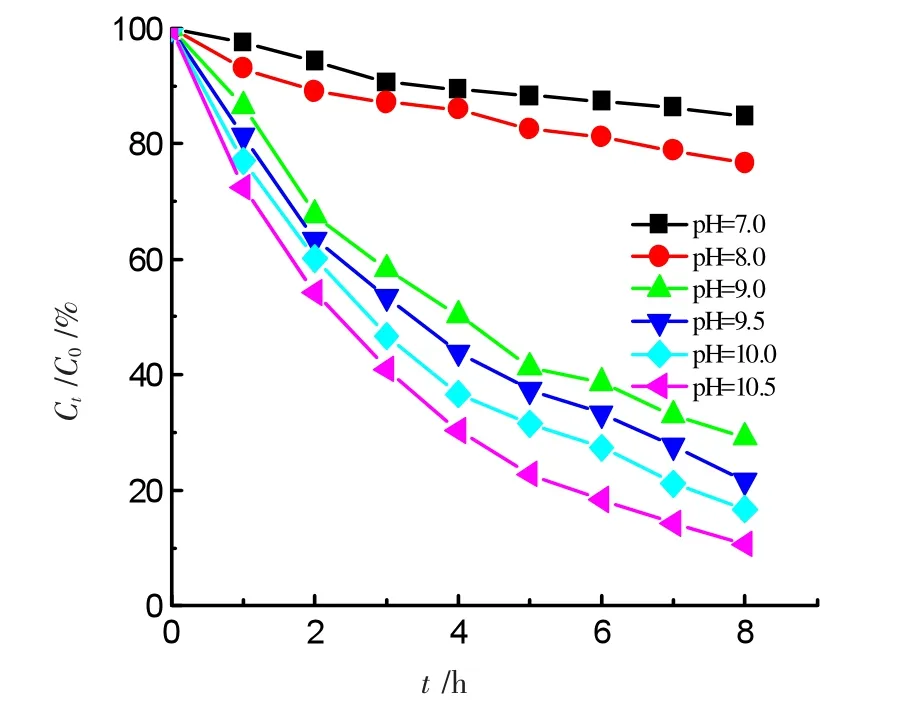
Figure 5 Effects of pH values on degradation ratio of ammonia during photo-Fenton process
2.5 Effects of H2O2concentration
The effect of the H2O2concentration on the degradation ratio was researched,a series of solutions containing 0.5 mmol·L-1,1.0 mmol·L-1and 10.0 mmol·L-1H2O2were tested.The results showed that the degradation ratios for ammonia approached to 71.3%,78.3%,and 85.3%at 8.0 h with respect to the three concentrations of H2O2,respectively.As seen in Figure 6,the degradation ratio for ammonia rose with the augment of H2O2concentration.50.0 mL solutions at pH 10.5 containing 50.0 mg·L-1ammonia-nitrogen+0.20 g GO-NiFe2O4+various concentrations of H2O2.It was attributed to the augment of hydorxyl radicals due to the more concentration of H2O2.However,the excess concentration of hydrogen peroxide could lead to the decrease of the degradation ratio due to the hydroxyl radical scavenging effect of H2Oas shown in the reactions below
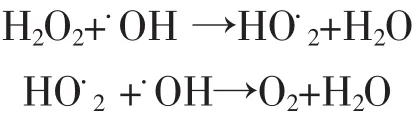
2.6 Effects of ammonia concentrations and degradation kinetics
The initial concentration of ammonia was varied as desired while 1.0 mmol·L-1of the H2O2concentration,0.2 g catalyst in 50.0 mL solution with pH 10.5 were constant.The degradation curves for the various ammonia concentrations were shown in Figure 7.A 50.0 mL solution at pH 10.5 containing 1.0 mmol·L-1H2O2+0.20 g GO-NiFe2O4catalyst with various ammonia concentrations.The corresponding parameter ln(C0/Ct)is linearly proportional to the irradiation time t,shown in Figure 8.The experimental conditions are the same as those in Figure 7.The data showed the ammonia degradation follows a pseudo-first order kinetic law.According to the integral equation(1)and experimental data,the average value of an apparent rate constant kappwas estimated to be equal to 3.854×10-3min-1,which shows the degradation rate for ammonia is fast.

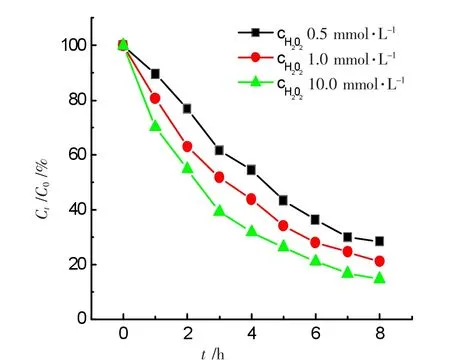
Figure 6 Effects of initial concentrations of H2O2on degradation ratio of ammonia
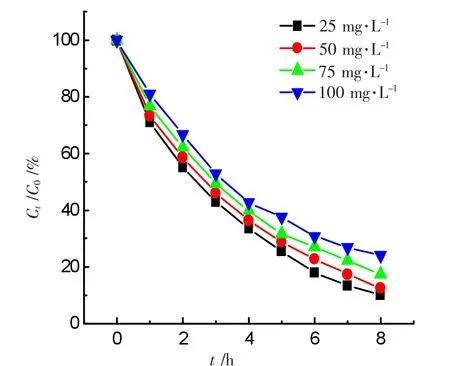
Figure 7 Effects of the initial concentrations of ammonia on degradation rate

Figure 8 The dependence of ln(C0/Ct)on irradiation time for ammonia degradation
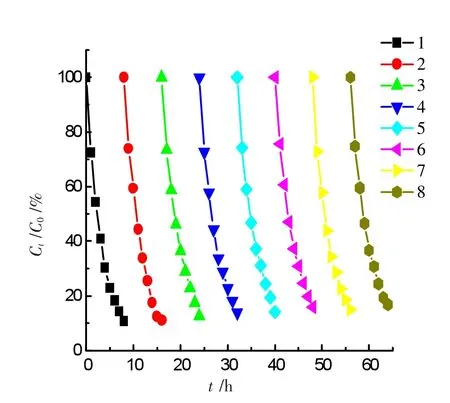
Figure 9 Cyclic tests of the GO-NiFe2O4catalyst
2.7 Stability and reuse
Figure 9 shows eight consecutive experiments using the fresh ammonia solutions.Experimental conditions:50 mL solutions at pH 10.5 containing 50 mg·L-1ammonia-nitrogen+1.0 mmol·L-1H2O2+0.2 g GO-NiFe2O4catalyst.The irradiation time was about 8 h each run.At the end of the previous experiment,the catalyst was collected by an external magnetite,separated and washed with deionized water for three times.The results showed that the degradation ratio was almost constant for eight runs,indicating the GO-NiFe2O4catalyst is very stable,recoverable and reusable.
3 Conclusion
GO-Nickel ferrite composite can photocatalytically degrade ammonia in the presence of hydrogen peroxide under visible irradiation.Graphene oxide improves the absorption of visible light and enhances the photocatalytic activity of nickel ferrite.The composite catalyst will play an important role in degrading ammonia in wastewaters due to its high activity and stability.
References:
[1]RODÍGUEZ E M,FERNÁNDEZ G,ÁLVAREZ P M,et al.Photocatalytic degradation of organics in water in the presence of iron oxides:Effects of pH and light source[J].Applied Catalysis B:Environ,2011,102:572-583.
[2]JYOTHI D,DESHPAND P A,VENUGOPAL B R,et al.Transition metal oxide loaded MCM catalysts for photocatalytic degradation of dyes[J].Journal of Chemical Sciences,2012,124:385-393.
[3]CASBEER E,SHARMA V K,LI X Z.Synthesis and photocatalytic activity of ferrites under visible light:A review[J].Separation and Purification Technology,2012,87:1-14.
[4]WANG W J,HAO Q L,LEI W,et al.Ternary nitrogen-doped graphene/nickel ferrite/polyaniline nanocomposites for high-performance supercapacitors[J].J Power Sources,2014,269:250-259.
[5]SUTKA A,MEZINSKIS G,LUSIS A,et al.Gas sensing properties of Zn-doped p-type nickel ferrite[J].Sensor Actuat B,2012,171/172:354-360.
[6]LIU S Q,FENG L R,XU N,et al.Magnetic nickel ferrite as a heterogeneous photo-Fenton catalyst for the degradation of rhodamine B in the presence of oxalic acid[J].Chem Eng J,2012,203:432-439.
[7]COSTA R C C,LELIS M F F,OLIVELIVEIRA L C A,et al.Novel active heterogeneous Fenton system based on Fe3-xMxO4(Fe,Co,Mn,Ni):the role of M2+species on the reactivity towards H2O2reactions[J].J Hazard Mater B,2006,129:171-178.
[8]LIU S Q,XIAO B,FENG L R,et al.Graphene oxide enhances the Fenton-like photocatalytic activity of nickel ferrite for degradation of dyes under visible light irradiation[J].Carbon,2013,64:197-206.
[9]WANG Y,SHI R,LIN J,et al.Significant photocatalytic enhancement in methylene blue degradation of TiO2photocatalysts via graphene-like carbon in situ hybridization[J].Appl Catal B:Environ,2010,100:179-183.
[10]SEOL Y,JAVANDEL I.Citric acid-modified Fenton's reaction for the oxidation of chlorinated ethylenes in soil solution systems[J].Chemosphere,2008,72:537-542.
[12]PARKHOMCHUK E V,VANINA M P,PREIS S.The activation of heterogeneous Fenton-type catalyst Fe-MFI[J].Catalysis Communications,2008,9:381-385.
[13]BONSEN E M,SCHROETER S,JACOBS H,et al.Photocatalytic degradation of ammonia with t102 as photocatalyst in the laboratory and under the use of solar radiation[J].Chemosphere,1997,35:1431-1445.
[14]HOIGNE J,BADER H.Ozonation of water:kinetics of oxidation of ammonia by ozone and hydroxyl radicals[J].Environ Sci Technol,1978,12:79-84.
[15]MUTHUVEL I,SWAMINATHAN M.Highly solar active Fe(III)immobilized alumina for the degradation of acid violet 7[J].Sol Energy Mater Sol Cells,2008,92:857-863.
2015-05-19
国家自然科学基金资助项目(21347006);江苏省自然科学基金资助项目(BK20141178);绿色催化四川省高校重点实验室开放项目(LZJ1304);苏州市科技局纳米专项(ZXG201429)
周姗姗(1989-),女,江苏盐城人,硕士研究生,研究方向:环境功能材料。
X506 Document code:A Article ID:1672-0687(2016)02-0023-07
*通信联系人:刘守清(1962-),教授,博士,硕士生导师,E-mail:shouqing_liu@hotmail.com。

